Abstract
Background
Although it has been previously reported that radiotherapy (RT) effectively reduced the incidence of local recurrence of ductal carcinoma in situ (DCIS) following breast-conserving surgery (BCS), little is known about the effect of RT on survival of patients with locally excised DCIS.
Patients and methods
Using Surveillance, Epidemiology, and End Results registry data, we selected 56,968 female DCIS patients treated with BCS between 1998 and 2007. Overall survival (OS) and breast cancer-specific survival (BCSS) were compared among patients who received RT or no RT using the Kaplan–Meier methods and Cox proportional hazards regression models.
Results
Median follow-up was 91 months. In the multivariable model, patients receiving postoperative RT had better OS than those undergoing BCS alone (hazard ratio [HR] 0.59, 95% confidence interval [CI] 0.53–0.67, P<0.001). This pattern remained after stratification by estrogen receptor (ER) status and age. In contrast, RT delivery was not significantly associated with improved BCSS (HR 0.71, 95% CI 0.48–1.03, P=0.073). However, after stratifying by the above two variables, RT contributed to better BCSS in ER-negative/borderline patients (HR 0.41, 95% CI 0.19–0.88, P=0.023) and younger patients (≤50 years old; HR 0.37, 95% CI 0.15–0.91, P=0.030).
Conclusion
Our analysis confirms the beneficial effect of RT on OS in women with locally excised DCIS and reveals the specific protective effect of RT on BCSS in ER-negative/borderline and younger patients.
Keywords: ductal carcinoma in situ, breast cancer, breast-conserving surgery, radiotherapy, survival
Introduction
Ductal carcinoma in situ (DCIS) is defined as a premalignant condition that involves proliferation of neoplastic mammary ductal epithelial cells without evidence of invasion beyond the basement membrane.1 Until the 1980s, mastectomy remained the reference treatment for patients with DCIS. However, with the introduction of breast-conserving surgery (BCS) for the treatment of early-stage breast cancer, local excision of DCIS began to be widely adopted. Currently, BCS has become the most common surgery for DCIS, constituting 74% of treated cases according to a query of the Surveillance, Epidemiology, and End Results (SEER) database.2 In addition, radiotherapy (RT) has become one of the main types of adjuvant therapy for DCIS.3
To date, four randomized controlled trials (RCTs) have investigated the effectiveness of RT in reducing local recurrence (LR) of DCIS after BCS.4–7 All four trials confirmed that postoperative RT reduced in situ or invasive recurrences by approximately 50%. However, long-term results of the NSABP B-17 trial showed that RT was not associated with overall mortality reduction.8 In addition, the EORTC and SweDCIS trials showed that the long-term prognosis of DCIS was not influenced by RT.9,10
Nevertheless, only two trials took survival as a study endpoint.9,10 In the SweDCIS trial, there existed a potential positive selection bias in determining the cause of death; the authors only retrieved the medical records of women with a previous ipsilateral or contralateral event.10 In the EORTC trial, there existed misclassification in the pathological assessments of the cases; 5% and 3% of the lesions were reclassified as benign disease and microinvasive carcinoma, respectively.11 The sample size of the aforementioned single trial was relatively small. The publication of data from the four RCTs did not settle the ongoing debates regarding the pros and cons of RT following BCS for DCIS treatment.
Therefore, we performed this SEER population-based analysis to investigate the effect of RT on survival of DCIS patients who had undergone BCS with or without postoperative RT, aiming to provide some evidence to assist clinical decision-making in the management of DCIS.
Materials and methods
Ethics statement
We have access to the data released from the SEER database by complying with data-use agreements for the SEER research data file. This study was approved by the Ethical Committee and Institutional Review Board of Fudan Cancer Center.
Data acquisition and patient selection
The study population was obtained from the records of the SEER database. Patients diagnosed with breast cancer between January 1, 1998 and December 31, 2007 were selected. Patients diagnosed with breast cancer before 1998 were excluded because of unavailable surgery information.12 Patients diagnosed with breast cancer after 2007 were excluded to guarantee an adequate follow-up time. Patients aged more than 79 years were excluded because RT is unlikely to benefit these patients due to the competing risk of death from comorbid disease.13
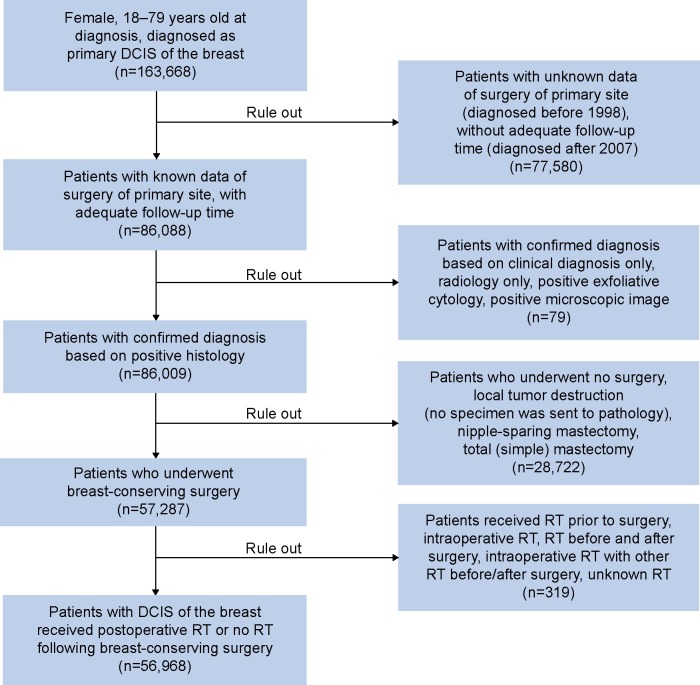
The specific inclusion criteria are listed as follows: female, age at diagnosis between 18 years and 79 years, pathologically confirmed DCIS, surgical treatment with BCS, and breast cancer as the first and only primary malignancy; only patients who received postoperative RT or no RT following BCS were included. There is a potential misclassification bias of clinicopathologic variables in the SEER database, so we only included patients with positive histology confirmation to minimize this bias. The algorithm for patient selection is shown in Figure 1.
Figure 1.
Algorithm for patient selection.
Notes: The Surveillance, Epidemiology, and End Results database was used to identify patients diagnosed with DCIS of the breast. Inclusion criteria were as follows: female, aged from 18 years to 79 years, diagnosed between 1998 and 2007, confirmed by positive histology, who underwent breast-conserving surgery, and who received postoperative RT or no RT. In all, 56,968 patients were included in this analysis.
Abbreviations: DCIS, ductal carcinoma in situ; RT, radiotherapy.
Outcome measurements
The primary outcomes of interest were overall survival (OS) and breast cancer-specific survival (BCSS). Vital status was obtained as alive or dead. BCSS was calculated from the date of diagnosis to the date of death attributed to breast cancer; patients were counted as censored if they died from other causes at the date of death, were lost to follow-up, or survived beyond December 31, 2007. OS was calculated from the date of diagnosis to the date of death attributed to any causes; patients were coded as censored if they were lost to follow-up or survived beyond December 31, 2007.
Covariates
Patient characteristics included age at diagnosis, year of diagnosis, race, marital status, and socioeconomic position (SEP). Age at diagnosis was dichotomized into ≤50 years and >50 years. Year of diagnosis was classified into 1998–2002 and 2003–2007. Race was categorized into white, black, and other (American Indian/Alaskan Native, Asian/Pacific Islander). Marital status was coded as married and non-married including divorced, widowed, single (never married), and separated. Three SEP measures derived from the 1990 and 2000 US censuses were used in this analysis. Percent of persons with an income below the federal poverty level was separated into <10%, 10%–19.99%, and ≥20%.14 Percent of adults (older than 25 years) with lower than high-school education level was divided into quartiles (≤15.99%, 16%–20.80%, 20.81%–28.76%, and >28.76%) based on the distribution of the variable across all counties in the US using the SEER county attributes database.15,16 A poverty–high school index combining the poverty and high-school variable was defined as low, middle, and high SEP categories as previously described by Schlichting et al.16 Tumor characteristics included laterality (left, right), primary site of lesions, histological grade, estrogen receptor (ER) status (negative/borderline, positive), progesterone receptor (PR) status (negative/borderline, positive), and RT delivery (yes, no). Primary site of lesions was classified into single-quadrant lesions and multi-quadrant lesions. Histological grade was coded as grade I (well differentiated), grade II (moderately differentiated), and grade III–IV (poorly differentiated and undifferentiated; anaplastic).
Statistical analysis
Comparisons of patient and tumor characteristics with respect to RT delivery were performed using the Pearson chi-square test. Survival outcomes were estimated using the Kaplan–Meier product-limit method, and survival difference was assessed with the log-rank test. A multivariate Cox proportional hazards model was applied to estimate the effect of covariates of interest on OS or BCSS. The estimated risks for OS or BCSS were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). All the P-values were two-sided, and P<0.05 was considered statistically significant. All the statistical analyses were performed using Stata version 12.0 (Stata Corporation, College Station, TX, USA) and SPSS version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Descriptive statistics
We identified 56,968 eligible patients for our study. In total, 35,092 (61.6%) patients received postoperative RT following BCS, 598 (1.0%) patients died from breast cancer, and 4,866 (8.5%) patients died from other causes. All the identified patients were at American Joint Committee on Cancer stage zero (TisN0M0). Comparisons of patient and tumor characteristics with respect to RT delivery are summarized in Table 1. Categories of RT delivery significantly differed with respect to age at diagnosis, year of diagnosis, race, percent below poverty, percent lower than high school education, poverty–high school index, marital status, ER status, PR status, primary site, and histological grade. Laterality was well balanced between the No-RT and RT groups.
Table 1.
Patient and tumor characteristics with respect to radiotherapy delivery
| Characteristics | Number (%) of patients
|
P-value* | ||
|---|---|---|---|---|
| Total (n=56,968) | No RT (n=21,876) | RT (n=35,092) | ||
| Age (years) | <0.001 | |||
| ≤50 | 15,554 (27.3) | 5,551 (25.4) | 10,003 (28.5) | |
| >50 | 41,414 (72.7) | 16,325 (74.6) | 25,089 (71.5) | |
| Year of diagnosis | <0.001 | |||
| 1998–2002 | 23,601 (41.4) | 9,975 (45.6) | 13,626 (38.8) | |
| 2003–2007 | 33,367 (58.6) | 11,901 (54.4) | 21,466 (61.2) | |
| Race | 0.013 | |||
| Black | 5,422 (9.5) | 2,137 (9.8) | 3,285 (9.4) | |
| White | 46,024 (80.8) | 17,626 (80.6) | 28,398 (80.9) | |
| Other† | 5,173 (9.1) | 1,896 (8.7) | 3,277 (9.3) | |
| Unknown | 349 (0.6) | 217 (1.0) | 132 (0.4) | |
| Marital status | <0.001 | |||
| Non-married‡ | 19,475 (34.2) | 7,934 (36.3) | 11,541 (32.9) | |
| Married | 35,283 (61.9) | 12,762 (58.3) | 22,521 (64.2) | |
| Unknown | 2,210 (3.9) | 1,180 (5.4) | 1,030 (2.9) | |
| % below poverty | <0.001 | |||
| <10 | 24,346 (44.5) | 8,859 (40.5) | 16,487 (47.0) | |
| 10–19.99 | 28,292 (49.7) | 11,708 (53.5) | 16,584 (47.3) | |
| ≥20 | 3,327 (5.8) | 1,307 (6.0) | 2,020 (5.8) | |
| Unknown | 3 (0.0) | 2 (0.0) | 1 (0.0) | |
| % with lower than high school education | <0.001 | |||
| ≤15.99 | 20,693 (36.3) | 7,174 (32.7) | 13,546 (38.6) | |
| 16–20.8 | 17,427 (30.6) | 6,441 (29.4) | 10,986 (31.3) | |
| 20.81–28.76 | 7,872 (13.8) | 3,021 (13.8) | 4,851 (13.8) | |
| >28.76 | 10,973 (19.3) | 5,265 (24.1) | 5,708 (16.3) | |
| Unknown | 3 (0.005) | 2 (0.003) | 1 (0.002) | |
| Poverty–high school index | <0.001 | |||
| Low SEP | 11,776 (20.7) | 5,542 (25.3) | 6,234 (17.8) | |
| Moderate SEP | 28,289 (49.7) | 10,522 (48.1) | 17,767 (50.6) | |
| High SEP | 16,900 (29.7) | 5,810 (26.6) | 11,090 (31.6) | |
| Unknown | 3 (0.0) | 2 (0.0) | 1 (0.0) | |
| Laterality | 0.755 | |||
| Left | 29,073 (51.0) | 11,140 (50.9) | 17,933 (51.1) | |
| Right | 27,874 (48.9) | 10,716 (49.0) | 17,158 (48.9) | |
| Unknown | 21 (0.037) | 20 (0.1) | 1 (0.0) | |
| Primary site | <0.001 | |||
| Single-quadrant lesions | 48,002 (84.3) | 17,269 (78.9) | 30,733 (87.6) | |
| Multi-quadrant lesions | 8,966 (15.7) | 4,607 (21.1) | 4,359 (12.4) | |
| ER status | <0.001 | |||
| Negative/borderline | 3,913 (6.9) | 1,035 (4.7) | 2,878 (8.2) | |
| Positive | 20,202 (35.5) | 6,298 (28.8) | 13,904 (39.6) | |
| Unknown | 32,853 (57.6) | 14,543 (66.5) | 18,310 (52.2) | |
| PR status | <0.001 | |||
| Negative/borderline | 6,145 (10.8) | 1,699 (7.8) | 4,446 (12.7) | |
| Positive | 16,470 (28.9) | 5,082 (23.2) | 11,388 (32.5) | |
| Unknown | 34,353 (60.3) | 15,095 (69.0) | 19,258 (54.9) | |
| Grade | <0.001 | |||
| I | 7,066 (12.4) | 3,616 (15.8) | 3,450 (10.3) | |
| II | 18,937 (33.2) | 7,706 (35.2) | 11,231 (32.0) | |
| III and IV | 19,730 (34.6) | 5,608 (25.6) | 14,122 (40.2) | |
| Unknown | 11,235 (19.7) | 5,112 (23.4) | 6,123 (17.4) | |
Notes:
P-value was calculated after unknown category was excluded.
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: RT, radiotherapy; SEP, socioeconomic position; ER, estrogen receptor; PR, progesterone receptor.
Comparison of survival between No-RT and RT groups
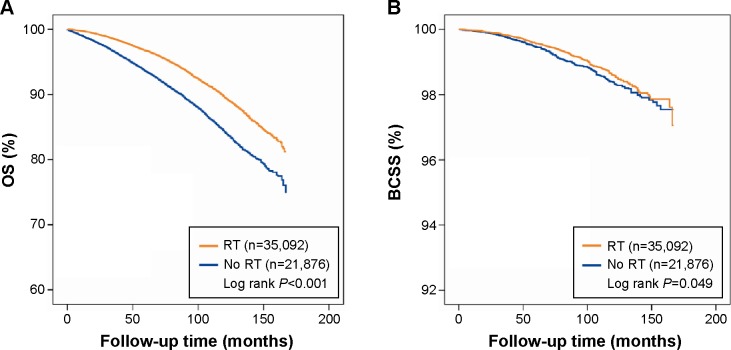
The median follow-up was 91 months (approximately 7.6 years). The 5-year and 10-year OS rates were 93.6% (95% CI 93.2–93.9) and 84.3% (95% CI 83.7–84.9), respectively, in the No-RT group versus 96.7% (95% CI 96.5–96.9) and 89.6% (95% CI 89.1–90.0), respectively, in the RT group. The 5-year and 10-year BCSS rates were 99.5% (95% CI 99.3–99.6) and 98.4% (95% CI 98.1–98.6), respectively, in the No-RT group versus 99.6% (95% CI 99.5–99.7) and 98.6% (95% CI 98.4–98.8), respectively, in the RT group. Survival curves were generated using the Kaplan–Meier method. Compared with patients not receiving RT, patients receiving RT had better OS (log rank, P<0.001; Figure 2A). However, this benefit was not obvious in BCSS (log rank, P=0.049; Figure 2B).
Figure 2.
Kaplan–Meier survival analysis within locally excised ductal carcinoma in situ according to the delivery of RT.
Notes: (A) OS. (B) BCSS.
Abbreviations: RT, radiotherapy; OS, overall survival; BCSS, breast cancer-specific survival.
Univariate and multivariate analyses of OS and BCSS using the Cox proportional hazards regression model are shown in Tables 2 and 3. With regard to OS, patients who received postoperative RT had better OS than those in the No-RT group in both univariate analysis (HR 0.62, 95% CI 0.59–0.66, P<0.001) and multivariate analysis (HR 0.59, 95% CI 0.53–0.67, P<0.001); in addition, both univariate and multivariate analyses showed that younger age, nonblack race, being married, single-quadrant lesions, and positive PR were significantly associated with improved OS. With regard to BCSS, RT did not provide benefit for BCSS in multivariate analysis (HR 0.71, 95% CI 0.48–1.03, P=0.073); factors associated with improved BCSS included younger age, more recent year of diagnosis, white race, being married, and right-side breast cancer based on multivariate analysis.
Table 2.
Cox proportional hazards regression model of overall survival
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| ≤50 | Reference | Reference | ||
| >50 | 4.98 (4.50–5.52) | <0.001 | 3.95 (3.20–4.88) | <0.001 |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.87 (0.82–0.93) | <0.001 | 0.82 (0.69–0.97) | 0.019 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.68 (0.63–0.75) | <0.001 | 0.76 (0.64–0.90) | 0.001 |
| Other* | 0.43 (0.37–0.49) | <0.001 | 0.59 (0.45–0.79) | <0.001 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.52 (0.50–0.55) | <0.001 | 0.55 (0.49–0.62) | <0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 0.93 (0.87–0.99) | 0.020 | 1.15 (0.98–1.34) | 0.083 |
| High SEP | 0.75 (0.70–0.81) | <0.001 | 0.92 (0.77–1.10) | 0.359 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.99 (0.94–1.05) | 0.822 | 1.02 (0.91–1.15) | 0.745 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 1.21 (1.13–1.29) | <0.001 | 1.21 (1.03–1.43) | 0.022 |
| ER status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.86 (0.76–0.98) | 0.023 | 1.07 (0.88–1.31) | 0.492 |
| PR status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.82 (0.73–0.92) | <0.001 | 0.84 (0.72–1.00) | 0.043 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 0.89 (0.82–0.97) | 0.009 | 0.99 (0.83–1.18) | 0.922 |
| III and IV | 0.84 (0.77–0.91) | <0.001 | 0.98 (0.82–1.18) | 0.848 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.62 (0.59–0.66) | <0.001 | 0.59 (0.53–0.67) | <0.001 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; ER, estrogen receptor; PR, progesterone receptor; RT, radiotherapy.
Table 3.
Cox proportional hazards regression model of breast cancer-specific survival
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| ≤50 | Reference | Reference | ||
| >50 | 1.86 (1.51–2.30) | <0.001 | 1.83 (1.12–2.97) | 0.015 |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.85 (0.71–1.03) | 0.096 | 0.62 (0.39–0.99) | 0.046 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.49 (0.40–0.61) | <0.001 | 0.51 (0.32–0.81) | 0.004 |
| Other* | 0.35 (0.24–0.51) | <0.001 | 0.50 (0.22–1.13) | 0.096 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.61 (0.52–0.72) | <0.001 | 0.52 (0.36–0.75) | <0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 0.99 (0.81–1.20) | 0.883 | 1.35 (0.85–2.13) | 0.204 |
| High SEP | 0.64 (0.50–0.81) | <0.001 | 0.58 (0.31–1.06) | 0.077 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.89 (0.75–1.04) | 0.139 | 0.67 (0.46–0.97) | 0.033 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 1.27 (1.04–1.56) | 0.020 | 1.07 (0.63–1.82) | 0.800 |
| ER status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.60 (0.42–0.86) | 0.006 | 0.99 (0.56–1.74) | 0.961 |
| PR status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.59 (0.43–0.83) | 0.002 | 0.69 (0.42–1.12) | 0.136 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 1.17 (0.86–1.58) | 0.316 | 1.82 (0.92–3.59) | 0.086 |
| III and IV | 1.34 (1.00–1.80) | 0.053 | 1.73 (0.86–3.47) | 0.122 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.85 (0.72–1.00) | 0.049 | 0.71 (0.48–1.03) | 0.073 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; ER, estrogen receptor; PR, progesterone receptor; RT, radiotherapy.
Determination of the effect of RT on survival after stratification by ER status and age
We hypothesized the existence of confounding factors that would affect the interaction between RT delivery and clinical outcomes. Therefore, we performed further multivariate analyses stratifying by potential factors including age and ER status.
After stratifying by ER status (Tables S1 and S2), 3,913 patients and 20,202 patients were found to have ER-negative/borderline and ER-positive disease, respectively. Consistent with results of the overall population analysis, RT had a favorable effect on OS in both ER-negative/borderline (HR 0.56, 95% CI 0.42–0.75, P<0.001) and ER-positive subgroups (HR 0.61, 95% CI 0.53–0.69, P<0.001). In contrast, we noted that RT delivery was specifically associated with improved BCSS in ER-negative/borderline patients (HR 0.41, 95% CI 0.19–0.88, P=0.023).
After stratifying by age (Tables S3 and S4), 15,554 patients aged ≤50 years and 41,414 patients aged >50 years were identified. Consistent with the results of the overall population analysis, RT provided benefit for OS in both subgroups (younger group, HR 0.47, 95% CI 0.31–0.72, P<0.001; older group, HR 0.61, 95% CI 0.54–0.69, P<0.001). By comparison, RT specifically contributed to better BCSS in the younger subgroup (HR 0.37, 95% CI 0.15–0.91, P=0.030).
Discussion
DCIS is a biologically and clinically heterogeneous disease with good prognosis; however, the treatment of DCIS after BCS remains controversial. In the National Comprehensive Cancer Network guidelines for treatment of breast cancer (version 1, 2015), RT is still an optional, but not a compulsory, treatment for DCIS following BCS. Therefore, we performed this analysis to investigate whether postoperative RT exerts a protective effect on the survival of DCIS patients after BCS.
In this population-based cohort of DCIS patients who underwent BCS, RT delivery was significantly associated with improved OS regardless of subgroup analysis after stratification, refuting the statement by two previous trials claiming that RT does not affect OS of DCIS patients.9,10 We speculated that one possible cause for this disparity was the sample size, for both trials lacked sufficient sample size to conclusively suggest effect of RT on OS. Another possible reason for the disparity was attributed to the year of diagnosis; the patients in our analysis were diagnosed in more recent years than those in the two trials, and thereby might benefit from more advanced RT techniques. Recently, Soeteman et al developed a simulation model integrating data from the published literature to simulate the clinical outcome after different treatments for women with newly diagnosed DCIS. The results of their model also suggested that RT offered a survival benefit for DCIS patients.17
In contrast, BCSS did not benefit from RT in the overall population, but after stratification by ER status and age, RT acted as a protective factor in ER-negative/borderline and younger patients. We speculated that the role of RT might be masked by endocrine therapy in ER-positive patients. On one hand, endocrine therapy has a definite role in reducing LR risk, and recent clinical trial data indicated that endocrine therapy may be an adequate substitute for RT in some ER-positive older patients.18–20 On the other hand, endocrine therapy is usually administered for 5 years and still exerts a carry-over effect after withdrawal.21 In contrast, the benefit from RT was highlighted in ER-negative/borderline patients who did not receive any endocrine therapy. Recently, a retrospective study also suggested that RT could be omitted in ER-positive DCIS.22
As with infiltrating breast cancer, young age (under 40 years or 50 years) has been shown to be associated with a higher LR rate of DCIS, as confirmed by several studies.4,5,7,23 Since the risk of recurrence in a conserved breast is much higher in younger than in older women, it could be reasonably reliably inferred that RT to a conserved breast would have a correspondingly greater effect on BCSS in younger than in older women. Moreover, avoidance of death from breast cancer gains more additional years of life expectancy for younger than for older women. Therefore, our results showed that only younger patients (≤50 years old) benefited from RT with respect to BCSS. However, our results differ from two clinical trials (SweDCIS and UK/ANZ) suggesting that older women benefit more from RT than younger women do.6,24 There are several possible explanations for this disparity: First, the primary endpoint of these two trials was ipsilateral LR; the benefit of LR reduction could not be translated into survival benefit directly as death due to breast cancer after DCIS is a tertiary-level effect after LR and distant metastases.25 Second, in the two trials, the older patients overwhelmingly accounted for a larger proportion than younger patients in both the RT group and overall population, especially in the UK/ANZ trial, in which over 90% of subjects were aged 50 years or more. Thus, clinical trials with age-balanced populations are required to solve this problem.
After adjusting for other known prognostic factors, our findings showed that married patients had better survival, which was consistent with another population-based study.26 In addition, DCIS patients with right-sided lesions had better BCSS, which was similar to what was seen in invasive breast cancer.27 Our analysis also supported the notion that black women had worse BCSS, as has been shown in existing studies.28–30 In addition, our analysis showed that patients diagnosed in more recent years had better BCSS after adjusting for other factors, which supported other published studies suggesting a significant decline in breast cancer mortality over time, which was mainly due to advances in adjuvant therapies.31,32
There are several limitations in our analysis. First, it was a retrospective analysis based on the SEER population, among which several clinicopathological variables were not well balanced. Second, the SEER database did not provide complete tumor characteristics (eg, HER2/neu status, breast subtype, and tumor size), cancer therapy (chemotherapy, endocrine therapy, and RT types), and clinical outcome (recurrence and metastasis) variables; thus, these potential confounding factors could not be adjusted, nor could recurrence-free survival and distant metastasis-free survival be assessed. Third, the SEER database did not provide surgery information on patients diagnosed before 1998, so DCIS patients who underwent BCS before 1998 were omitted, leading to limited sample size for our analysis; the individual subgroups became smaller after stratification (especially by ER status), yielding limited statistical power. Finally, the current SEER database is only updated till 2011, limiting the identification of potential long-term survival differences based on current follow-up because breast cancer recurrence may occur after up to 15 years in the natural history of the disease.18
Despite these limitations, our analysis confirms that postoperative RT contributes to improvement in OS in patients with locally excised DCIS. Regarding BCSS, our analysis reveals that women younger than 50 years benefit substantially from RT after local resection, and the protective role of RT becomes more evident in ER-negative/borderline patients. Therefore, in the clinical practice, RT should be selectively recommended to the younger or ER-negative/borderline patients to avoid overtreatment of other patients. At the same time, it is important to note the fatal adverse effects of RT including contralateral breast cancer and cardiac or lung damage. But these adverse effects of local treatment would be gradually avoided by the progress of RT techniques.
Taken together, the results of our analysis would be helpful to clinical decision-making in the management of DCIS, but our findings need to be further validated in other databases. DCIS is a complex disease process with heterogeneous biological and clinical characteristics, requiring personalized therapy for the patients. Therefore, further biological and clinical studies should be sought to identify a “low-risk” subgroup of DCIS patients for whom RT may be avoided.
Supplementary materials
Table S1.
Multivariate analysis of overall survival after stratification by ER status
| Variable | ER positive
|
ER negative/borderline
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| <50 | Reference | Reference | ||
| >50 | 4.29 (3.38–5.43) | <0.001 | 2.73 (1.72–4.35) | <0.001 |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.84 (0.70–1.01) | 0.069 | 0.76 (0.53–1.09) | 0.137 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.75 (0.62–0.91) | 0.003 | 0.81 (0.43–1.54) | 0.317 |
| Other* | 0.55 (0.40–0.76) | <0.001 | 0.80 (0.52–1.24) | 0.519 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.55 (0.48–0.62) | <0.001 | 0.60 (0.45–0.79) | <0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 1.10 (0.93–1.30) | 0.278 | 1.35 (0.94–1.92) | 0.101 |
| High SEP | 0.93 (0.76–1.12) | 0.435 | 0.89 (0.58–1.36) | 0.576 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 1.03 (0.91–1.18) | 0.645 | 0.98 (0.74–1.29) | 0.871 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 1.16 (0.97–1.40) | 0.114 | 1.43 (1.00–2.04) | 0.047 |
| PR status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.81 (0.68–0.96) | 0.013 | 1.33 (0.82–2.17) | 0.250 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 0.98 (0.82–1.18) | 0.836 | 1.15 (0.51–2.58) | 0.735 |
| III and IV | 0.97 (0.81–1.18) | 0.782 | 1.12 (0.52–2.40) | 0.776 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.61 (0.53–0.69) | <0.001 | 0.56 (0.42–0.75) | <0.001 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: ER, estrogen receptor; HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; PR, progesterone receptor; RT, radiotherapy.
Table S2.
Multivariate analysis of breast cancer-specific survival after stratification by ER status
| Variable | ER positive
|
ER negative/borderline
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| <50 | Reference | Reference | ||
| >50 | 1.98 (1.13–3.45) | 0.017 | 1.24 (0.46–3.31) | 0.674 |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.60 (0.35–1.04) | 0.068 | 0.71 (0.28–1.79) | 0.468 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.46 (0.28–0.77) | 0.003 | 0.93 (0.27–3.17) | 0.904 |
| Other* | 0.29 (0.10–0.86) | 0.026 | 1.92 (0.42–8.83) | 0.400 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.46 (0.30–0.70) | <0.001 | 0.75 (0.35–1.63) | 0.471 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 1.26 (0.74–2.14) | 0.389 | 1.64 (0.65–4.14) | 0.295 |
| High SEP | 0.72 (0.37–1.39) | 0.327 | 0.13 (0.02–1.08) | 0.058 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.76 (0.50–1.16) | 0.191 | 0.42 (0.18–0.97) | 0.042 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 1.08 (0.58–1.98) | 0.815 | 1.05 (0.36–3.07) | 0.930 |
| PR status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.66 (0.40–1.09) | 0.103 | 1.06 (0.25–4.54) | 0.943 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 1.99 (0.97–4.06) | 0.060 | 0.47 (0.05–4.55) | 0.514 |
| III and IV | 1.70 (0.81–3.57) | 0.163 | 0.94 (0.13–7.03) | 0.953 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.81 (0.52–1.25) | 0.340 | 0.41 (0.19–0.88) | 0.023 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: ER, estrogen receptor; HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; PR, progesterone receptor; RT, radiotherapy.
Table S3.
Multivariate analysis of overall survival after stratification by age
| Variable | <50 years old
|
>50 years old
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.69 (0.41–1.16) | 0.162 | 0.84 (0.71–1.00) | 0.050 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.61 (0.34–1.10) | 0.099 | 0.77 (0.64–0.92) | 0.004 |
| Other* | 0.77 (0.35–1.68) | 0.512 | 0.56 (0.41–0.77) | <0.001 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.77 (0.50–1.17) | 0.217 | 0.54 (0.47–0.61) | <0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 0.94 (0.58–1.53) | 0.802 | 1.17 (1.00–1.38) | 0.055 |
| High SEP | 0.62 (0.34–1.11) | 0.106 | 0.96 (0.80–1.15) | 0.651 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.82 (0.54–1.22) | 0.325 | 1.04 (0.92–1.18) | 0.516 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 0.84 (0.44–1.57) | 0.576 | 1.25 (1.06–1.49) | 0.010 |
| ER status | ||||
| Negative | Reference | Reference | ||
| Positive | 1.03 (0.54–1.97) | 0.928 | 1.08 (0.87–1.33) | 0.492 |
| PR status | ||||
| Negative | Reference | Reference | ||
| Positive | 0.56 (0.32–0.97) | 0.038 | 0.88 (0.74–1.04) | 0.129 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 0.80 (0.41–1.55) | 0.502 | 1.01 (0.84–1.21) | 0.912 |
| III and IV | 1.04 (0.54–1.99) | 0.916 | 0.98 (0.81–1.18) | 0.794 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.47 (0.31–0.72) | <0.001 | 0.61 (0.54–0.69) | <0.001 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never marcried), and separated.
Abbreviations: HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; ER, estrogen receptor; PR, progesterone receptor; RT, radiotherapy.
Table S4.
Multivariate analysis of breast cancer-specific survival after stratification by age
| Variable | <50 years old
|
<50 years old
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.51 (0.17–1.50) | 0.220 | 0.64 (0.38–1.09) | 0.100 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.39 (0.12–1.29) | 0.122 | 0.53 (0.32–0.88) | 0.014 |
| Other* | 1.45 (0.37–5.73) | 0.599 | 0.25 (0.07–0.83) | 0.024 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.59 (0.24–1.48) | 0.265 | 0.50 (0.33–0.75) | 0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 1.35 (0.46–4.00) | 0.586 | 1.36 (0.82–2.26) | 0.235 |
| High SEP | 0.73 (0.19–2.78) | 0.641 | 0.55 (0.27–1.09) | 0.087 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.44 (0.17–1.15) | 0.093 | 0.72 (0.48–1.08) | 0.110 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 0.66 (0.15–2.89) | 0.576 | 1.16 (0.65–2.05) | 0.617 |
| ER status | ||||
| Negative | Reference | Reference | ||
| Positive | 0.97 (0.26–3.65) | 0.963 | 0.99 (0.53–1.86) | 0.973 |
| PR status | ||||
| Negative | Reference | Reference | ||
| Positive | 0.43 (0.13–1.39) | 0.158 | 0.75 (0.44–1.29) | 0.299 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 2.83 (0.36–22.50) | 0.325 | 1.72 (0.83–3.54) | 0.144 |
| III and IV | 2.42 (0.30–19.71) | 0.408 | 1.67 (0.79–3.48) | 0.179 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.37 (0.15–0.91) | 0.030 | 0.80 (0.52–1.21) | 0.285 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; RT, radiotherapy.
Acknowledgments
The authors thank Dr Wei Deng for her assistance in statistical analysis and Dr Cissy Yu for her assistance in manuscript preparation.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchio C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 2010;57(2):171–192. doi: 10.1111/j.1365-2559.2010.03568.x. [DOI] [PubMed] [Google Scholar]
- 2.Duggal S, Robin J, Julian TB. Ductal carcinoma in situ: an overview. Expert Rev Anticancer Ther. 2013;13(8):955–962. doi: 10.1586/14737140.2013.820557. [DOI] [PubMed] [Google Scholar]
- 3.Sakorafas GH, Farley DR, Peros G. Recent advances and current controversies in the management of DCIS of the breast. Cancer Treat Rev. 2008;34(6):483–497. doi: 10.1016/j.ctrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Emdin SO, Granstrand B, Ringberg A, et al. Swedish Breast Cancer Group SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45(5):536–543. doi: 10.1080/02841860600681569. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12(1):21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EORTC Breast Cancer Cooperative Group; EORTC Radiotherapy Group; Bijker N, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853 – a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 8.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 ran-domized phase III trial. J Clin Oncol. 2013;31(32):4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 10.Wärnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS trial. J Clin Oncol. 2014;32(32):3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 11.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of protocol B-17: intraductal carcinoma. Cancer. 1999;86(3):429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results (SEER) Program. Research Data (1973–2011) Surveillance Research Program, Surveillance Systems Branch, National Cancer Institute, DCCPS; released April 2014, based on the November 2013 submission. Available from: www.seer.cancer.gov. [Google Scholar]
- 13.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98(10):681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 14.Singh GK, Miller BA, Hankey BF, Edwards BK. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. Bethesda, MD: National Cancer Institute; 2003. (NCI Cancer Surveillance Monograph Series, Number 4). [Google Scholar]
- 15.Surveillance, Epidemiology, and End Results (SEER) Program . SEER*Stat database: county attributes – total U.S., 1969–2007 counties. Bethesda, MD: Surveillance Research Program, Cancer Statistics Branch, National Cancer Institute, DCCPS; Available from: www.seer.cancer.gov/seerstat/variables/countyattribs. [Google Scholar]
- 16.Schlichting JA, Soliman AS, Schairer C, et al. Association of inflammatory and noninflammatory breast cancer with socioeconomic characteristics in the surveillance, epidemiology, and end results database, 2000–2007. Cancer Epidemiol Biomarkers Prev. 2012;21(1):155–165. doi: 10.1158/1055-9965.EPI-11-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soeteman DI, Stout NK, Ozanne EM, et al. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J Natl Cancer Inst. 2013;105(11):774–781. doi: 10.1093/jnci/djt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Bryant J, Dignam JJ, et al. National Surgical Adjuvant Breast and Bowel Project Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20(20):4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 20.Hughes KS, Schnaper LA, Berry D, et al. Cancer and Leukemia Group B. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 21.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim T, Park HK, Lee KH, et al. Is radiotherapy necessary for intermediate risk ductal carcinoma in situ after breast conserving surgery? Springerplus. 2014;3:405. doi: 10.1186/2193-1801-3-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinder SE, Duggan C, Ellis IO, et al. UK Coordinating Committee on Cancer Research (UKCCCR) Ductal Carcinoma In Situ (DCIS) Working Party A new pathological system for grading DCIS with improved prediction of local recurrence: results from the UKCCCR/ANZ DCIS trial. Br J Cancer. 2010;103(1):94–100. doi: 10.1038/sj.bjc.6605718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26(8):1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 25.Cutuli B, Bernier J, Poortmans P. Radiotherapy in DCIS, an underestimated benefit? Radiother Oncol. 2014;112(1):1–8. doi: 10.1016/j.radonc.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao J, Yu KD, Jiang YZ, Shao ZM, Di GH. The effect of laterality and primary tumor site on cancer-specific mortality in breast cancer: a SEER population-based study. PLoS One. 2014;9(4):e94815. doi: 10.1371/journal.pone.0094815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 30.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101(14):993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry DA, Cronin KA, Plevritis SK, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 32.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Multivariate analysis of overall survival after stratification by ER status
| Variable | ER positive
|
ER negative/borderline
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| <50 | Reference | Reference | ||
| >50 | 4.29 (3.38–5.43) | <0.001 | 2.73 (1.72–4.35) | <0.001 |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.84 (0.70–1.01) | 0.069 | 0.76 (0.53–1.09) | 0.137 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.75 (0.62–0.91) | 0.003 | 0.81 (0.43–1.54) | 0.317 |
| Other* | 0.55 (0.40–0.76) | <0.001 | 0.80 (0.52–1.24) | 0.519 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.55 (0.48–0.62) | <0.001 | 0.60 (0.45–0.79) | <0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 1.10 (0.93–1.30) | 0.278 | 1.35 (0.94–1.92) | 0.101 |
| High SEP | 0.93 (0.76–1.12) | 0.435 | 0.89 (0.58–1.36) | 0.576 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 1.03 (0.91–1.18) | 0.645 | 0.98 (0.74–1.29) | 0.871 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 1.16 (0.97–1.40) | 0.114 | 1.43 (1.00–2.04) | 0.047 |
| PR status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.81 (0.68–0.96) | 0.013 | 1.33 (0.82–2.17) | 0.250 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 0.98 (0.82–1.18) | 0.836 | 1.15 (0.51–2.58) | 0.735 |
| III and IV | 0.97 (0.81–1.18) | 0.782 | 1.12 (0.52–2.40) | 0.776 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.61 (0.53–0.69) | <0.001 | 0.56 (0.42–0.75) | <0.001 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: ER, estrogen receptor; HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; PR, progesterone receptor; RT, radiotherapy.
Table S2.
Multivariate analysis of breast cancer-specific survival after stratification by ER status
| Variable | ER positive
|
ER negative/borderline
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| <50 | Reference | Reference | ||
| >50 | 1.98 (1.13–3.45) | 0.017 | 1.24 (0.46–3.31) | 0.674 |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.60 (0.35–1.04) | 0.068 | 0.71 (0.28–1.79) | 0.468 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.46 (0.28–0.77) | 0.003 | 0.93 (0.27–3.17) | 0.904 |
| Other* | 0.29 (0.10–0.86) | 0.026 | 1.92 (0.42–8.83) | 0.400 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.46 (0.30–0.70) | <0.001 | 0.75 (0.35–1.63) | 0.471 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 1.26 (0.74–2.14) | 0.389 | 1.64 (0.65–4.14) | 0.295 |
| High SEP | 0.72 (0.37–1.39) | 0.327 | 0.13 (0.02–1.08) | 0.058 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.76 (0.50–1.16) | 0.191 | 0.42 (0.18–0.97) | 0.042 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 1.08 (0.58–1.98) | 0.815 | 1.05 (0.36–3.07) | 0.930 |
| PR status | ||||
| Negative/borderline | Reference | Reference | ||
| Positive | 0.66 (0.40–1.09) | 0.103 | 1.06 (0.25–4.54) | 0.943 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 1.99 (0.97–4.06) | 0.060 | 0.47 (0.05–4.55) | 0.514 |
| III and IV | 1.70 (0.81–3.57) | 0.163 | 0.94 (0.13–7.03) | 0.953 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.81 (0.52–1.25) | 0.340 | 0.41 (0.19–0.88) | 0.023 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: ER, estrogen receptor; HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; PR, progesterone receptor; RT, radiotherapy.
Table S3.
Multivariate analysis of overall survival after stratification by age
| Variable | <50 years old
|
>50 years old
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.69 (0.41–1.16) | 0.162 | 0.84 (0.71–1.00) | 0.050 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.61 (0.34–1.10) | 0.099 | 0.77 (0.64–0.92) | 0.004 |
| Other* | 0.77 (0.35–1.68) | 0.512 | 0.56 (0.41–0.77) | <0.001 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.77 (0.50–1.17) | 0.217 | 0.54 (0.47–0.61) | <0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 0.94 (0.58–1.53) | 0.802 | 1.17 (1.00–1.38) | 0.055 |
| High SEP | 0.62 (0.34–1.11) | 0.106 | 0.96 (0.80–1.15) | 0.651 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.82 (0.54–1.22) | 0.325 | 1.04 (0.92–1.18) | 0.516 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 0.84 (0.44–1.57) | 0.576 | 1.25 (1.06–1.49) | 0.010 |
| ER status | ||||
| Negative | Reference | Reference | ||
| Positive | 1.03 (0.54–1.97) | 0.928 | 1.08 (0.87–1.33) | 0.492 |
| PR status | ||||
| Negative | Reference | Reference | ||
| Positive | 0.56 (0.32–0.97) | 0.038 | 0.88 (0.74–1.04) | 0.129 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 0.80 (0.41–1.55) | 0.502 | 1.01 (0.84–1.21) | 0.912 |
| III and IV | 1.04 (0.54–1.99) | 0.916 | 0.98 (0.81–1.18) | 0.794 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.47 (0.31–0.72) | <0.001 | 0.61 (0.54–0.69) | <0.001 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never marcried), and separated.
Abbreviations: HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; ER, estrogen receptor; PR, progesterone receptor; RT, radiotherapy.
Table S4.
Multivariate analysis of breast cancer-specific survival after stratification by age
| Variable | <50 years old
|
<50 years old
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Year of diagnosis | ||||
| 1998–2002 | Reference | Reference | ||
| 2003–2007 | 0.51 (0.17–1.50) | 0.220 | 0.64 (0.38–1.09) | 0.100 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.39 (0.12–1.29) | 0.122 | 0.53 (0.32–0.88) | 0.014 |
| Other* | 1.45 (0.37–5.73) | 0.599 | 0.25 (0.07–0.83) | 0.024 |
| Marital status | ||||
| Non-married† | Reference | Reference | ||
| Married | 0.59 (0.24–1.48) | 0.265 | 0.50 (0.33–0.75) | 0.001 |
| Poverty–high school index | ||||
| Low SEP | Reference | Reference | ||
| Middle SEP | 1.35 (0.46–4.00) | 0.586 | 1.36 (0.82–2.26) | 0.235 |
| High SEP | 0.73 (0.19–2.78) | 0.641 | 0.55 (0.27–1.09) | 0.087 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 0.44 (0.17–1.15) | 0.093 | 0.72 (0.48–1.08) | 0.110 |
| Primary site | ||||
| Single-quadrant lesions | Reference | Reference | ||
| Multi-quadrant lesions | 0.66 (0.15–2.89) | 0.576 | 1.16 (0.65–2.05) | 0.617 |
| ER status | ||||
| Negative | Reference | Reference | ||
| Positive | 0.97 (0.26–3.65) | 0.963 | 0.99 (0.53–1.86) | 0.973 |
| PR status | ||||
| Negative | Reference | Reference | ||
| Positive | 0.43 (0.13–1.39) | 0.158 | 0.75 (0.44–1.29) | 0.299 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 2.83 (0.36–22.50) | 0.325 | 1.72 (0.83–3.54) | 0.144 |
| III and IV | 2.42 (0.30–19.71) | 0.408 | 1.67 (0.79–3.48) | 0.179 |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.37 (0.15–0.91) | 0.030 | 0.80 (0.52–1.21) | 0.285 |
Notes:
Including American Indian/Alaskan Native and Asian/Pacific Islander.
Including divorced, widowed, single (never married), and separated.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HR, hazard ratio; CI, confidence interval; SEP, socioeconomic position; RT, radiotherapy.