Abstract
Objective
To estimate the cost-effectiveness of using an extensively hydrolyzed casein formula (eHCF) containing the probiotic Lactobacillus rhamnosus GG, (eHCF + LGG; Nutramigen LGG) as first-line management for cow’s milk allergy (CMA) compared with eHCF alone, soy-based formulae (SBF), hydrolyzed rice formulae (HRF), and amino acid formulae (AAF) in Italy, from the perspective of the Italian National Health Service (INHS) and parents.
Methods
Decision modeling was used to estimate the probability of infants developing tolerance to cow’s milk by 18 months, based on an observational study dataset. The model also estimated the cost (at 2012/2013 prices) of health care resource use funded by the INHS and formulae paid for by parents over 18 months after starting a formula, as well as the relative cost-effectiveness of each of the formulae.
Results
The probability of developing tolerance to cow’s milk by 18 months was higher among infants with either IgE-mediated or non-IgE-mediated allergy who were fed eHCF + LGG compared to those fed one of the other formulae. The total health care cost of initially feeding infants with eHCF + LGG was less than that of feeding infants with one of the other formulae. Hence, eHCF + LGG affords the greatest value for money to both the INHS and parents of infants with either IgE-mediated or non-IgE-mediated CMA.
Conclusion
Using eHCF + LGG instead of eHCF, SBF, HRF, or an AAF for first-line management of newly diagnosed infants with CMA in Italy affords a cost-effective use of publicly funded resources, and is cost-effective from the parents’ perspective, since it improves outcome for less cost. A randomized controlled study showing faster tolerance development in children receiving a probiotic-containing formula is required before this conclusion can be confirmed.
Keywords: amino acid formula, extensively hydrolyzed formula, soy-based formulae, hydrolyzed rice formulae
Introduction
Cow’s milk allergy (CMA) is one of the most common food allergies in early childhood, with an estimated incidence ranging between 0.02 and 0.03.1,2 Recent evidence suggests that the natural history of this allergy is changing, with an increased risk of persistence until later ages3,4 and severity.1,5 Guidelines addressing the management of infants with CMA recommend the use of substitutive hypoallergenic formulae.6,7 However, the potential impact of these formulae on disease duration has historically not been considered due to a lack of comparative data.
In a recent observational study, the addition of the probiotic Lactobacillus rhamnosus GG (LGG) to an extensively hydrolyzed casein formula (eHCF + LGG; Nutramigen LGG) was found to accelerate the development of tolerance in infants with CMA compared with those receiving eHCF alone, soy-based formulae (SBF), hydrolyzed rice formulae (HRF), or amino acid formulae (AAF).8 The study’s findings are consistent with a previous study.9
The comparative health economic impact of these different formulae is unknown, and therefore, dietetic choices are based largely on their safety, nutritional value, and purchase cost. Hence, the objective of the current study was to use data from the aforementioned observational study8 to estimate the cost-effectiveness of using eHCF + LGG as a first-line formula for CMA compared with eHCF, HRF, SBF, and AAF in Italy, from the perspective of the Italian National Health Service (INHS) and parents.
Methods
Economic model
A decision model was constructed using TreeAge Pro 2009 (TreeAge Software Inc., Williamstown, MA, USA) depicting the management of infants with CMA who are managed first-line with eHCF + LGG, eHCF, SBF, HRF, or an AAF. The model was populated with 1) the patient-level data from the aforementioned observational study,8 kindly provided by the authors of the study and 2) estimates of health care resource use derived from interviews with Italian pediatricians. The period of the model was up to 18 months or when an infant developed tolerance to cow’s milk if that occurred earlier.
Model inputs – clinical outcomes
The observational study was an open, nonrandomized intervention conducted between July 2010 and June 2012. The study prospectively evaluated otherwise healthy infants (n=260; mean age at recruitment of 5.92 months; 64% male; mean body weight 6.66 kg; 43% with IgE-mediated CMA) who were referred to a tertiary pediatric allergy center for a diagnostic double-blind, placebo-controlled food challenge (DBPCFC) for suspected CMA 15–30 days after starting a formula. Prior to referral, all infants had been managed with a formula that was selected and prescribed by a family pediatrician or physician. Management following study entry did not vary depending upon formula type. Infants were excluded from the study if they were fed a pre-probiotic product in the previous 4 weeks or if they experienced cow milk protein–induced anaphylaxis, eosinophilic disorders of the gastrointestinal tract, food protein–induced enterocolitic syndrome, or other chronic comorbidities.8
The endpoint of the study was the percentage of infants who developed tolerance to cow’s milk at 12 months from the start of a formula. Tolerance was confirmed following the results of a full anamnestic and clinical evaluation, skin prick test, atopy patch test, and oral food challenge.8 All food challenges were performed in a DBPCFC manner. Clinical acquisition of tolerance was defined by the presence of a negative DBPCFC over a 7-day post-challenge observation period. Infants with negative DBPCFC were reevaluated after 6 months to check the persistence of tolerance to cow’s milk.
The study found that significantly more infants in the eHCF + LGG group developed oral tolerance to cow’s milk after 12 months (78.9%; P<0.05) compared with those fed with one of the other formulae: eHCF (43.6%), HRF (32.6%), SBF (23.6%), and AAF (18.2%). Binary logistic regression revealed that the rate of infants developing tolerance at the end of the study was influenced by both IgE-mediated mechanism (odds ratio: 0.12; P<0.001) and choice of eHCF + LGG formula (odds ratio: 28.62; P<0.001). Time series forecasting was used to extrapolate the probability of developing tolerance to cow’s milk with each formula up to 18 months. These percentages were used to populate the model with the probability of infants developing tolerance to cow’s milk at 6-monthly intervals up to 18 months.
Model inputs – resource use
The model was populated with estimates of health care resource use pertaining to the management of infants with CMA in Italy. These estimates were derived from a series of interviews with five Italian pediatricians who managed infants with CMA according to the recommendations of the Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA)10 and the National Institute for Health and Care Excellence (NICE).11
The interviewed general pediatricians each saw a mean of <20 infants with suspected CMA per annum, with a mean age at presentation of ~5 months (range 3–6 months). According to the interviewees, ~75% of infants would have their CMA diagnosed by a general pediatrician. The other 25% of infants would be referred to a pediatric specialist for further investigations and confirmation of diagnosis. Time from referral to seeing a pediatric specialist would be ~1 week. The interviewed pediatric specialists each saw a mean of 300 infants with CMA per annum, with a mean age at presentation of ~6 months (range: 2–9 months). Estimates of resource use derived from the clinician interviews were incorporated into the model.
According to the interviewees, an infant would generally start a formula ~3 weeks after the initial visit to a pediatrician and ~2 weeks after the initial visit to a pediatric specialist. The infants would not generally receive other prescriptions (such as for gastrointestinal drugs and topical medication) for CMA.
In Italy, parents of affected infants pay for prescriptions of nutritional formulae. However, the prescriptions may be free, depending on the region, when CMA is associated with other comorbidities such as malnutrition or there is evidence of anaphylaxis.
The interviewed physicians prescribe formula based on an infants’ age and weight. Hence, up to 3 months of age, infants received ~150 mL/kg/day (500–1,000 mL/day) decreasing to ~120 mL/kg/day (800–900 mL/day) at 6 months of age. Between 7 and 9 months of age, infants received ~600 mL/day, decreasing to ~400 mL/day at >1 year of age.
In accordance with the infants’ age in the study, infants enter the model at a mean age of 5.92 months. Hence, it was estimated that infants would be prescribed: 48×400 g cans of formula in the first 6 months of the model, 36×400 g cans of formula in the next 6 months of the model, and 36×400 g cans of formula between 13 and 18 months.
Statistical analyses
Using analysis of covariance (ANCOVA), differences in tolerance acquisition between formulae were adjusted for any differences in the following baseline variables: age, sex, presenting symptoms, and baseline values of the diagnostic tests. The analysis found that the five groups were balanced and no adjustments were necessary. All statistical analyses were performed using IBM SPSS software (v21.0; IBM Corporation, Armonk, NY, USA).
Model outputs
The primary measure of clinical effectiveness was the probability of infants developing tolerance to cow’s milk by 18 months.
Unit costs at 2012/2013 prices12 were assigned to the estimates of resource use in the model in order to calculate the cost over 18 months from starting a formula of:
health care resource use funded by the INHS and
formulae paid for by parents.
The model was used to estimate the cost-effectiveness of using one formula compared with another in terms of the incremental cost per additional infant who developed tolerance to cow’s milk by 18 months in Italy. This was calculated as the difference between the expected costs of the two dietetic strategies divided by the difference between the expected outcomes of the two strategies in terms of the probability of developing tolerance to cow’s milk. If one of the formulae improved the probability of developing tolerance to cow’s milk for less cost, it was considered to be the dominant (cost-effective) dietetic strategy.
Sensitivity analyses
To assess uncertainty within the model, probabilistic sensitivity analysis was undertaken (100,000 iterations of the model) by simultaneously varying the probabilities, clinical outcomes, resource use values, and unit costs within the model. A beta distribution was used to represent uncertainty in probability values by assuming a 5% standard deviation around the mean values. Clinical outcomes and resource use estimates were varied randomly according to a log-normal distribution by assuming a 5% standard deviation around the mean values. Unit costs were varied randomly according to a gamma distribution by assuming a 10% standard deviation around the mean values. The outputs from these analyses were used to estimate the probability of being cost-effective at different thresholds of cost per additional infant who developed tolerance to cow’s milk by 18 months.
In addition, deterministic sensitivity analyses were performed to identify how the incremental cost-effectiveness of one strategy over the other would change by varying different parameters in the model. The budget impact and resource implications of starting infants with eHCF + LGG compared to current practice were also estimated for the annual cohort of newly diagnosed infants with CMA in Italy.
Results
Probability of developing tolerance to cow’s milk
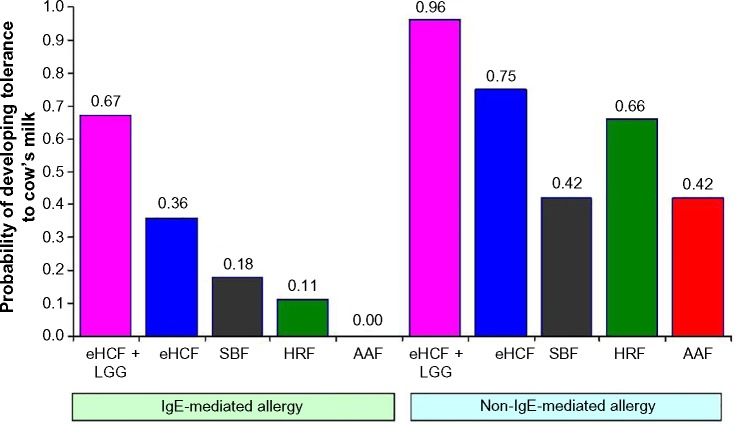
The probability of developing tolerance to cow’s milk was higher among infants who were initially fed with eHCF + LGG (Figure 1). Also, the probability of developing tolerance to cow’s milk was higher among those infants with non-IgE-mediated CMA compared to those with IgE-mediated allergy.
Figure 1.
Expected probability of infants developing tolerance to cow’s milk by 18 months after starting a formula.
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; LGG, Lactobacillus rhamnosus GG; SBF, soy-based formula.
Health care resource use and corresponding costs
An infant who is initially managed with eHCF + LGG is expected to consume fewer health care resources than infants managed with the other formulae (Table 1). Hence, initially feeding infants with eHCF + LGG instead of the other formulae is expected to free-up health care resources for alternative use by other patients. Consequently, the total health care cost of initially feeding infants with eHCF + LGG is expected to be less than that of feeding infants with one of the other formulae (Table 1). Similarly, the cost to parents of infants managed with eHCF + LGG is expected to be less than that to parents of infants fed with one of the other formulae (Table 1).
Table 1.
Expected levels of health care resource use and corresponding costs over 18 months from starting a formula
| eHCF + LGG
|
eHCF
|
SBF
|
HRF
|
AAF
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgE-mediated | Non-IgE-mediated | IgE-mediated | Non-IgE-mediated | IgE-mediated | Non-IgE-mediated | IgE-mediated | Non-IgE-mediated | IgE-mediated | Non-IgE-mediated | |
| Mean resource per patient | ||||||||||
| Number of visits to a pediatrician | 4.23 | 1.32 | 4.68 | 1.52 | 4.85 | 1.66 | 4.93 | 1.74 | 4.97 | 1.69 |
| Number of visits to a pediatric specialist | 0.74 | 0.38 | 0.78 | 0.41 | 0.79 | 0.44 | 0.80 | 0.45 | 0.79 | 0.44 |
| Number of skin prick tests | 0.43 | 0.77 | 0.48 | 0.84 | 0.50 | 0.89 | 0.51 | 0.91 | 0.51 | 0.89 |
| Number of RAST | 1.12 | 1.02 | 1.17 | 1.05 | 1.18 | 1.07 | 1.19 | 1.09 | 1.19 | 1.08 |
| Number of atopy tests | 0.92 | 0.62 | 0.92 | 0.62 | 0.92 | 0.62 | 0.92 | 0.62 | 0.92 | 0.62 |
| Number of oral food challenges | 2.52 | 0.63 | 3.03 | 0.89 | 3.22 | 1.08 | 3.31 | 1.18 | 3.41 | 1.11 |
| Mean cost of health service resource use per patient | ||||||||||
| Pediatrician visits | €62.30 | €24.74 | €68.22 | €27.32 | €70.40 | €29.21 | €71.36 | €30.20 | €71.87 | €29.51 |
| Pediatric allergist visits | €15.28 | €7.75 | €16.05 | €8.52 | €16.29 | €9.09 | €16.43 | €9.38 | €16.30 | €9.18 |
| Tests | €146.21 | €72.46 | €166.32 | €84.00 | €173.89 | €92.44 | €177.46 | €96.88 | €179.37 | €93.77 |
| Total | €223.79 | €104.95 | €250.60 | €119.85 | €260.58 | €130.73 | €265.25 | €136.46 | €267.53 | €132.45 |
| Mean cost of formula per patient to parents | €2,178 | €1,640 | €2,643 | €2,179 | €2,223 | €2,043 | €2,353 | €1,983 | €4,745 | €4,136 |
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; LGG, Lactobacillus rhamnosus GG; RAST, radioallergosorbent test; SBF, soy-based formula.
Cost-effectiveness analyses
From the INHS’ perspective
Of the five formulae, use of eHCF + LGG resulted in a lower 18-month cost and a greater probability of developing tolerance than the other four formulae among infants with both IgE-mediated and non-IgE-mediated CMA. Hence, starting feeding with this formula was found to be the dominant strategy.
Among infants with IgE-mediated CMA, initial feeding with eHCF was found to be a dominant strategy when compared to starting feeding with SBF, HRF, or an AAF. The analysis also found that:
➢ initially feeding infants with SBF was a dominant strategy when compared to starting feeding with HRF or an AAF;
➢ initially feeding infants with HRF was a dominant strategy compared to an AAF.
Among infants with non-IgE-mediated CMA, initially feeding with eHCF was a dominant strategy when compared to starting feeding with SBF, HRF, or an AAF. The analysis also found that:
➢ Initially feeding infants with an AAF instead of SBF increased the probability of developing tolerance to cow’s milk by 0.003, but increased INHS costs by €2. Hence, the cost for each additional patient who developed tolerance to cow’s milk with AAF was €667.
➢ Initially feeding infants with HRF instead of an AAF increased the probability of developing tolerance to cow’s milk by 0.237, but increased INHS costs by €4. Hence, the cost for each additional patient who developed tolerance to cow’s milk with HRF was €17.
From parents’ perspective
From the parents’ perspective, eHCF + LGG is the preferred dietetic choice for both infants with IgE-mediated and non-IgE-mediated CMA, since it improved outcome for less cost (ie the dominant formula).
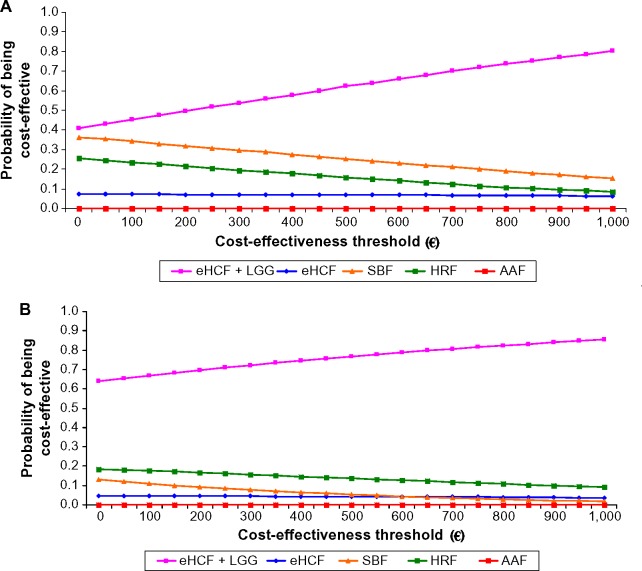
Sensitivity analyses
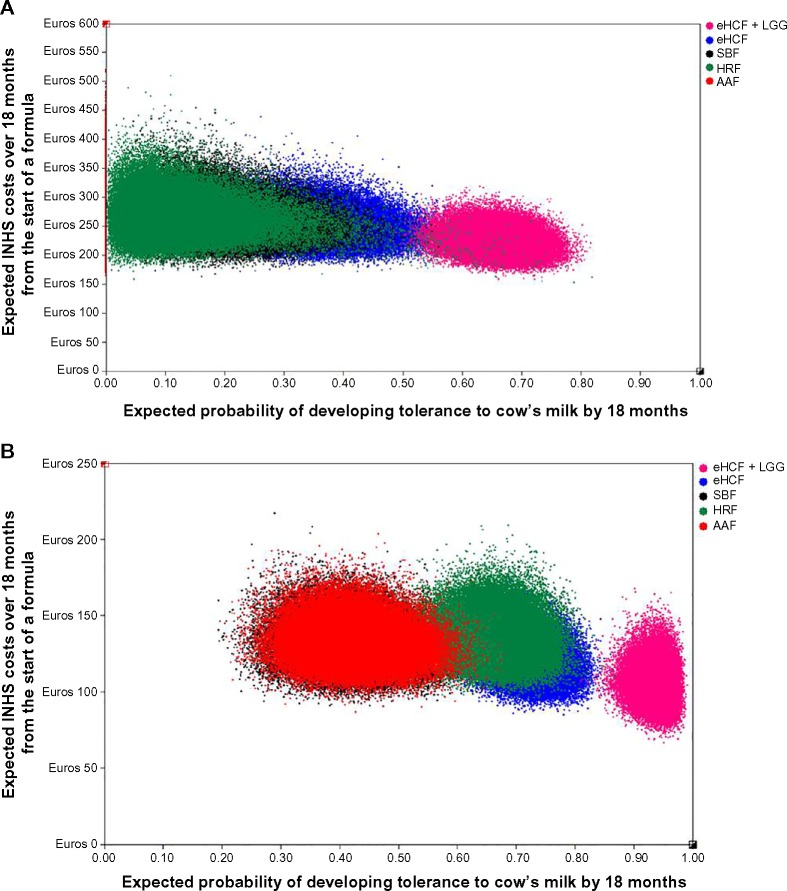
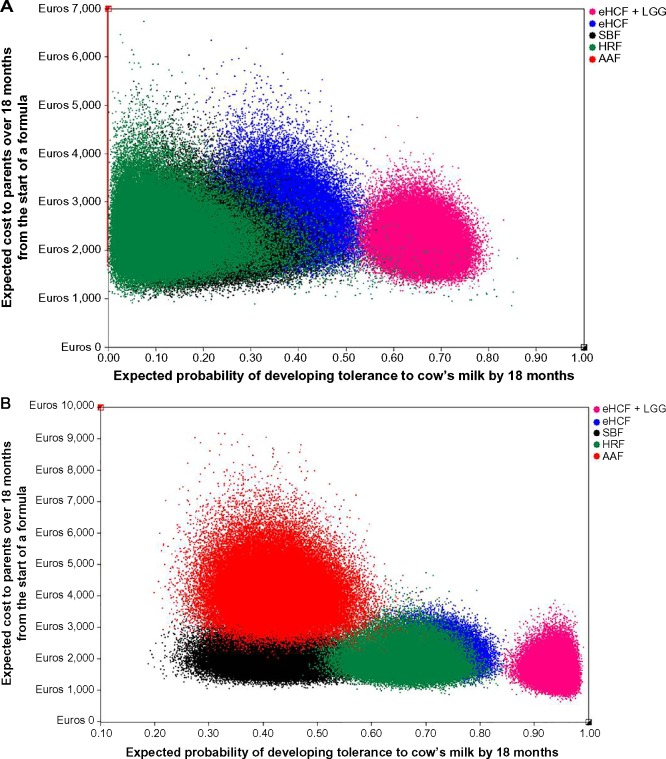
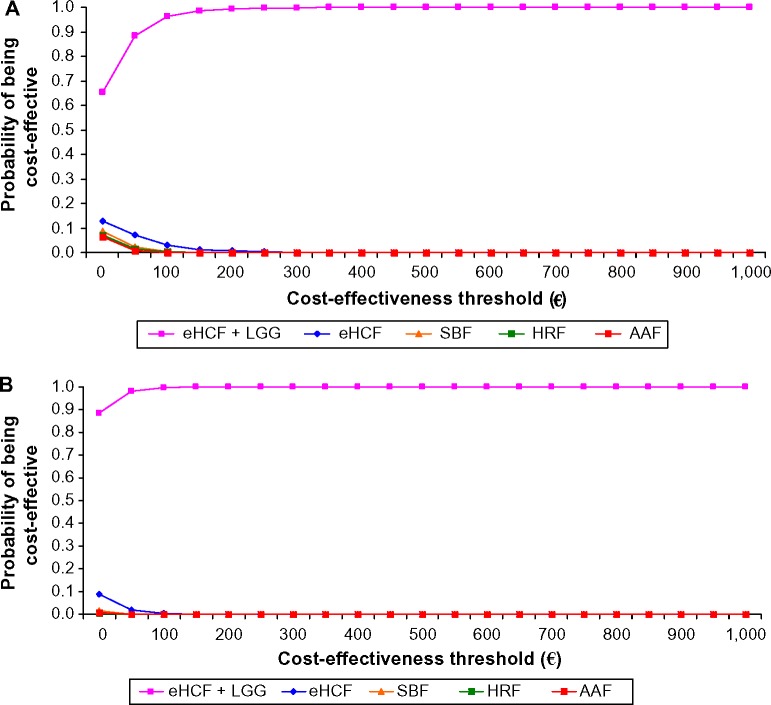
Probabilistic sensitivity analyses were performed to estimate the distribution of expected INHS costs (Figure 2) and parents’ costs (Figure 3) over 18 months from starting a formula and probability of developing tolerance to cow’s milk by 18 months. Using these distributions, the probability of each formula being cost-effective at different cost-effectiveness thresholds was estimated (Figures 4 and 5). These graphs showed that the probability of eHCF + LGG being cost-effective was greater than with the other formulae for both IgE-mediated and non-IgE-mediated allergic infants, from the perspective of both the health service and parents. Moreover, these graphs suggest that neither eHCF, SBF, HRF, nor AAF would afford a cost-effective use of resources when compared with eHCF + LGG.
Figure 2.
(A) Distribution of expected INHS costs over 18 months from starting a formula and expected probability of developing tolerance to cow’s milk by 18 months among IgE-mediated allergic infants, generated by 100,000 iterations of the model. (B) Distribution of expected INHS costs over 18 months from starting a formula and expected probability of developing tolerance to cow’s milk by 18 months among non-IgE-mediated allergic infants, generated by 100,000 iterations of the model.
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; INHS, Italian National Health Service; LGG, Lactobacillus rhamnosus GG; SBF, soy-based formula.
Figure 3.
(A) Distribution of expected costs to parents over 18 months from starting a formula and expected probability of developing tolerance to cow’s milk by 18 months among IgE-mediated allergic infants, generated by 100,000 iterations of the model. (B) Distribution of expected costs to parents over 18 months from starting a formula and expected probability of developing tolerance to cow’s milk by 18 months among non-IgE-mediated allergic infants, generated by 100,000 iterations of the model.
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; LGG, Lactobacillus rhamnosus GG; SBF, soy-based formula.
Figure 4.
(A) Probability of being cost-effective at different cost-effectiveness thresholds for IgE-mediated allergy infants, from the perspective of the INHS. (B) Probability of being cost-effective at different cost-effectiveness thresholds for non-IgE-mediated allergy infants, from the perspective of the INHS.
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; INHS, Italian National Health Service; LGG, Lactobacillus rhamnosus GG; SBF, soy-based formula.
Figure 5.
(A) Probability of being cost-effective at different cost-effectiveness thresholds for IgE-mediated allergy infants, from parents’ perspective. (B) Probability of being cost-effective at different cost-effectiveness thresholds for non-IgE-mediated allergy infants, from parents’ perspective.
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; LGG, Lactobacillus rhamnosus GG; SBF, soy-based formula.
These analyses also indicate that eHCF + LGG affords the greatest value for money to the INHS followed by eHCF, SBF, HRF, and AAF in that order for managing infants with IgE-mediated allergy, but followed by eHCF, HRF, SBF, and AAF in that order for managing infants with non-IgE-mediated allergy. From the parents’ perspective, eHCF + LGG affords the greatest value for money followed by SBF, HRF, eHCF, and AAF in that order for managing infants with IgE-mediated allergy, but followed by HRF, SBF, eHCF, and AAF in that order for managing infants with non-IgE-mediated allergy. Irrespective of the perspective, eHCF + LGG is ranked as the preferred formula, and AAF the last formula of choice.
Deterministic sensitivity analyses (Table 2) demonstrated that inclusion/exclusion of the probability of developing tolerance to cow’s milk after 12 months has minimal impact on the results. However, exclusion of the probability of developing tolerance to cow’s milk after 6 months has the potential to yield misleading results. Additionally, changes in resource use can potentially change costs incurred by the INHS, but they are unlikely to change the ranking of dietetic choices. The relative cost-effectiveness of the five formulae was not sensitive to changes in any other model input.
Table 2.
Sensitivity analyses
| Scenario | Formula | Range in expected probability of developing tolerance to cow’s milk
|
Range in expected INHS costs
|
Range in expected parents’ costs
|
|||
|---|---|---|---|---|---|---|---|
| IgE-mediated | Non-IgE-mediated | IgE-mediated | Non-IgE-mediated | IgE-mediated | Non-IgE-mediated | ||
| Assume no more infants develop tolerance to cow’s milk after 12 months | eHCF + LGG | 0.70–0.55 | 0.95–0.90 | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline |
| eHCF | 0.35–0.25 | 0.75–0.60 | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | |
| SBF | 0.20–0.15 | 0.45–0.30 | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | |
| HRF | 0.15–0.10 | 0.65–0.60 | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | |
| AAF | 0.00–0.00 | 0.45–0.30 | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | Unchanged from baseline | |
| Assume no incremental improvement after 6 months and no more infants develop tolerance to cow’s milk after 12 months | eHCF + LGG | 0.65–0.30 | 0.95–0.85 | €220–260 | €100–140 | €2,100–2,700 | €1,600–2,300 |
| eHCF | 0.35–0.10 | 0.75–0.40 | €240–270 | €110–140 | €2,600–2,900 | €2,100–2,700 | |
| SBF | 0.20–0.10 | 0.45–0.20 | €250–270 | €120–140 | €2,200–2,300 | €2,000–2,200 | |
| HRF | 0.15–0.10 | 0.65–0.55 | €260–270 | €130–140 | €2,300–2,400 | €1,900–2,100 | |
| AAF | 0.00–0.00 | 0.45–0.25 | €260–270 | €130–140 | €4,700–4,800 | €4,100–4,500 | |
| The number of follow-up visits to a pediatrician ranges from 50% below to 50% above the base case value | eHCF + LGG | Unchanged from baseline | Unchanged from baseline | €210–260 | €90–100 | Unchanged from baseline | Unchanged from baseline |
| eHCF | Unchanged from baseline | Unchanged from baseline | €240–290 | €110–120 | Unchanged from baseline | Unchanged from baseline | |
| SBF | Unchanged from baseline | Unchanged from baseline | €250–300 | €120–130 | Unchanged from baseline | Unchanged from baseline | |
| HRF | Unchanged from baseline | Unchanged from baseline | €250–300 | €120–130 | Unchanged from baseline | Unchanged from baseline | |
| AAF | Unchanged from baseline | Unchanged from baseline | €250–300 | €120–130 | Unchanged from baseline | Unchanged from baseline | |
| The number of follow-up visits to a pediatric specialist ranges from 50% below to 50% above the base case value | eHCF + LGG | Unchanged from baseline | Unchanged from baseline | €210–250 | €90–100 | Unchanged from baseline | Unchanged from baseline |
| eHCF | Unchanged from baseline | Unchanged from baseline | €240–290 | €110–120 | Unchanged from baseline | Unchanged from baseline | |
| SBF | Unchanged from baseline | Unchanged from baseline | €250–300 | €120–130 | Unchanged from baseline | Unchanged from baseline | |
| HRF | Unchanged from baseline | Unchanged from baseline | €250–300 | €120–130 | Unchanged from baseline | Unchanged from baseline | |
| AAF | Unchanged from baseline | Unchanged from baseline | €250–300 | €120–130 | Unchanged from baseline | Unchanged from baseline | |
| The number of diagnostic tests ranges from 50% below to 50% above the base case value | eHCF + LGG | Unchanged from baseline | Unchanged from baseline | €160–300 | €60–140 | Unchanged from baseline | Unchanged from baseline |
| eHCF | Unchanged from baseline | Unchanged from baseline | €180–400 | €70–160 | Unchanged from baseline | Unchanged from baseline | |
| SBF | Unchanged from baseline | Unchanged from baseline | €180–400 | €80–170 | Unchanged from baseline | Unchanged from baseline | |
| HRF | Unchanged from baseline | Unchanged from baseline | €180–400 | €80–170 | Unchanged from baseline | Unchanged from baseline | |
| AAF | Unchanged from baseline | Unchanged from baseline | €190–400 | €80–170 | Unchanged from baseline | Unchanged from baseline | |
Abbreviations: AAF, amino acid formula; eHCF, extensively hydrolyzed casein formula; HRF, hydrolyzed rice formula; INHS, Italian National Health Service; LGG, Lactobacillus rhamnosus GG; SBF, soy-based formula.
Budget impact and resource implications of using eHCF + LGG
There are an estimated 0.53 million live births in Italy per annum13 and the incidence of CMA is reported to be 0.025.1,2 Hence, there are an estimated 16,000 new CMA-affected infants per annum in Italy. Assuming the distribution of formula use is as depicted in the aforementioned study,8 current management of all 16,000 newly diagnosed infants results in 52% of the cohort developing tolerance to cow’s milk by 18 months, 46,300 visits to pediatricians, and a cost to the INHS of €2.83 million. If all these infants were initially managed with eHCF + LGG, it is expected that 84% of the cohort would develop tolerance to cow’s milk by 18 months, there would be 5,300 fewer visits to pediatricians, and a cost reduction to the INHS of €0.35 million.
If the budget impact analysis only considered a period of 12 months following the start of a formula, current management of all 16,000 newly diagnosed infants results in 44% of the cohort developing tolerance to cow’s milk by 12 months, 39,500 visits to pediatricians, and a cost to the INHS of €2.49 million. If all these infants were initially managed with eHCF + LGG, it is expected that 84% of the cohort would develop tolerance to cow’s milk by 12 months, there would be 5,300 fewer visits to pediatricians, and a cost reduction to the INHS of €0.35 million. The minimal difference in the budget impact of managing CMA over 12 and 18 months reflects the fact that most resources are used during the first 12 months of management following the start of a formula.
Discussion
To our knowledge, this is the first health economic study to estimate the relative cost-effectiveness of using eHCF + LGG as first-line management for infants with CMA compared to eHCF, SBF, HRF, and AAF in Italy. Accordingly, the basis of the analysis was the only comparative dataset currently available.8 The advantage of using this observational dataset is that the dietary effect was measured under controlled conditions. However, infants were not randomized to their formula, sample sizes were small in absolute terms and unbalanced between the groups, and resource use was not recorded. The study’s authors made every attempt to account for baseline differences between the groups and to overcome the nonrandomized study design. Differences in developing tolerance to cow’s milk between treatments were adjusted for any heterogeneity in baseline variables by the study’s authors. Additionally, we performed ANCOVA and found that no further adjustments were necessary. Nevertheless, there may have been some differences that have not been accounted for. The inherent variability and uncertainty of using data from this small and unequal sample of patients was addressed to some extent by our extensive sensitivity analyses. Notwithstanding this, power calculations showed that the sample sizes were sufficiently large to detect the observed differences, with 90% power and a Type I (alpha) error of 0.05 between the eHCF + LGG groups and the other groups, except the eHCF group among the infants with IgE-mediated CMA. The sample sizes in the IgE-mediated group fed with eHCF + LGG and eHCF had <80% power to detect the observed differences between the two groups. The results from the observational study8 are consistent with another study that showed that in both IgE- and non-IgE-mediated CMA, the addition of LGG to eHCF resulted in a higher rate of developing tolerance after 12 months of feeding.9 Additionally, we recently reported that in the US, significantly more eHCF + LGG-fed CMA infants in clinical practice were successfully managed compared with those who were fed with eHCF or AAF.14 There were no other published studies assessing the health economic impact of alternative formula for the management of CMA, except for our previous UK study.15 This UK study, which was also based on actual clinical practice, supports the current findings that eHCF affords a cost-effective use of health care resources when compared to AAF.
In order to estimate the health economic benefit of developing tolerance at 12 months, the model was constructed over a period of 18 months. However, sensitivity analyses showed that inclusion/exclusion of the probability of developing tolerance to cow’s milk after 12 months has minimal impact on the results, since the majority of resources associated with managing infants with CMA are used in the first 12 months following diagnosis.
While the study results are compelling, the model may not necessarily reflect clinical outcomes associated with managing a large cohort of infants in clinical practice. Hence, the results should be viewed with some caution until more data become available which can be used to update the model. In particular, this study’s findings should provide a framework for a randomized, controlled study to measure the cost-effectiveness of tolerance development in children receiving a probiotic-containing formula compared to other formulae.
The study has several other limitations. The model was informed with assumptions about treatment patterns from pediatricians based at five centers. Hence, the levels of health care resource use may not be indicative of Italy as a whole. There was insufficient published clinical evidence to enable us to extrapolate the model beyond 18 months. Therefore, the analysis estimated the cost and consequences of managing infants up to 18 months and does not consider the potential impact of managing infants who continue to suffer from CMA beyond that period. Infants in the observational study8 were well matched and those with comorbidities were excluded. Hence, the model used resource estimates for the “average infant” and does not consider the impact of other factors that may affect the results, such as comorbidities, underlying disease severity, and pathology of the underlying disease. Additionally, the analysis does not take into account the suitability of infants to receive different formulae. The model only considered direct health care costs borne by payers and excluded indirect costs incurred by society as a result of employed parents taking time off work. Also excluded are changes in quality of life and improvements in general well-being of sufferers and their parents as well as parents’ preferences. Consequently, this study may have underestimated the relative cost-effectiveness of eHCF + LGG.
Despite these limitations, the model shows that over the first 18 months, proportionally more infants fed with eHCF + LGG than with the other formulae would develop tolerance to cow’s milk. Consequently, they cost the health service less to manage and the cost incurred by parents for the formulae is less. This is an expected finding since, according to the interviewed pediatricians, infants who develop tolerance to cow’s milk would no longer require any management or feeding with a hypoallergenic formula. Accordingly, treating the annual cohort of 16,000 new CMA-affected infants in Italy with eHCF + LGG instead of the current mix of formulae could increase the percentage of infants developing tolerance to cow’s milk from 52% to 84% and free up 5,300 visits to pediatricians and reduce health service costs by up to €0.35 million. Clearly, initial use of eHCF + LGG has the potential to release health care resources for alternative use within the system.
LGG administration is associated with a complex response in intestinal mucosa, reflected by the up- and downregulation of several genes involved in immune response, inflammation, cell–cell signaling, signal transcription, and transduction.16 Additionally, LGG is known to modulate immune functions via various pathways17–20 to alter cytokine levels that may be involved in IgE- or non-IgE-mediated CMA, thereby modulating the major pathways involved in CMA pathogenesis17–21 and to alter the composition of the intestinal microbial community with a large increase in the number of taxa previously associated with less development of allergy and atopy.22 Moreover, in infants with CMA, the addition of LGG to eHCF compared with eHCF alone has been shown to more effectively attenuate increased intestinal permeability, and to decrease fecal calprotectin and the persistence of occult fecal blood losses.23,24
In conclusion, within the limitations of the observational dataset, first-line management of newly diagnosed infants with CMA with eHCF + LGG instead of eHCF, SBF, HRF, or an AAF improves outcome, releases health care resources for alternative use, reduces costs to the INHS, affords a cost- effective use of publicly funded resources, and is cost-effective from the parents’ perspective. Hence, eHCF + LGG is the preferred first-line formula for newly diagnosed infants compared to the other dietetic choices. However, a randomized controlled study showing faster tolerance development in children receiving a probiotic-containing formula is required before this conclusion can be confirmed.
Acknowledgments
This study was supported with an unrestricted research grant from Mead Johnson Nutrition, Glenview, IL, USA. However, Mead Johnson Nutrition had no influence on: 1) the study design; 2) the collection, analysis, and interpretation of data; 3) the writing of the manuscript; and 4) the decision to submit the manuscript for publication.
The authors wish to thank Professor Roberto Berni Canani, Department of Translational Medical Science, Pediatric Section, University of Naples “Federico II”, Naples, Italy and co-workers for providing their observational study dataset in order to perform the health economic evaluation.
The authors also wish to thank the following clinicians for their contributions to this study: Dr Marina Govoni, pediatrician, Via Di Villa Pardò, Bologna; Dr Francesco Savino, pediatrician, Ospedale Infantile Regina Margherita, Torino; Dr Massimo Landi, pediatrician, Pediatria di Gruppo AslTo1, Torino; Dr Silvia Salvatore, pediatric allergist, Clinica Pediatrica di Varese, Varese; and Dr Silvia Caimmi, pediatric allergist, Università degli Studi di Pavia, Pavia.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored expert panel report. J Allergy Clin Immunol. 2010;126(6):1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–1177. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Levy Y, Segal N, Garty B, Danon YL. Lessons from the clinical course of IgE-mediated cow milk allergy in Israel. Pediatr Allergy Immunol. 2007;18(7):589–593. doi: 10.1111/j.1399-3038.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 5.Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(Suppl 15):23–28. doi: 10.1034/j.1399-3038.13.s.15.7.x. [DOI] [PubMed] [Google Scholar]
- 6.Koletzko S, Niggemann B, Arato A, et al. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–229. doi: 10.1097/MPG.0b013e31825c9482. [DOI] [PubMed] [Google Scholar]
- 7.Venter C, Arshad SH. Guideline fever: an overview of DRACMA, US NIAID and UK NICE guidelines. Curr Opin Allergy Clin Immunol. 2012;12(3):302–315. doi: 10.1097/ACI.0b013e3283535893. [DOI] [PubMed] [Google Scholar]
- 8.Berni Canani R, Nocerino R, Terrin G, et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163(3):771–777.e1. doi: 10.1016/j.jpeds.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Berni Canani R, Nocerino R, Terrin G, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580–582. 582.e1–5. doi: 10.1016/j.jaci.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s milk Allergy (DRACMA) Guidelines. Milwaukee, WI: WAO; 2010. [Accessed March 4, 2015]. Available from: http://www.worldallergy.org/publications/WAO_DRACMA_guidelines.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE) Food Allergy in Children and Young People. London, UK: NICE; 2011. [Accessed February 5, 2015]. Available from: http://www.nice.org.uk/guidance/cg116/resources/cg116-food-allergy-in-children-and-young-people-full-guideline3. [Google Scholar]
- 12.Ministry of Health (Italy) Nomenclatore tariffario dell’assistenza specialistica ambulatoriale. Rome, Italy: Ministry of Health (Italy); 2010. [Accessed October 1, 2013]. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1767&area=programmazioneSanitariaLea&menu=lea. [Google Scholar]
- 13.Italian National Institute of Statistics (ISTAT) [homepage on the Internet] Rome, Italy: ISTAT; 2009. [Accessed October 1, 2013]. Available from: http://demo.istat.it/bil2012/index_e.html. [Google Scholar]
- 14.Ovcinnikova O, Panca M, Guest JF. Cost-effectiveness of using an extensively hydrolyzed casein formula plus the probiotic Lactobacillus rhamnosus GG compared to an extensively hydrolyzed formula alone or an amino acid formula as first-line dietary management for cow’s milk allergy in the US. Clinicoecon Outcomes Res. 2015;2015(7):145–152. doi: 10.2147/CEOR.S75071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor RR, Sladkevicius E, Panca M, Lack G, Guest JF. Cost- effectiveness of using an extensively hydrolysed formula compared to an amino acid formula as first-line treatment for cow milk allergy in the UK. Pediatr Allergy Immunol. 2012;23(3):240–249. doi: 10.1111/j.1399-3038.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 16.Di Caro S, Tao H, Grillo A, et al. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig Liver Dis. 2005;37(5):320–329. doi: 10.1016/j.dld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Pan SJ, Kuo CH, Lam KP, Chu YT, Wang WL, Hung CH. Probiotics and allergy in children – an update review. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e659–e666. doi: 10.1111/j.1399-3038.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghadimi D, Fölster-Holst R, de Vrese M, Winkler P, Heller KJ, Schr-ezenmeir J. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology. 2008;213(8):677–692. doi: 10.1016/j.imbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Donato KA, Gareau MG, Wang YJ, Sherman PM. Lactobacillus rham-nosus GG attenuates interferon-{gamma} and tumor necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiology. 2010;156(Pt 11):3288–3297. doi: 10.1099/mic.0.040139-0. [DOI] [PubMed] [Google Scholar]
- 20.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One. 2009;4(9):e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai S, Bay BH, Lee YK, Lu J, Mahendran R. Live and lyophilized Lactobacillus species elicit differential immunomodulatory effects on immune cells. FEMS Microbiol Lett. 2010;302(2):189–196. doi: 10.1111/j.1574-6968.2009.01853.x. [DOI] [PubMed] [Google Scholar]
- 22.Cox MJ, Huang YJ, Fujimura KE, et al. Lactobacillus casei abundance is associated with profound shifts in the infant gut microbiome. PLoS One. 2010;5(1):e8745. doi: 10.1371/journal.pone.0008745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldassarre ME, Laforgia N, Fanelli M, Laneve A, Grosso R, Lifschitz C. Lactobacillus GG improves recovery in infants with blood in the stools and presumptive allergic colitis compared with extensively hydrolyzed formula alone. J Pediatr. 2010;156(3):397–401. doi: 10.1016/j.jpeds.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Pohjavuori E, Viljanen M, Korpela R, et al. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow’s milk allergy. J Allergy Clin Immunol. 2004;114(1):131–136. doi: 10.1016/j.jaci.2004.03.036. [DOI] [PubMed] [Google Scholar]