Abstract
Introduction
Inflammatory mediators are key players in the pathogenesis of osteoarthritis (OA) and bone destruction. Conventional drugs suppress symptomatic activity and have no therapeutic influence on disease. Cissus quadrangularis and Withania somnifera are widely used for the treatment of bone fractures and wounds; however, the cellular and molecular mechanisms regulated by these herbals are still unclear.
Methods
We established an in vitro OA culture model by exposing human chondrocytes to proinflammatory cytokine and interleukin (IL)-1β for 36 hours prior to treatment with the herbals: C. quadrangularis, W. somnifera, and the combination of the two herbals. Cell viability, toxicity, and gene expression of OA modifying agents were examined. In addition, expression of survivin, which is crucial for cell growth, was analyzed. In vivo work on osteotomized rats studied the bone and cartilage regenerative effects of C. quadrangularis, W. somnifera, and the combination therapy.
Results
Exposure of chondrocytes to IL-1β induced significant toxicity and cell death. However, herbal treatment alleviated IL-1β induced cell toxicity and upregulated cell growth and proliferation. C. quadrangularis inhibited gene expression of cytokines and matrix metalloproteinases, known to aggravate cartilage and bone destruction, and augmented expression of survivin by inhibiting p38 MAPK. Interestingly, osteotomized rats treated with C. quadrangularis drastically enhanced alkaline phosphatase and cartilage tissue formation as compared to untreated, W. somnifera only, or the combination of both herbals.
Conclusion
Our findings demonstrate for the first time the signaling mechanisms regulated by C. quadrangularis and W. somnifera in OA and osteogenesis. We suggest that the chondroprotective effects and regenerative ability of these herbals are via the upregulation of survivin that exerts inhibitory effects on the p38 MAPK signaling pathway. These findings thus validate C. quadrangularis as a potential therapeutic for rheumatic disorders.
Keywords: cytokines, inflammation, osteoarthritis, Withania somnifera, alternative therapy, herbal
Introduction
Osteoarthritis (OA) is a chronic disease directly associated to cartilage degeneration, joint inflammation, and bone sclerosis.1–3 Studies have shown significant superinduction of proinflammatory agents like interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and reactive oxygen species (ROS) during the osteoarthritic process. These mediators consequentially induce a destructive cascade of events that lead to a positive stimulatory loop of the cytokines as well as the synthesis of matrix metalloproteinase (MMP) and prostaglandins.4–6 Current therapeutics such as nonsteroidal anti-inflammatory drugs and glucocorticoids offer symptomatic relief but disregard disease modification or progression. In addition, biologic treatments including TNF-α antagonist, etanercept (TNF inhibitor), and tanezumab (monoclonal antibody for β-nerve growth factors) enhance cartilage restoration, but their extended use induces severe side effects such as infections and gastrointestinal disorders.7 Therefore, long-term, nontoxic therapeutics for the treatment of chronic arthritis are imperative.
Studies worldwide have shown 60%–90% of arthritic patients are resolving to undertake complementary and alternative treatments as an alternative to conventional therapeutics.8 Herbals such as Cissus quadrangularis (Asthi Shrinkhala) and Withania somnifera (Ashwagandha) used for their bone healing and anti-inflammatory activities have recently been gaining worldwide recognition for their anti-arthritic effects. W. somnifera was reported to induce protective effects on mice with adjuvant-induced arthritis by significantly reducing urinary constituents, lipid peroxidation, and glycoproteins and by increasing bone deposition.9 In addition, immunomodulatory effects of W. somnifera reported by Rasool and Varalakshmi10 demonstrated significant anti-inflammatory activity where W. somnifera drastically reduced complement activity, proliferation of lymphocytes, and inflammatory responses as a results of delayed-type hypersensitivity in arthritic mice as compared to control groups.10 The multifunctional herb C. quadrangularis was recently shown to induce proliferation, matrix mineralization, and differentiation of human osteoblastic cells.11 C. quadrangularis significantly relieved pain in patients suffering with OA by hindering the production of prostaglandins, the molecule associated with pain.12 These results demonstrate extensive therapeutic potentials of these herbals; however, more studies examining the molecular and cellular mechanisms remain to be elucidated.
Our lab has worked extensively with natural bioactive molecules as therapies for chronic diseases including cancer, brain disorders, and arthritis.13–18 Recently, we reported for the first time the therapeutic activity of the widely used herbal Lakshadi Guggul (LG) in collagen-induced arthritic mice.19 In this study, LG significantly improved chondroprotectivity in cells exposed to inflammatory conditions and inhibited the production of prostaglandins and ROS. Furthermore, LG administered orally to collagen-induced arthritic mice significantly lowered inflammation by inhibiting proinflammatory cytokines and induced cartilage regeneration.19 Although these herbal formulations have been used for many decades, the mechanisms by which they ameliorate anti-inflammatory and bone regenerative activities are vaguely understood. Therefore, by exploiting gene and protein expression of inflammatory markers, the molecular and cellular activity of C. quadrangularis and W. somnifera in chondrocytes were examined, and bone regenerative effects in osteotomized rats were elucidated.
Materials and methods
Animal model
All animal procedures were approved by the Indian National Science Academy’s (INSA) Ethics committee and performed in accordance with the standards and guidelines for animal research set out by the academy in Ayurevedic College, Paprola, Kangra, Himachal Pradesh, India. Swiss Albino rats more than 3-months old of both sexes were used in this study. Animals were housed in a temperature controlled room under a 12-hour light/dark cycle. Rats had free access to a standard pellet diet and water. All animals were allowed to adapt to the new environment before the start of the experiment. To induce cartilage and bone damage, rats were first anesthetized with ketamine (60 mg/kg) and xylazine (8 mg/kg) and then osteotomized. The radial mid diaphyses of the left limb underwent open transverse osteotomy with a sharp osteotome. Rats were divided into four groups of six animals and each group was given either the control diet, C. quadrangularis, W. somnifera, or the combination of C. quadrangularis and W. somnifera at a dose of 0.018× human adult dose.20 Rats were fed treatment diets for 4 weeks before being euthanized according to INSA guidelines.
Radiographic assessment of osteotomy
Following treatment with herbals, radiographic examinations of operated rat limbs from all four groups were analyzed. Craniocaudal and lateral views to evaluate the presence of bone callus were imaged. Radiographic changes were scaled from 0 to 5 depending on the severity of the osteotomy line observed (0= sharp or sclerotic line throughout; 1= in more than 75% of the diameter; 2= well-defined line extended in both projections; 3= extended only in one projection; 4= osteotomy line seen faintly; 5= osteotomy not seen).
Histopathological analysis of bone callus
Rat limbs subjected to osteotomy were dissected and fixed in 10% formalin for 24 hours. Bones were decalcified in solution containing 10% sodium citrate and 14% EDTA for 5–7 days with gentle rocking. Following decalcification, tissues were dehydrated through a graded ethanol series and embedded in paraffin by routine procedures. Tissues were sectioned into 10 μm slices and stained with hematoxylin and eosin. Tissue sections were evaluated and scored on a scale of 1–11, in which 1= all fibrous tissue; 2= more fibrous tissue than cartilage; 3= fibrous and cartilaginous tissue in equal proportion; 4= fibrous tissue with more cartilaginous tissue than woven bone; 5= fibrous tissue with equal cartilage and woven bone; 6= fibrous tissue with more woven bone than cartilage; 7= cartilaginous tissue and woven bone in almost equal proportions; 8= less cartilage and more woven bone; 9= entirely woven bone; 10= woven bone and some mature bone; and 11= lamellar (mature) bone.
Alkaline phosphatase and histological assessment of bone structure
Four weeks postsurgery, rats were euthanized, and blood samples collected from heart and serum were used to analyze levels of alkaline phosphatase (ALP), following protocols as per manufacturer’s instructions.
Primary human OA chondrocyte culture
Chondrocytes isolated from the cartilage in the knee joint of patients diagnosed with OA were purchased from Asterand, Detroit, MI, USA. Cells were cultured in Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 media supplemented with 15% fetal bovine serum and 1% antibiotic–antimycotic. Cell passages between two and four were used for experiments. Prior to each assay, cells were plated at a density appropriate for the particular experiment and allowed to settle for 24 hours. Chondrocyte media was then replaced with media containing transforming growth factor-beta (TGF-β) (Lonza Group Ltd., Basel, Switzerland) to induce differentiation. Media were changed three times a week for approximately 10–12 days or until cells redifferentiated from fibroblastic morphology to a polygonal structure reported to be the structural characteristics of chondrocyte cells.21 Following differentiation, chondrocytes were treated with 10 ng/mL of IL-1β (Biovision, Waterloo, NSW, Australia) for 36 hours before being treated with different concentrations of herbal extracts.
Cell proliferation and cytotoxicity assay
Chondrocytes pretreated with IL-1β were treated for 24 hours with increasing concentrations of C. quadrangularis and W. somnifera. Proliferation of cells by quantifying DNA replication (nuclear activity) was evaluated by CyQuant assay (Thermo Fisher Scientific, Waltham, MA, USA) which incorporates a fluorescence dye into dividing DNA. In order to evaluate the cytotoxicity induced by herbals, lactate dehydrogenase (LDH) cytotoxic detection kit (Hoffman-La Roche Ltd., Basel, Switzerland) was utilized. Briefly, cells pretreated with IL-1β and then incubated with herbal were briefly centrifuged at 800 rpm for 5 minutes. One hundred microliters of conditioned media was transferred to a 96-well plate and incubated with LDH reaction mixture for 30 minutes at room temperature (RT). Absorbance was read at 620 nm and percentage cell cytotoxicity was calculated relatively to IL-1β-only treated control.
Glycosaminoglycan production analysis
Glycosaminoglycans (GAGs) can be classified as the building blocks that make up the cartilage and help maintain its structure and integrity. During OA, reduced amount of GAG is reported in cartilage as compared to normal hyaline cartilage.22 Therefore, the production of GAG following treatment with herbal extracts was examined using the Blyscan sulfated assay (Biocolor Ltd, Carrickfergus, UK). Briefly, IL-1β-exposed chondrocytes were treated with different concentration of herbal samples and incubated for 24 hours. One milliliter of Blyscan dye reagent (1,9-dimethyl-methylene blue) was mixed with 100 μL of conditioned media and incubated for 30 minutes with gentle shaking. The mixture was centrifuged and the supernatant discarded carefully. Five hundred microliters of dissociation reagent was added and concentration of GAG was quantified by measuring the absorbance at 656 nm.
Nitric oxide quantification
Total nitrite released from chondrocytes was measured using spectrophotometric methods utilizing the Griess reagent kit (Thermo Fisher Scientific). Supernatants from treated chondrocytes were mixed with the Griess reagent and incubated for 30 minutes at RT. Absorbance was measured at 540 nm and concentration calculated by comparing to a standard curve of NaNO2.
Quantitative real-time-polymerase chain reaction analysis
Cells preexposed to IL-1β were treated with herbal samples for 24 hours, following which total RNA was isolated using TRI-zol (Thermo Fisher Scientific) method. In addition, specific inhibitions of nuclear factor kappa B (NF-κB), (Bay 11-7082 at 5 μM) and p38-mitogen-activated protein kinase (MAPK) (SB203580 at 50 μM) or vehicle (dimethyl sulfoxide; 0.1% v/v final concentration) were treated for 24 hours at 37°C and total RNA was isolated using the same method described above. Five micrograms of total RNA was reverse transcribed with SuperScript III reverse-transcriptase (Thermo Fisher Scientific) and synthesized cDNA was amplified in duplicate with SYBR green master mix (Bio-Rad Laboratories Inc., Hercules, CA, USA). The following PCR protocol was used: 60 cycles of 95°C for 30 seconds, annealing at 55°C for 30 seconds, and 72°C for 40 seconds. Data was analyzed using 2−ΔΔCt method23 with actin as the reference gene. PCR products were electrophoresised on 1% agarose gels containing SYBR safe DNA stain (Thermo Fisher Scientific, Waltham) and visualized under ultraviolet light.
Fluorescence-activated cell sorting analysis
Primary chondrocytes cultured in IL-1β for 36 hours were treated with 100 μg/mL of herbals. Following treatment, cells were washed briefly with 1X phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 10 minutes at RT, and blocked for 30 minutes with 3% bovine serum albumin. Following blocking, cells were incubated with primary antibody purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) at a dilution of 1:100 and incubated for 1 hour at RT. Cells were washed with PBS, centrifuged briefly, and resuspended in either anti-mouse–FITC (Sigma-Aldrich Co., St Louis, MO, USA) or anti-goat–FITC (Sigma-Aldrich Co.) at a 1:150 dilution for 1 hour in the dark. Cell were then analyzed using fluorescence-activated cell sorting to examine the expression of proteins.
Immunofluorescence for the expression of survivin
Expression of survivin in chondrocytes preexposed to IL-1β and then treated with herbals was analyzed using immunofluorescence techniques. Briefly, following treatment, cells were washed with PBS, fixed with 4% paraformaldehyde for 20 minutes, and incubated with 0.2% Triton X-100 for 10 minutes to permeabilize cells. Cells were then blocked with 3% horse serum for 1 hour at RT before being incubated for 1 hour with mouse-anti–survivin primary antibody (Santa Cruz Biotechnology Inc.). Cells were washed carefully and then incubated with anti-mouse secondary antibody labeled with tetramethylrhodamine (Sigma-Aldrich Co.) for 1 hour. Cells were washed carefully and mounted with DAPI mounting media (Sigma-Aldrich Co.) before being imaged with confocal microscopy.
Statistical analysis
Results from in vitro data are expressed as mean ± standard error of the mean from three independent experiments. P-values were evaluated by one-way analysis of variance with Tukey’s honestly significantly different post hoc tests. In vivo experimental data are presented as the mean ± standard error of the mean with the difference between groups assessed using Student’s t-test. P<0.05 was considered significant and P<0.01 considered to be highly significant.
Results
Herbal extracts enhanced chondrocyte proliferation
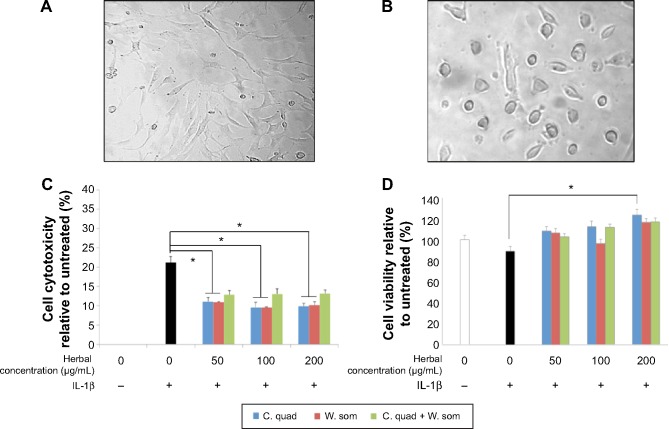
Undifferentiated chondrocytes that were fibroblastic in morphology (Figure 1A) were dedifferentiated with TFG-β until cells attained a spherical morphology (Figure 1B). Chondrocytes were then treated with IL-1β for 36 hours to activate osteoarthritic progressions. Initial experiments were to examine whether the herbal extracts, C. quadrangularis, W. somnifera, and the combination, had an effect on cell proliferation and toxicity following IL-1β exposure. As shown in Figure 1C, cytotoxicity analyzed using LDH revealed a significant increase in toxicity of 21.2%±1.6% with IL-1β treatment; however, upon treatment with herbal samples, significant reduction of IL-1β-induced toxicity were observed. With 50 μg/mL concentration, cytotoxicity was reduced by 11.4%±0.5% with C. quadrangularis, 11.1%±0.85% with W. somnifera, and by 7.98%±0.98% with the combination as compared to IL-1β-only treatment. Proliferative effects induced by herbals demonstrated significant upregulation of cell growth in a dose-dependent manner (Figure 1D). IL-1β-only treated cells lowered growth by 10.9%±4.2% as compared to untreated cells; however, with herbals, IL-1β−inhibited cell growth was diminished. Highest proliferation was observed at 200 μg/mL, with C. quadrangularis significantly increasing cell viability by 34.4%±5.1% as compared to IL-1β-only treated cells.
Figure 1.
Morphological changes of chondrocytes and chondroprotective activity of herbals.
Notes: (A) Osteoarthritic chondrocytes grown in monolayer tend to attain a fibroblastic morphology. (B) Incubation with culture media containing TGF-β for 10–12 days induce redifferentiation to chondrocytes confirmed by the spherical morphology. (C) LDH release was used to determine cytotoxicity effects induced by the herbal extracts and (D) cell viability analyzed using CyQUANT assay. Figure shows representative data of three independent experiments run in triplicate. Values correspond to means ± standard error; *P<0.05. “−” means the IL-1β was not added to the treatment whereas “+” denotes that IL-1β was added before herbal treatment.
Abbreviations: C. quad, Cissus quadrangularis; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; TGF-β, transforming growth factor-β; W. som, Withania somnifera.
C. quadrangularis induces chondroprotective activity
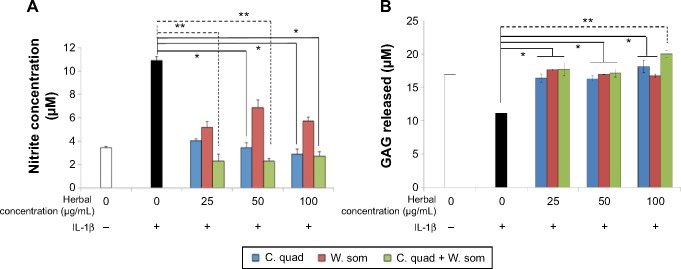
In order to examine the protective effects of herbal extracts, the regulation of nitric oxide (NO) and GAGs produced by chondrocytes was examined. As shown in Figure 2A, treatment with IL-1β increased nitrite levels to 10.91±0.32 μM which was significantly reduced with increasing concentrations of herbals. At 100 μg/mL, C. quadrangularis and the combination treatment reduced nitrite levels by 3.8-fold, whereas W. somnifera reduced nitrite levels by 1.9-fold relative to IL-1β-only treated cells. To further analyze the protective effects of herbals, the production of GAG by chondrocytes was examined (Figure 2B). Untreated chondrocytes produced GAG at levels of 16.90±0.13 μM; however, upon exposure to IL-1β, drastic reduction of GAG was observed with levels reducing to 11.16±0.23 μM. However, herbal treatment was found to induce a dose-dependent increase in GAG production. At 100 μg/mL, chondrocytes treated with C. quadrangularis expressed 18.15±1.48 μM of GAG, W. somnifera induced 16.74±0.23 μM production of GAG, and the combination significantly increased levels to 20.04±0.01 μM as compared to IL-1β-only treated cells.
Figure 2.
Regulation of nitric oxide and glycosaminoglycan production with herbal treatments.
Notes: IL-1β pretreated chondrocytes were incubated with increasing concentrations of herbal sample for 24 hours. Conditioned media was collected and measured for the production of NO (A) and GAG (B). Figure shows representative data of two independent experiments run in triplicate and corresponds to means ± standard error; *P<0.05; **P<0.01. “−” means the IL-1β was not added to the treatment whereas “+” denotes that IL-1β was added before herbal treatment.
Abbreviations: C. quad, Cissus quadrangularis; GAG, glycosaminoglycan; IL-1β, interleukin-1β; NO, nitric oxide; W. som, Withania somnifera.
Downregulation of catabolic genes with C. quadrangularis and combination therapy
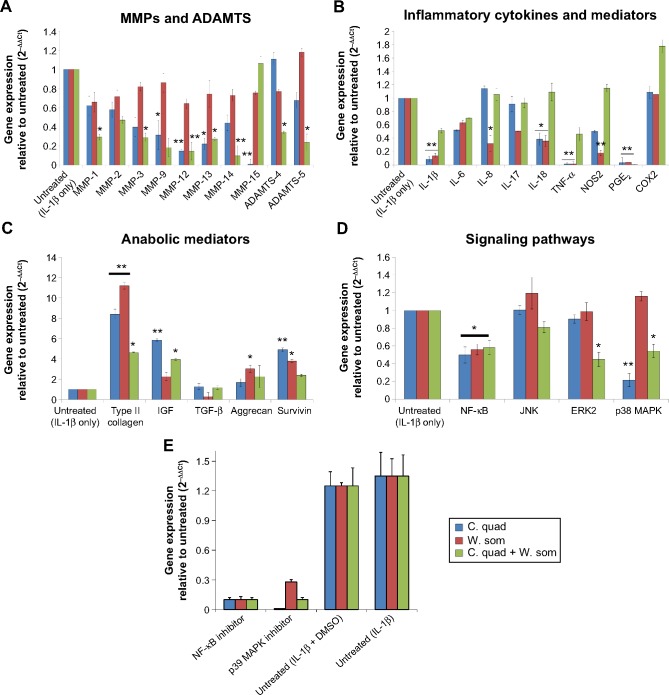
Human articular chondrocytes were stimulated with IL-1β (10 ng/mL) for 36 hours before treatment with the herbal extracts at a concentration of 100 μg/mL. As observed in Figure 3A and B, cells exposed to IL-1β alone showed an upregulation of gene expression of the catabolic factors, MMP-1, MMP-3, MMP-9, MMP-12, MMP-13, MMP-14, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5, nitric oxide synthase 2, and TNF-α. However, treatment with herbals significantly hindered expression of these catabolic factors. C. quadrangularis and combination therapy, at a concentrations of 100 μg/mL, downregulated gene expression of MMP-3, MMP-9, MMP-12, MMP-13, and prostaglandins 2 (PGE2). In addition, C. quadrangularis downregulated gene expression of MMP-15, IL-1β, IL-18, TNF-α, and PGE2. Treatment with W. somnifera downregulated IL-1β, TNF-α, and PGE2, however failed to show noteworthy regulation of MMPs and ADAMTs.
Figure 3.
Gene expression of cartilage-modifying agents in herbal-treated chondrocytes.
Notes: Cells preexposed to IL-1β were treated with 100 μg/mL of herbals extracts. Gene expression of MMPs (A), inflammatory cytokines (B), anabolic agents (C), signaling pathways (D), and the activity of inhibitors on signaling pathways (E) were evaluated using quantitative real-time-polymerase chain reaction. Values correspond to means ± standard error from two independent experiments run in triplicate; *P<0.05; **P<0.01.
Abbreviations: ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; C. quad, Cissus quadrangularis; COX2, cyclooxygenase-2; DMSO, dimethyl sulfoxide; MMPs, matrix metalloproteinases; NOS2, nitric oxide synthase 2; W. som, Withania somnifera; IL-1β, interleukin-1β; MMP, matrix metal loproteinase; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa B; TGF-β, transforming growth factor-β; TNF-α, tumour necrosis factor-α; IGF, insulin-like growth factor; PGE2, prostaglandins 2; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinases.
Herbal extracts upregulated gene expression of anabolic factors and inhibited NF-κB
To overcome OA disease progression, production of cartilage regenerative molecules that assist cartilage resynthesis is critical. Gene expression of type II collagen and aggrecan, the building blocks of cartilage, was evaluated and was observed to significantly increase in levels upon treatment with herbal extracts (Figure 3C). W. somnifera significantly increased gene expression of type II collagen and aggrecan by tenfold and twofold, respectively, as compared to IL-1β-only treated cells. C. quadrangularis showed similar activity in which type II collagen and aggrecan were upregulated seven- and twofold, respectively. Survivin, a member of the inhibitor of apoptosis family, is associated with inhibition of cell apoptosis and promoting cell growth.24 Recent work has shown upregulation of survivin aids in chondrocyte proliferation and viability.13,19 As shown in Figure 3C, treatment with C. quadrangularis and W. somnifera induced survivin expression by fourfold and 2.5-fold, respectively, as compared to untreated (IL-1β-only) cells. Interestingly, inhibitory activity of herbals on inflammation-inducing pathways such as NF-κB and MAPK were also observed (Figure 3D). NF-κB was downregulated with all herbals and the combination treatment reduced expression of extracellular signal-regulated kinases (ERK)2 and p38 MAPK signaling. No significant change, however, was observed with the JNK pathway. The involvement of NF-κB (Bay 11-7082 at 5 μM) and p38 MAPK was further confirmed by using specific inhibitors (Figure 3E). There was a complete inhibition of these two important cell-signaling molecules confirming their roles in C. quadrangularis and W. somnifera treatments.
C. quadrangularis significantly induced cartilage regenerative proteins
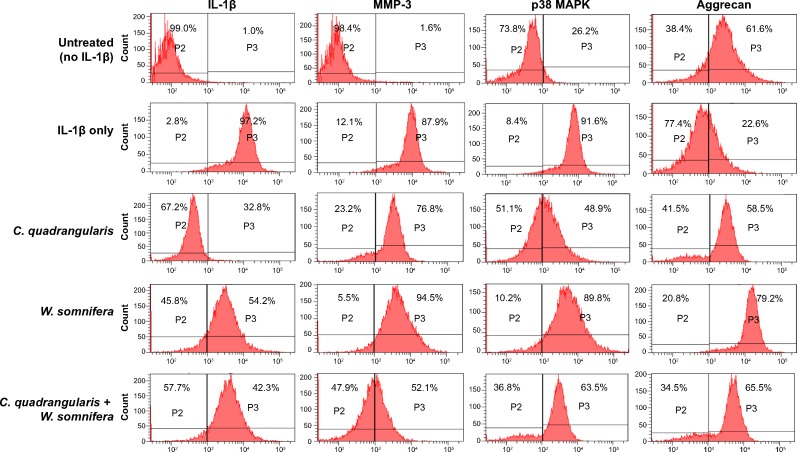
Protein expressions of key osteoarthritic markers were examined via fluorescence-activated cell sorting analysis to evaluate the cartilage regenerative activity of C. quadrangularis. As shown in Figure 4, untreated chondrocytes expressed low levels of IL-1β (1.0%), MMP-3 (1.6%), and p38 MAPK (26.2%). However, as observed with gene expression, exposure to IL-1β significantly increased expression of these proteins by 96.2% (IL-1β), 86.3% (MMP-3), and 65.4% (p38 MAPK), respectively. Evidently, treatment with C. quadrangularis and combination induced substantial protective effects on cells in which levels were drastically reduced by 64.4% (IL-1β), 11.1% (MMP-3), and 42.7% (p38 MAPK) with C. quadrangularis and 54.9% (IL-1β), 35.8% (MMP-3), and 28.4% (p38 MAPK) with the combination therapy as compared to IL-1β only. W. somnifera was observed to show similar protective effects, however, to a much lesser potency compared to that of C. quadrangularis and the combination. To further demonstrate the cartilage regenerative ability of herbals, expression of aggrecan was examined. As anticipated, IL-1β diminished levels of aggrecan; however, upon treatment with herbals, an increase of 35.9% with C. quadrangularis, 56.6% with W. somnifera, and 42.9% with the combination was observed as compared to IL-1β-only treated chondrocytes.
Figure 4.
Protein expression of cartilage-modifying agents in herbal-treated chondrocytes.
Notes: Cells preexposed to IL-1β were treated with 100 μg/mL of herbals extracts. Protein expression in chondrocytes was analyzed with fluorescence-activated cell sorting analysis. Values correspond to the mean ± standard error expression of survivin calculated from approximately 300 cells for each treatment.
Abbreviations: C. quadrangularis, Cissus quadrangularis; W. somnifera, Withania somnifera; IL-1β, interleukin-1β; MMP-3, matrix metalloproteinase-3; MAPK, mitogen-activated protein kinase.
Upregulation of survivin with C. quadrangularis
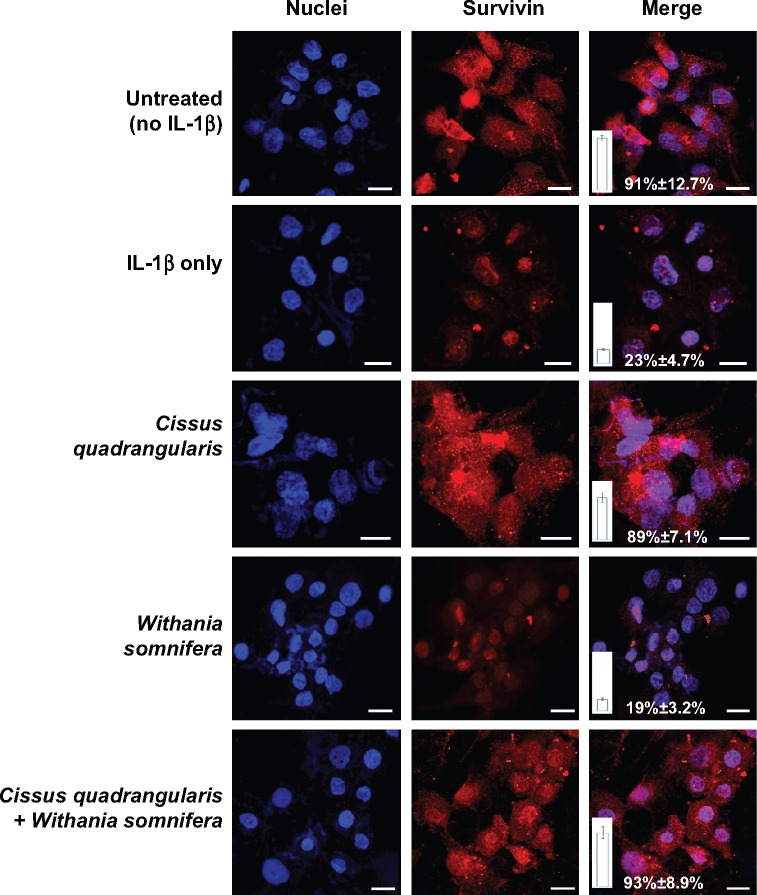
Expression of survivin, as an indicator of antiapoptotic activity, was examined in IL-1β-exposed chondrocytes treated with herbals. As shown in Figure 5, survivin was present in untreated chondrocytes and was localized in the cytoplasm and nucleus. However, upon exposure to IL-1β drastic reduction in survivin was observed and was predominantly present in the nuclei. Interestingly, C. quadrangularis and combination treatment reinstated levels of survivin to much higher levels than untreated cells, demonstrating the ability of herbals to inhibit IL-1β-induced cell toxicity and apoptosis.
Figure 5.
Immunofluorescence analysis of survivin in chondrocytes.
Notes: OA chondrocytes were examined for the expression of survivin following treatment with herbals. Cells were stained with antigen-specific primary antibody followed by secondary antibody conjugated to tetramethylrhodamine dye. Nuclei were counterstained with DAPI. Cells were imaged using Leica confocal microscopy and processed in Photoshop. Representative images from two independent experiments are shown.
Abbreviations: IL-1β, interleukin-1β; OA, osteoarthritis.
C. quadrangularis and combination therapy stimulated cartilaginous tissue
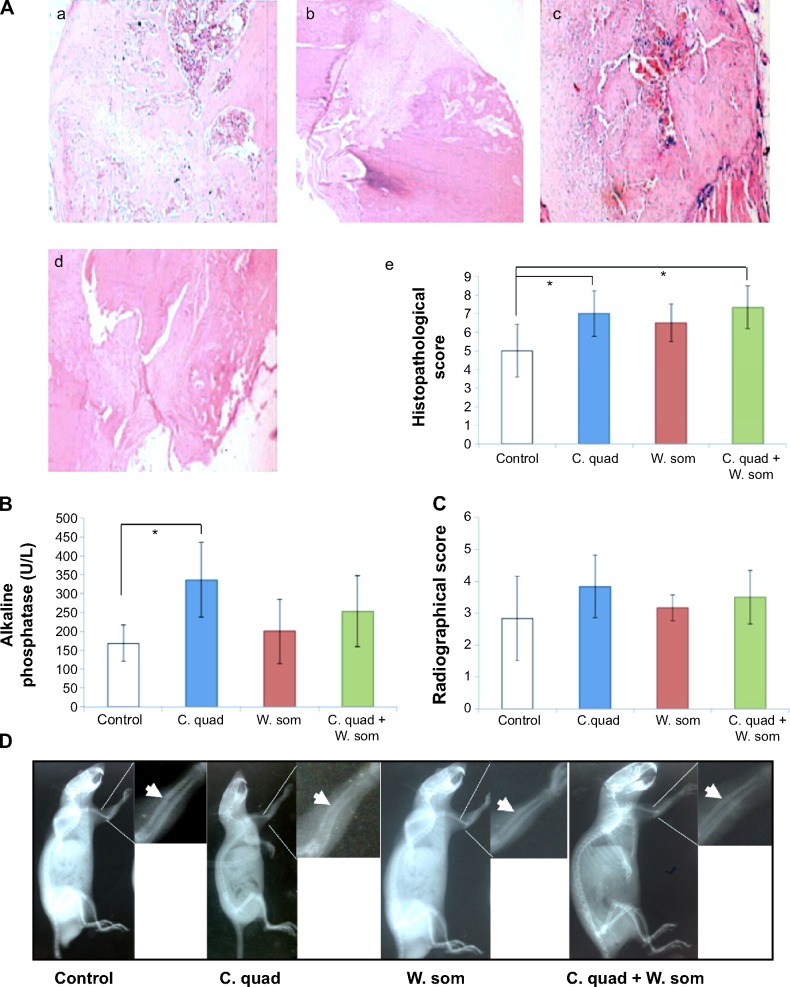
The healing and regrowth capacity of herbals were evaluated by examining stained bone sections of osteotomized rats treated with C. quadrangularis, W. somnifera, or combination of the two herbals. Histological scoring assessed for the formation of fibrous tissue, cartilaginous tissue, woven bone, and mature bone in bone calluses (Figure 6A). As compared to the control (untreated) group (Figure 6Aa) that showed high levels of fibrous tissue, rats treated with C. quadrangularis (Figure 6Ab) and combination (Figure 6Ad) showed enhanced levels of cartilaginous tissue and woven bone. W. somnifera therapy (Figure 6Ac), however, induced high levels of fibrous tissue, similar to what was observed in untreated rats, with increased woven bone tissue compared to cartilage. Figure 6Ae shows a histogram of the histopathological scoring of rats following herbal treatments.
Figure 6.
Representative histopathological images of bone tissue and ALP activity in rats that underwent osteotomy.
Notes: (A) (a) Stained tissue sections of the control group, (b) Cissus quadrangularis-treated group, (c) Withania somnifera-treated group, and (d) combination group (original magnification ×10); (e) representative histopathological scores in osteotomized rats scaled from 0 to 11. Representative images from bone sections are presented. (B) Swiss albino rats surgically osteotomized and treated with herbal sample for 4 weeks were evaluated for levels of ALP in serum. (C) The structural differences of the osteotomized area of the limb were evaluated through X-ray and the observed severity of bone callus scaled between 0 and 5. (D) Representative radiographs showing the effects of different treatments; arrows indicate the area rats were osteotomized. Values correspond to means ± standard error (n=6 per group); *P<0.05.
Abbreviations: ALP, alkaline phosphatase; C. quad, Cissus quadrangularis; W. som, Withania somnifera.
C. quadrangularis upregulated ALP in surgically osteotomized rats
Levels of ALP were analyzed to evaluate whether herbal samples on osteotomized rats had an effect on bone differentiation and proliferation. Serum samples collected from rats revealed increased levels of ALP with all herbal treatment as compared to rats fed on control diets (Figure 6B and C). Similar results were shown with different treatments (Figure 6D). C. quadrangularis induced a significantly high level of ALP at a concentration of 336.3±99.7 U/L; however, W. somnifera and combination showed no significant difference to the control group (168.3±48.0 U/L) with concentrations increasing only by 45.3±46.1 U/L and 84.73±37.6 U/L, respectively. Furthermore, radiographic changes observed in osteotomized rats revealed no significant difference between herbal treated and control rats (Figure 6C). Osteotomy line was observed in rats given the combination herbal therapy; however, animals fed on C. quadrangularis showed faint osteotomy sclerosis.
Discussion
OA involves complex physiologies that lead to an imbalance of anabolic and catabolic factors present in the cartilage and surrounding bone.2 Key factors that promote degeneration involve proinflammatory mediators such as cytokines, prostaglandins, and oxidative species.6 Drugs like nonsteroidal anti-inflammatory drugs and glucocorticoids are usually prescribed for chronic inflammatory and degenerative arthritis; however, their long term use leads to major discrepancies as effective therapeutics. Herbal therapies are recently gaining worldwide recognition by patients suffering from rheumatic disorders as alternative and complementary treatment strategies. Products such as gammalinolenic acid from seed oils,25 curcumin,26 and capsaicin27 have shown reduced joint swelling, pain, stiffness, and tenderness in patient with arthritis. However, it was also noted these treatments caused side effects such as gastrointestinal complications and burning sensations.
C. quadrangularis and W. somnifera are ayurvedic, natural compounds used widely for many decades. Popular for it antioxidant, anti-inflammatory, analgesic, and bone healing abilities, these herbals have proven to be beneficial in the treatment of inflammatory and rheumatic disorders. However, the mechanisms utilized by these compounds remains indistinct. Therefore, the present study was set to investigate the anti-inflammatory, cartilage-regenerative, and anti-arthritic effects of C. quadrangularis and W. somnifera on an osteoarthritic in vitro model and in surgically osteotomized rats. It was demonstrated that all three herbals (C. quadrangularis, and W. somnifera, and the combination of both) were able to induce significant anti-inflammatory and chondroprotective mechanisms on human chondrocytes preexposed to IL-1β cytokine. However, among the three samples, C. quadrangularis and the combination exhibited supreme inhibitory effects on disease progression. Augmented cell growth, inhibited ROS production, and diminished gene expression of catabolic factors were perceived, making them potential candidates for arthritis treatment.
IL-1β, a well-known proinflammatory cytokine, stimulates the production of various other inflammatory cytokines along with cartilage degenerative agents such as MMPs and ROS. Their elevated levels stimulate production of PGE2, NO, MMP-1, MMP-3, MMP-9, MMP-12, and MMP-13 in osteoarthritic cartilage explants.28 Hence, utilizing IL-1β exposure as a mediator of OA progression, our in vitro model focused on the pretreatment of chondrocytes with IL-1β to initiate OA. Our results were similar to those of Yang et al28 in that, upon exposure of cells to IL-1β, upregulation of cytokines, MMPs, and ROS was observed. This validated the in vitro OA model as a practicable system to examine the therapeutic potentials of drugs for OA.
Chondrocyte viability and growth are vital features that are essential for the maintenance of cartilage structure and integrity. In this study, downregulation of proliferative activity and enhanced cytotoxicity in response to IL-1β treatment was evident. We demonstrated that incubation of chondrocytes with herbals significantly improved cell viability and proliferation and inhibited IL-1β-induced toxic effects on cells. C. quadrangularis showed higher proliferative activity as compared to untreated; however, the combination therapy, along with inducing cell proliferation, inhibited NO production and stimulated GAG synthesis. This enhanced therapeutic activity of the combination therapy may be associated to the synergistic chondroprotective effects of both herbals. Recently, work by Gupta and Singh reported the anti-inflammatory effects of W. somnifera in rats induced with collagen-induced arthritis.29 It was shown that oral treatment of W. somnifera significantly reduced joint swelling and improved motor activity in arthritic rats as compared to untreated and methotrexate treated rats. It was suggested that the protective effects induced by W. somnifera may be due to its antioxidant, anti-inflammatory, and immunomodulatory activity.9,10,29 Similarly, studies with C. quadrangularis have shown enhanced bone healing and nontoxic effects in in vitro and in vivo models. It was reported to stimulate bone matrix mineralization, calcium deposition, anti-inflammation, and antioxidant activity.11 In addition, proliferation activity observed by osteoblasts treated with C. quadrangularis was via the upregulation of the MAPK pathway.30
Downstream cellular responses such as proliferation, inflammation, and apoptosis as a result of MAPK activation are strongly associated to various stimuli. Proinflammatory cytokines such as IL-1β, and TNF-α, NO, and cyclooxygenases play essential roles in the activation of p38 MAPK and c-Jun N-terminal kinases (JNK) pathways. This in turn leads to inflammation, further cytokine production, osteoclast differentiation, and chondrocyte apoptosis.31,32 On the other hand, growth factors, such as TGF-β and insulin-like growth factor (IGF) modulate chondrocyte proliferation via inhibition of p38 MAPK and upregulation of ERK signaling.33 Our study showed similar results where exposure of chondrocytes to IL-1β significantly increased expression of proinflammatory cytokines, NO, and p38 MAPK. However, treatment of cells with herbals, in particularly C. quadrangularis and the combination therapy, considerably alleviated production of catabolic agents by inhibiting activation of p38 MAPK and ERK2 signaling. Hence, it can be suggested that C. quadrangularis and the combination therapy act as growth factors by synergistically inducing cellular protection against inflammatory responses.
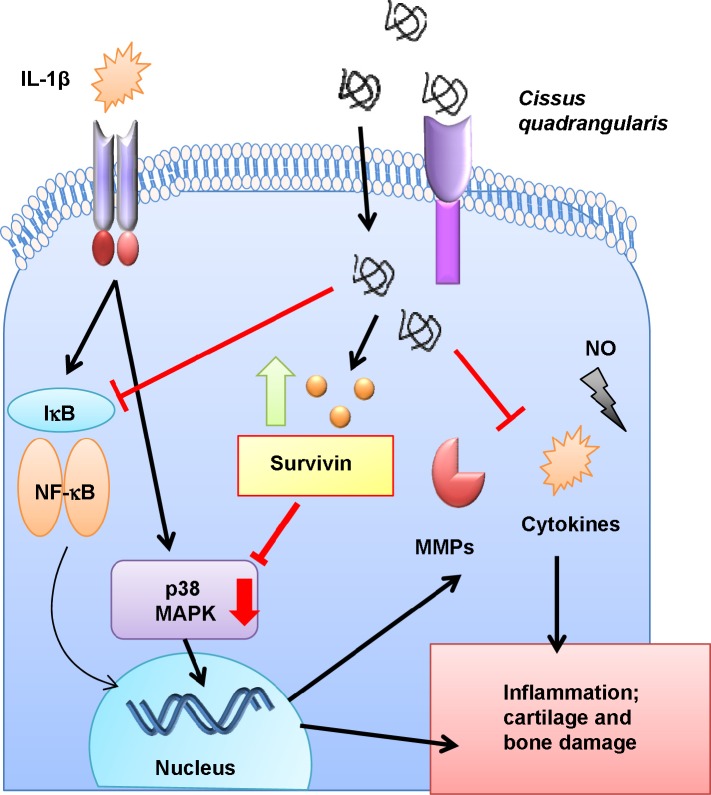
Survivin, a member of the inhibitor of apoptosis family, is closely associated to the regulation of most cancers in which its high expression promotes tumor growth, chemotherapy drug resistance, and inflammation.24,34 Extensive work from our laboratory has shown the therapeutic potentials of targeting highly expressed survivin in colon, breast, and neuronal cancers using dominant negative survivin and naturally derived milk proteins such as iron-saturated bovine lactoferrin.15,35,36 Alternatively, it was also reported that survivin upregulation in normal cells promotes protection from detrimental stimuli such as ROS and proinflammatory cytokines.37,38 In addition, increased levels of survivin in human chondrocytes was shown to induce cell proliferation and inhibit apoptosis.39 Our study demonstrates similar results in which proinflammatory cytokine IL-1β inhibits the production of endogenous survivin in human chondrocytes that in turn leads to reduced cell viability and proliferation. However, upon treatment with C. quadrangularis and W. somnifera, a significant increase in endogenous survivin was observed, thus stimulating cell growth. These reports further validate the signaling pathways activated by the herbals wherein treatment of cytokine-stressed chondrocytes with C. quadrangularis suppress p38 MAPK signaling via the activation of survivin (Figure 7).
Figure 7.
Schematic diagram illustrating the therapeutic activity of Cissus quadrangularis.
Notes: C. quadrangularis inhibits catabolic activity of IL-1β-induced inflammation and cartilage damage. Reduction of proinflammatory cytokines and MMPs are regulated by treatment of C. quadrangularis.
Abbreviations: IL-1β, interleukin-1β; MAPK, mitogen-activated protein kinase; MMPs, matrix metalloproteinases; NO, nitric oxide; NF-κB, nuclear factor kappa B.
In this study, Swiss albino rats were osteotomized surgically and used as an animal model to study the healing effects of the herbal extracts. This model of bone destabilizing effectively mimics the structural damage seen in humans that have bone fractures or who suffer from OA as it provides similar morphological changes and underlying disease mechanisms as observed in patients. Treatment of rats with C. quadrangularis, W. somnifera, or combination resulted in an increased production of ALP in serum. ALP is an enzyme elevated in serum due to rapid bone growth and mineralization. Produced by osteoblasts as a result of increased activity and together with other proteins, ALP assists with bone mineralization.40 Previous work with C. quadrangularis has shown increased ALP activity with different osteoblastic cells line.11,30,41 It was shown that C. quadrangularis and its components were able to increase production of ALP by inducing proliferation, differentiation, and growth of osteoblasts cells. Results from our study correlated with these finding where it was shown that, as compared to the other treatments, C. quadrangularis increased levels of ALP in serum.
Radiographic and histopathology analysis in our study further revealed the healing activity of the herbal extracts. We found that with C. quadrangularis and combination, bone healing was significantly enhanced with rapid formation of woven bone and cartilaginous tissue within 4 weeks of treatment. The healing property of C. quadrangularis has been previously shown in various in vivo models in which its use helped rapid restoration of bone strength and induced denser mineralization.42,43 Similarly, C. quadrangularis and W. somnifera are known for their use in bone fractures and as a therapy for OA. Clinical study with these herbals on patients suffering with OA showed effective therapeutic benefits.44 Possible mechanisms for the heightened therapeutic activity observed by these herbals is suggested to be due to their interactions with the transcriptional factor Runx2,20 increased production of IGF,45 and through the regulation of the MAPK signaling pathways.30 Further, our study demonstrates for the first time the interaction of these herbals with collagenases, nitric oxides, prostaglandins, and apoptotic proteins.
Conclusion
Taken together, the results presented in this study confirm the anti-inflammatory and cartilage-regenerative properties of C. quadrangularis and its mode of action via the inhibition of MMP and ROS. In addition, we propose that survivin plays a vital role in chondroprotection and osteogenesis by inhibiting p38 MAPK signaling pathways (Figure 7). Therefore, understanding the mechanisms by which these herbals mediate signaling molecules could have significant benefits for personalized treatment options for arthritic and inflammatory diseases.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Burr DB, Schaffler MB. The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc Res Tech. 1997;37:343–357. doi: 10.1002/(SICI)1097-0029(19970515)37:4<343::AID-JEMT9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 3.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Zayed N, Afif H, Chabane N, et al. Inhibition of interleukin-1beta-induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum. 2008;58:3530–3540. doi: 10.1002/art.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16:2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 7.Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol. 2002;20:S152–S157. [PubMed] [Google Scholar]
- 8.Rao JK, Mihaliak K, Kroenke K, Bradley J, Tierney WM, Weinberger M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med. 1999;131:409–416. doi: 10.7326/0003-4819-131-6-199909210-00003. [DOI] [PubMed] [Google Scholar]
- 9.Rasool M, Varalakshmi P. Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundam Clin Pharmacol. 2007;21:157–164. doi: 10.1111/j.1472-8206.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 10.Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: An in vivo and in vitro study. Vascul Pharmacol. 2006;44:406–410. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Muthusami S, Senthilkumar K, Vignesh C, et al. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. J Cell Biochem. 2011;112:1035–1045. doi: 10.1002/jcb.23016. [DOI] [PubMed] [Google Scholar]
- 12.Unnati S. Pharmacognostical investigation on cissus quadrangularis linn. Int J Pharma Bio Sci. 2010;1:1–5. [Google Scholar]
- 13.Samarasinghe RM, Kanwar RK, Kanwar JR. The effect of oral administration of iron saturated-bovine lactoferrin encapsulated chitosan-nanocarriers on osteoarthritis. Biomaterials. 2014;35:7522–7534. doi: 10.1016/j.biomaterials.2014.04.109. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar JR, Palmano KP, Sun X, et al. ‘Iron-saturated’ lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immunol Cell Biol. 2008;86:277–288. doi: 10.1038/sj.icb.7100163. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar JR, Mahidhara G, Roy K, et al. Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine (Lond) 2015;10(1):35–55. doi: 10.2217/nnm.14.132. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahim F, Shankaranarayanan JS, Kanwar JR, Gurudevan S, Krishnan UM, Kanwar RK. Identification of unprecedented anticancer properties of high molecular weight biomacromolecular complex containing bovine lactoferrin (HMW-bLf) PLoS One. 2014;9:e106568. doi: 10.1371/journal.pone.0106568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrow H, Kanwar RK, Mahidhara G, Kanwar JR. Effect of selenium-saturated bovine lactoferrin (Se-bLF) on antioxidant enzyme activities in human gut epithelial cells under oxidative stress. Anticancer Agents Med Chem. 2011;11:762–771. doi: 10.2174/187152011797378616. [DOI] [PubMed] [Google Scholar]
- 18.Burrow H, Kanwar RK, Kanwar JR. Antioxidant enzyme activities of iron-saturated bovine lactoferrin (Fe-bLf) in human gut epithelial cells under oxidative stress. Med Chem. 2011;7:224–230. doi: 10.2174/157340611795564286. [DOI] [PubMed] [Google Scholar]
- 19.Samarasinghe RM, Kanwar RK, Kumar K, Kanwar JR. Antiarthritic and chondroprotective activity of Lakshadi Guggul in novel alginate-enclosed chitosan calcium phosphate nanocarriers. Nanomedicine (Lond) 2014;9(6):819–837. doi: 10.2217/nnm.13.219. [DOI] [PubMed] [Google Scholar]
- 20.Laurence DR, Bacharach AL. Evaluation of drug activities: pharmacometrics. London, New York: Academic Press; 1964. [Google Scholar]
- 21.Stokes DG, Liu G, Dharmavaram R, Hawkins D, Piera-Velazquez S, Jimenez SA. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. Biochem J. 2001;360:461–470. doi: 10.1042/0264-6021:3600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastiaansen-Jenniskens YM, Koevoet W, Jansen KM, Verhaar JA, DeGroot J, VanOsch GJ. Inhibition of glycosaminoglycan incorporation influences collagen network formation during cartilage matrix production. Biochem Biophys Res Commun. 2009;379:222–226. doi: 10.1016/j.bbrc.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Samarasinghe RM, Gibbons J, Kanwar RK, Kanwar JR. Nanotechnology based platforms for survivin targeted drug discovery. Expert Opin Drug Discov. 2012;7:1083–1092. doi: 10.1517/17460441.2012.719869. [DOI] [PubMed] [Google Scholar]
- 25.Leventhal LJ, Boyce EG, Zurier RB. Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med. 1993;119:867–873. doi: 10.7326/0003-4819-119-9-199311010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–634. [PubMed] [Google Scholar]
- 27.Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383–395. [PubMed] [Google Scholar]
- 28.Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Singh S. Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm Biol. 2014;52:308–320. doi: 10.3109/13880209.2013.835325. [DOI] [PubMed] [Google Scholar]
- 30.Parisuthiman D, Singhatanadgit W, Dechatiwongse T, Koontongkaew S. Cissus quadrangularis extract enhances biomineralization through up-regulation of MAPK-dependent alkaline phosphatase activity in osteoblasts. In Vitro Cell Dev Biol Anim. 2009;45:194–200. doi: 10.1007/s11626-008-9158-1. [DOI] [PubMed] [Google Scholar]
- 31.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 32.Geng Y, Valbracht J, Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest. 1996;98:2425–2430. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studer RK, Bergman R, Stubbs T, Decker K. Chondrocyte response to growth factors is modulated by p38 mitogen-activated protein kinase inhibition. Arthritis Res Ther. 2004;6:R56–R64. doi: 10.1186/ar1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coumar MS, Tsai FY, Kanwar JR, Sarvagalla S, Cheung CH. Treat cancers by targeting survivin: Just a dream or future reality? Cancer Treat Rev. 2013;39:802–811. doi: 10.1016/j.ctrv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Sriramoju B, Kanwar RK, Kanwar JR. Nanoformulated cell-penetrating survivin mutant and its dual actions. Int J Nanomedicine. 2014;9:3279–3298. doi: 10.2147/IJN.S60169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanwar JR, Mahidhara G, Kanwar RK. Novel alginate-enclosed chitosan-calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine (Lond) 2012;7:1521–1550. doi: 10.2217/nnm.12.29. [DOI] [PubMed] [Google Scholar]
- 37.Baratchi S, Kanwar RK, Kanwar JR. Novel survivin mutant protects differentiated SK-N-SH human neuroblastoma cells from activated T-cell neurotoxicity. J Neuroimmunol. 2011;233:18–28. doi: 10.1016/j.jneuroim.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Baratchi S, Kanwar RK, Cheung CH, Kanwar JR. Proliferative and protective effects of SurR9-C84A on differentiated neural cells. J Neuroimmunol. 2010;227:120–132. doi: 10.1016/j.jneuroim.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Lechler P, Schaumburger J, Köck FX, et al. The oncofetal gene survivin promotes cell proliferation and survival in primary human osteoblastic cells. Calcif Tissue Int. 2011;89:211–220. doi: 10.1007/s00223-011-9508-y. [DOI] [PubMed] [Google Scholar]
- 40.Leung KS, Fung KP, Sher AH, Li CK, Lee KM. Plasma bone-specific alkaline phosphatase as an indicator of osteoblastic activity. J Bone Joint Surg Br. 1993;75:288–292. doi: 10.1302/0301-620X.75B2.8444951. [DOI] [PubMed] [Google Scholar]
- 41.Prouillet C, Mazière JC, Mazière C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67:1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Udupa KN, Prasad GC. Further studies on the effect of Cissus quadrangularis in accelerating fracture healing. Indian J Med Res. 1964;52:26–35. [PubMed] [Google Scholar]
- 43.Deka DK, Lahon DC, Saikia J, Mukit A. Effect of Cissus quadrangularis in accelerating healing process of experimentally fractured radius-ulna of dog: a preliminary study. Indian J Pharm. 1994;26:44–45. [Google Scholar]
- 44.Sharma VD, Sharma A, Kushwah HK. An indigenous approach to manage the osteoarthritis of knee joint with lakshadi guggulu, kalka-patra bandhan and knee traction. Anc Sci Life. 2007;26:23–29. [PMC free article] [PubMed] [Google Scholar]
- 45.Muthusami S, Ramachandran I, Krishnamoorthy S, Govindan R, Narasimhan S. Cissus quadrangularis augments IGF system components in human osteoblast like SaOS-2 cells. Growth Horm IGF Res. 2011;21:343–348. doi: 10.1016/j.ghir.2011.09.002. [DOI] [PubMed] [Google Scholar]