Abstract
Poly[methacrylic acid-grafted-poly(ethylene glycol)] [P(MAA-g-EG)] is a complexation hydrogel molecularly designed for oral peptide delivery. In this work, the cytotoxicity and insulin-transport enhancing effect of P(MAA-g-EG) microparticles on intestinal epithelial cells were evaluated using Caco-2 cell monolayers. A series of P(MAA-g-EG) microparticles with different polymer compositions were prepared by a photo-initiated free radical solution polymerization and subsequent pulverization. The hydrogel microparticles were preswollen in either Ca2+-containing (CM+) or Ca2+-free medium (CM-; pH 7.4) and applied to the apical side of the Caco-2 monolayers. No significant cytotoxic effects, as determined by a calorimetric assay with P(MAA-g-EG) microparticles preswollen in the CM+, were observed at doses ranging from 3 to 31 mg/cm2 of cell monolayer. Transepithelial electrical resistance (TEER) measurements showed that the P(MAA-g-EG) microparticles induced a Ca2+ concentration-dependent lowering in TEER values. The reduction effect in CM− media was greater than that in CM+ media (17 ± 2% reduction in CM+ and 45 ± 3% reduction in CM− , respectively). Insulin transport in the presence of the preswollen P(MAA-g-EG) microparticles was also strongly depended on the Ca2+ concentration in the medium. The respective estimated permeability for insulin alone and the insulin with hydrogels in CM+ were 0.77 and 1.16 × 10−8 cm/s, whereas those in CM− were 1.18 and 24.78 × 10−8 cm/s. The results demonstrate that the P(MAA-g-EG) hydrogel microparticles could be used as a cytocompatible carrier possessing the transport-enhancing effect of insulin on the intestinal epithelial cells.
Keywords: pH-sensitive hydrogels, oral peptide delivery, Caco-2 cells, cytotoxicity, insulin transport, transepithelial electrical resistance
INTRODUCTION
In the last few decades, considerable research has been done in the design of oral release systems for delivery of therapeutic peptides and proteins. Difficulties encountered for delivery of these drugs via the oral route result mainly from their delicate physicochemical properties, their degradation by proteolytic enzymes, and their poor permeability through the intestinal tissue. Thus, the system requires specific functions so as to deliver the drugs in an effective way. For this purpose, versatile polymeric materials such as azopolymers,1 poly(alkyl cyanoacrylates),2 chitosan,3 poly(acrylic acid) and its derivatives,4,5 polyanhydrides,6 and graft copolymers with hydrophobic backbone and hydrophilic branches 7 have been utilized to fabricate the delivery systems with such specific functions.
Copolymer networks of poly[methacrylic acid-grafted-poly(ethylene glycol)] [P(MAA-g-EG)] are hydrogels exhibiting hydrogen bonding and molecularly designed to achieve such specific functions in oral delivery of peptides and proteins.8-13 These hydrogels were shown to exhibit swelling behavior due to the formation of interpolymer complexes in acidic media as a result of hydrogen bonding between etheric groups of the graft PEG chains and the protons of the carboxylic groups on the PMAA network.8,9 At low pH values corresponding to a gastric juice, the networks form complexes and thereby do not swell to a high degree. The network mesh size at the low pH is small enough to prevent diffusion of peptide drugs such as insulin through the network.10 Hence, drugs incorporated into the hydrogels can be protected from degradation by digestive enzymes.
In the higher pH environments of the intestine, the pendant acid groups on the PMAA network become ionized, and the hydrogen bonds break down. Consequently, the network swells to a high degree because of the electric repulsion generated within the network. The large mesh size resulting from the highly swollen state allows drug release from the network at more favorable sites for their absorption.10 Additionally, the uncomplexed hydrogels demonstrate mucoadhesive characteristics due to the PEG tethered chains act as anchors for the mucin layer 11 and inhibitory effects of calcium-dependent luminal enzymes such as trypsin and α-chymotrypsin by the calcium chelating capability of the carboxylic acid pendant groups.12 These characteristics can lead to the locally increased drug concentration at its absorption sites and thereby the enhanced absorption. Indeed, insulin-loaded P(MAA-g-EG) microparticles have demonstrated strong hypoglycemic effects when they were orally administered to diabetic rats.13
Previous findings in our laboratory motivated us to further investigate the enhancing effect of insulin absorption in a model biological environment for better understanding of the performance of P(MAA-g-EG) hydrogels in vivo. In the present study, the effect of P(MAA-g-EG) microparticles on the cellular response involving peptide absorption behavior was thus evaluated using the Caco-2 cell line. Viability and integrity of the cell monolayers, which affect the permeability of cell monolayers for peptide molecules, were assessed in the presence of P(MAA-g-EG) microparticles. The transport behavior of insulin employed as a model peptide drug through the cell monolayers was also examined.
MATERIALS AND METHODS
Materials
Methacrylic acid (MAA) and 2,2-dimethoxy-2-phenylacetophenon (DMPA) were purchased from Aldrich Chemical Co. (Milwaukee, WI). Methoxy-terminated poly(ethylene glycol) monomethacrylate (PEGMA) with PEG chains of molecular weight 200, 400, and 1000, and tetraethylene glycol dimethacrylate (TEGDMA) were obtained from Polysciences Inc. (Warrington, PA). The other reagents were used without further purification.
Preparation of hydrogels
P(MAA-g-EG) hydrogels were synthesized by free radical solution polymerization according to a previous report.12 The monomer MAA was purified by vacuum distillation prior to use. The MAA and PEGMA were mixed in the molar ratios of 1:2, 1:1, 2:1, and 4:1 MAA/EG repeating units with PEG1000, whereas they were mixed at a molar ratio of 1:1 for hydrogels with different molecular weights of the PEG chains (200, 400, and 1000). One wt % (based on the total amount of MAA and PEGMA) of TEGDMA, a crosslinking agent, was added to the monomer mixture. The resulting mixture was diluted with 50 wt % ethanolic solution to provide 66.7 wt % concentration of the monomer mixture. Nitrogen was bubbled through the mixture for 30 min. Thereafter, the photoinitiator DMPA was dissolved in the monomer mixture in the amount of 2 wt % of the total monomers. The solution was cast between two flat glass plates with a 0.9-mm-thickness spacer.
The glass plates were exposed with a UV light source (Ultracure 100, Efos USA., Inc., Williamsville, NY) with an intensity of 800 mW/cm2 at 365 nm for 15 min in a nitrogen atmosphere. The polymer films thus obtained were immersed in deionized water for 7 days; the water was changed twice daily in order to remove unreacted monomers, crosslinking agent, and initiator. The washed films were dried at room temperature for 48 h. The dried films were pulverized by a mortar and pestle and then finely ground using a mill.
The pulverized hydrogels were passed through a stainless sieve with an opening of 150 μm. Two hundred milligrams of the hydrogel powders were dispersed in 10 mL of purified water in a 30-mL volume glass vial, autoclaved at 121°C for 35 min, and used for all experiments. The hydrogel particles thus autoclaved were washed with Hanks’ balanced salt solution (HBSS; Sigma, St. Louis, MO) under aseptic conditions until pH value of the suspensions reached >7.0. For certain experiments, the hydrogel particles were washed with calcium-and magnesium-free HBSS [HBSS-CM(−), Sigma] in the same manner.
Cell culture
The Caco-2 cell line was purchased from the American Tissue Culture Collection (Rockville, MD). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma), supplemented with 10% fetal bovine serum (Life Technologies, Gaithersburg, MD), 1% nonessential amino acids (Life Technologies), 1% l-glutamine (Life Technologies), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma). The medium was changed every other day. The cells were passaged weekly after exposure to 0.25% trypsin plus 0.02% EDTA solution and routinely grown in 75-cm2 culture flasks at 37°C in a humidified atmosphere of 5% CO2 in air. For all experiments, cells were used between passage number 66 and 72.
Viability test
Cell viability was determined using a CellTiter 96 Aqueous One Solution Reagent (Promega, Madison, WI) containing a novel tetrazolium compound, 3-(4,5-dimethylthiazole-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), and an electron coupling reagent, phenazine ethosulfate. Briefly, the MTS tetrazolium compound is bioreduced by cells into a colored formazan product that is soluble in cell culture medium. This conversion was accomplished by NADPH or NADH produced by dehydrogenase enzymes in metabolically active cells. Cells were seeded in 96-well culture plates at a density of 31,250 cells/cm2 and cultivated for 7 days. The cells were washed twice with HBSS and incubated at 37°C for 2.5 h with 0.2 mL of HBSS containing the hydrogel particles prewashed with HBSS at a given concentration of 1, 5, and 10 mg/mL [corresponding to 3, 16, and 31 mg polymer (dry basis) per unit area (cm2) of cell monolayers], rinsed twice with HBSS and further incubated with the MTS assay solution for 1 more hour. The color intensity at 490 nm was determined with a microplate reader (Elx800NB BioTek Instruments, Winooski, VT). Cell viability (%) was expressed as the ratio of the color intensity in the treated groups to that in the control (untreated) group.
Measurement of the transepithelial electrical resistance
The integrity of cell monolayers upon contact with the hydrogel particles was assessed by measuring the transepithelial electrical resistance (TEER). The cells were seeded on polycarbonate filters with a surface area of 4.71 cm2 and a pore size of 0.4 μm in Costar Transwell 6-well plates at a seeding density of 52,500 cells/cm2. The cells were left for 21 days to reach confluence and to differentiate, while the culture medium was changed every other day. An EVOM volt-ohm meter with a chopstick-type electrode (World Precise Instruments, Sarasota, FL) was used to measure the TEER values. A preliminary confirmation revealed that the electrical resistance of the filter itself in the presence of the P(MAA-g-EG) particles was not different from that in the absence of the P(MAA-g-EG) particles, indicating no disturbance. The resistance of the monolayer was obtained by subtracting the intrinsic resistance of the system (filter insert alone) from the total resistance (monolayer plus filter insert). The TEER of the untreated monolayers reached a value of around 1200 Ωcm2.
The cell monolayers were washed twice with PBS (pH 7.4) and then equilibrated in HBSS containing 0.05 w/v% bovine serum albumin (BSA, Fraction-V, fatty acids free, Sigma) for 60 min. Thereafter, the apical medium was replaced with 1.5 mL of the HBSS dispersion containing 10 mg of the P(MAA-g-EG) microparticles, and the cell monolayers were incubated for 2.5 h. At specific time points, the TEER was measured by the EVOM. After 2.5-h incubation, the P(MAA-g-EG) microparticles were removed from the apical side, replaced with fresh HBSS and incubated for an additional 30 min. Then, both the apical and basolateral media were replaced with an equal volume of DMEM and the change of the TEER values of the cell monolayers were further monitored for a 21-h incubation.
The TEER measurements were also carried out under a calcium-deficient condition using the P(MAA-g-EG) microparticles prewashed with the HBSS-CM(−) media. In this experiment, the HBSS-CM(−) and the HBSS-CM(−) containing 0.2 mM calcium chloride were used as the apical media and the basolateral media, respectively. This solution is henceforth designated as HBSS-CM(0.2). This value is slightly above the minimum calcium concentration (0.1 mM) required to maintain monolayer integrity.14
Insulin transport
The cell monolayers were obtained as described in the preceding section, except that a polycarbonate filter with a pore size of 3.0 μm was used for the insulin transport experiments. Preliminary experiments using the Diff-Quick staining kit (Dade International Inc., Miami, FL) revealed that cell migration to the pores of the filter was negligible. The TEER values of the monolayers formed on the filters were in the range of 580–610 Ωcm2 before use.
Ten milligrams of bovine insulin (27.6 IU/mg, Sigma) was dissolved in 0.2 mL of 0.1N HCl. The insulin solution was diluted with 20 mL of HBSS containing 0.05 w/v% BSA (pH 7.4) medium and neutralized with 0.2 mL of 0.1N NaOH. This stock solution (15 mL) was further diluted with the HBSS and sterilized by passing the diluted solution through a 0.2-μm membrane filter in an aseptic condition. The cell monolayers were washed and equilibrated by the same procedure described in the preceding section. After the apical medium was removed, the insulin solution with the P(MAA-g-EG) particles dispersed in the corresponding medium was added so that the final insulin concentration was 161 μg/mL and 10 mg of the particles was present in each apical side.
At specific time points, 0.1-mL samples were withdrawn from the basolateral side and replaced with an equal volume of the corresponding fresh media. Control experiments were carried out with the insulin solution without the P(MAA-g-EG) particles. The samples were diluted with PBS containing 0.05 w/v% BSA and analyzed by using ELISA-kits (ALPCO, Windham, NH). In order to examine the calcium concentration dependence on the insulin transport-enhancing effect of the P(MAA-g-EG) particles, another experiments were performed under a calcium deficient condition with the similar manner described as the preceding section.
The apparent insulin permeability coefficient, Papp, was estimated by the following equation:
where Q is the amount of insulin in the basolateral side, C0 is the initial concentration of insulin in the apical chamber, and A is the surface area of the filter.
RESULTS
Cytotoxicity of P(MAA-g-EG) hydrogels
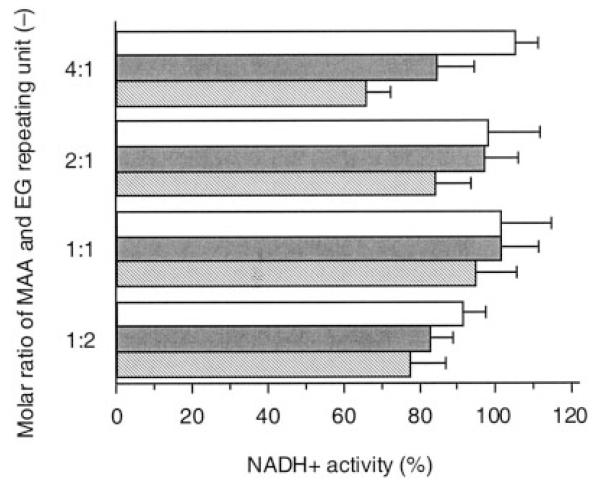
In order to examine a maximum dose of P(MAA-g-EG) hydrogels in contact with the Caco-2 cell monolayers, the cell viability was evaluated after exposure to the P(MAA-g-EG) particles for 2.5 h. The results are shown in Figure 1 for P(MAA-g-EG) particles with different molar ratios of MAA and EG repeating units.
Figure 1.
Viability of Caco-2 cells as determined by NADH+ activity after exposure to P(MAA-g-EG) microparticles with different MAA-to-EG molar ratios for 2.5 h in HBSS. Concentration of P(MAA-g-EG) microparticles (mg/mL): 1 (open bar), 5 (shaded bar), 10 (hatched bar). Each bar represents the mean ± SD of at least three experiments.
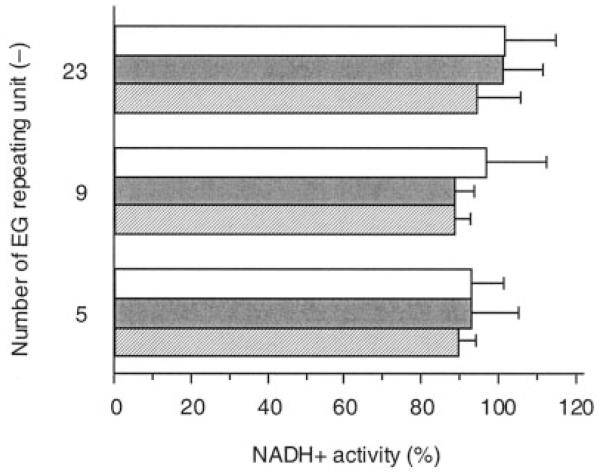
Overall, the P(MAA-g-EG) particles exhibited no significant cytotoxicity within the dose range studied here. At the low dose of 3 mg/cm2, >90% of the cells remained viable regardless of the polymer composition. As the dose increased, the cell viability tended to decrease, depending on the polymer composition. This tendency was most remarkable in the P(MAA-g-EG) with 4:1 molar ratio of MAA/EG repeating units. As shown in Figure 2, on the other hand, no significant dose-dependent effect on the cell viability was observed in the P(MAA-g-EG) with different length of PEG graft chains at 1:1 molar ratio of MAA/EG repeating units. In all cases, the cell viability was >90%, indicating that these hydrogels had a good compatibility with the cells.
Figure 2.
Viability of Caco-2 cells as determined by NADH+ activity after exposure to P(MAA-g-EG) microparticles with different number of EG repeating units for 2.5 h in HBSS. Concentration of P(MAA-g-EG) microparticles (mg/mL): 1 (open bar), 5 (shaded bar), 10 (hatched bar). Each bar represents the mean ± SD of at least three experiments.
Effect of P(MAA-g-EG) on TEER of cell monolayers
Change of the cell monolayer permeability was investigated by measuring the TEER in the presence of P(MAA-g-EG) microparticles with 1:1 molar ratio of MAA/EG units and EG repeating units of 23, which were the formulation employed for in vivo trials of the previous study.13 It is well known that Ca2+ ions have an important role to maintain the paracellular permeability of epithelial cell monolayers by modulating the tight junctions.14-16 Because the P(MAA-g-EG) hydrogels have the ability to bind calcium,12 their effect on the monolayer integrity was anticipated to be highly dependent on calcium concentration in the extracellular medium. Therefore, in order to clarify this, the TEER experiments were performed using calcium-rich and calcium-deficient media, respectively.
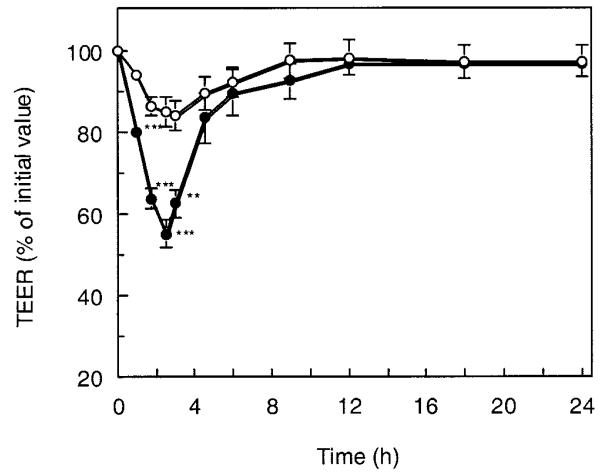
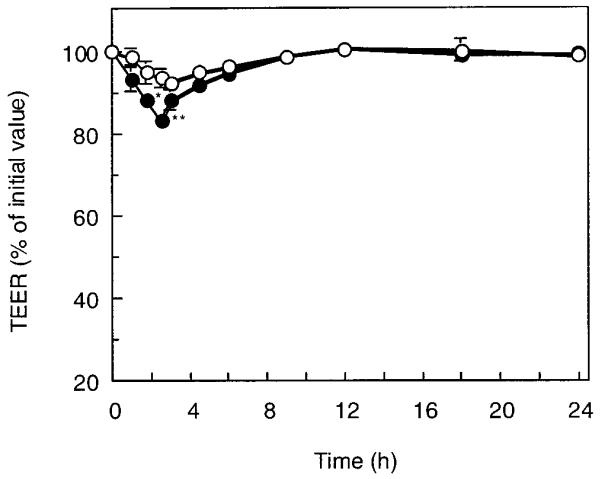
Figure 3 shows the change of the TEER of the cell monolayers in calcium-deficient conditions after the P(MAA-g-EG) microparticles were applied to the apical side at a dose of 2.1 mg/cm2. The TEER value dropped significantly in the presence of the P(MAA-g-EG) microparticles, when compared with that in the control. At 2.5 h after incubation, it reached 55% of the initial value. After the particles were removed from the apical side, the rapid recovery of TEER value was observed for ~1.5 h. The cell monolayer recovered its original TEER at 12 h followed by gradual recovery. This recovery stage lasted for 8 h, indicating that this TEER-lowering behavior was a reversible process. In contrast, it was found that this TEER-lowering effect was weaker in calcium-rich conditions as shown in Figure 4. The TEER value was simply reduced by 80% of its initial value after 2.5-h incubation, though the effect was still statistically significant compared with the control group.
Figure 3.
Time course change of the TEER of monolayers exposed to P(MAA-g-EG) microparticles with MAA-to-EG molar ratio of 1:1 and EG repeating units of 23 under calcium-deficient condition at a feed amount of 2.1 mg/cm2 for 2.5 h at 37°C. (○) Control, (●) P(MAA-g-EG). Culture medium: apical, HBSS-CM(−); basolateral, HBSS-CM(0.2). Each point represents the mean ± SD (n = 3). * * *p < 0.001, * *p < 0.01, significantly different from control result.
Figure 4.
Time course change of the TEER of monolayers exposed to P(MAA-g-EG) microparticles with MAA-to-EG molar ratio of 1:1 and EG repeating units of 23 under calcium-rich condition at a feed amount of 2.1 mg/cm2 for 2.5 h at 37°C. (○) Control, (●) P(MAA-g-EG). Culture medium: apical, HBSS; basolateral, HBSS. Each point represents the mean ± S.D. (n = 3). * *p < 0.01, *p < 0.05, significantly different from control result.
Effect of the P(MAA-g-EG) on the transport of insulin across cell monolayers
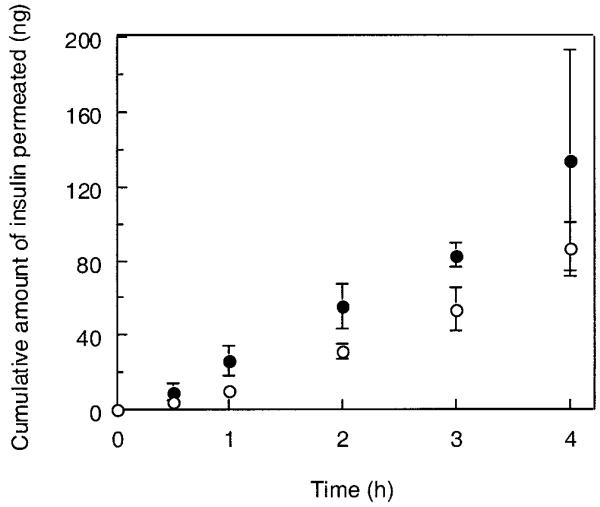
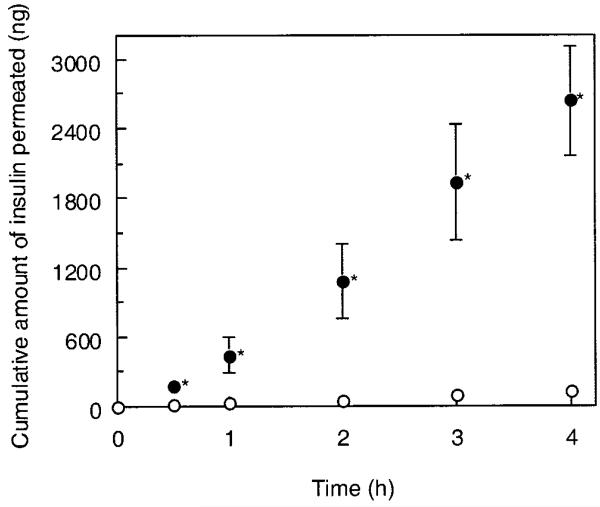
The apical-to-basolateral transport of insulin across the cell monolayers was compared in the presence or absence of the P(MAA-g-EG) particles with 1:1 molar ratio of MAA/EG and 23 EG repeating units. The experiments were performed under either calciumrich (Fig. 5) or calcium-deficient conditions (Fig. 6). No significant enhancement of the insulin transport by the P(MAA-g-EG) microparticles was detected in the calcium-rich conditions. In contrast, the P(MAA-g-EG) microparticles in the calcium-deficient conditions effectively improved the insulin transport across the cell monolayers. The cumulative amount of insulin transported for 4 h in the presence of the P(MAA-g-EG) microparticles reached ~1.0% of the total amount of insulin applied.
Figure 5.
Effect of the addition of P(MAA-g-EG) microparticles with MAA-to-EG molar ratio of 1:1 and EG repeating units of 23 to the apical side of monolayers on cumulative transport of insulin under calcium-rich condition at 37°C. (○) control, (●) P(MAA-g-EG). Each datum point represents the mean ± SD (n = 3).
Figure 6.
Effect of the addition of P(MAA-g-EG) microparticles with MAA-to-EG molar ratio of 1:1 and EG repeating units of 23 to the apical side of monolayers on cumulative transport of insulin under calcium-deficient condition at 37°C. (○) control, (●) P(MAA-g-EG). Each datum point represents the mean ± SD (n = 3). *p < 0.05, significantly different from insulin solution.
The apparent permeability coefficients of insulin were estimated from the data shown in Figures 5 and 6. The results are listed in Table I with the corresponding TEER values measured immediately before and after the transport experiments. Regardless of whether the P(MAA-g-EG) microparticles coexisted with insulin or not, the Papp values were found to be low in the calcium-rich conditions where there was no pronounced decrease in TEER up to 4 h, even with hydrogel application. However, in the calcium-deficient conditions, the Papp value in the presence of the P(MAA-g-EG) particles was significantly higher (24.78 ± 4.85 × 10−8 cm/sec), compared with that in the absence of the P(MAA-g-EG) particles (1.18 ± 0.30 × 10−8 cm/sec). This significant increase in the Papp value is probably ascribed from the substantial TEER reducing-effect of the P(MAA-g-EG) particles.
TABLE I. Apparent Permeability Coefficients of Insulin and the Corresponding TEER Measured Immediately before and after the Transport Experiments.
| Ca2+ Concentration (mM) in Transport Media |
TEER (Ω×cm2)a |
Papp ×10−8 (cm/sec) |
|||
|---|---|---|---|---|---|
| Apical | Basolateral | Before test (0 h) |
After test (4 h) |
||
| Control | 1.26 | 1.26 | 609 ± 7 | 546 ± 12 | — |
| Insulin solution | 1.26 | 1.26 | 581 ± 12 | 555 ± 15 | 0.77 |
| Insulin + P(MAA-g-EG) | 1.26 | 1.26 | 597 ± 14 | 485 ± 14 | 1.16 |
| Control | 0 | 0.2 | 586 ± 12 | 515 ± 7 | — |
| Insulin solution | 0 | 0.2 | 590 ± 12 | 504 ± 12 | 1.18 |
| Insluin + P(MAA-g-EG) | 0 | 0.2 | 597 ± 7 | 316 ± 12 | 24.78 |
Each value represents the mean ± SD (n = 3).
DISCUSSION
Several strategies for effective delivery of peptide drugs via oral route have been proposed so far. Use of absorption enhancers is a widely employed way to improve the absorption of peptides across the epithelial intestinal membrane.17,18 Ideally, the absorption enhancers used for this purpose must be capable of enhancement of peptide absorption, whereas they do not damage the intestinal membrane. The same is true for any class of oral peptide delivery systems based on an absorption-enhancing mechanism. The utility of the P(MAA-g-EG) hydrogels as devices for oral peptide delivery was thus assessed using the Caco-2 cell monolayers to determine the cytocompatibility as measured by intracellular enzyme activity, electrophysiology, and transport studies of insulin in the presence of the P(MAA-g-EG) microparticles.
The P(MAA-g-EG) microparticles were found to have a good cytocompatibility within the dose range and the monomer compositions studied here. The effect of the P(MAA-g-EG) hydrogels on intracellular enzyme activity was far lower than that observed with other commonly studied absorption enhancers, such as bile salts, fatty acids, chelating agents, and some other hydrophilic polymers.19-22 In general, such absorption enhancers are applied in the form of solution, so that they can easily access and more or less penetrate into the cells. Consequently, some absorption enhancers affect to the mucosal cell membrane and some induce essential cytotoxicity, depending on their type and/or their concentration. Unlike traditional absorption enhancers applied in the form of solution, it is reasonable to consider that the present hydrogels would be hardly absorbed into the Caco-2 cells monolayers because of their infinitely large macromolecular size.23-40
It is interesting to note that the P(MAA-g-EG) hydrogels are able to decrease the TEER of Caco-2 monolayers in a reversible manner (Figs. 3 and 4). Significantly larger drop of TEER value was observed when the cell monolayers were exposed to the P(MAA-g-EG) in the calcium-deficient condition, indicating that the integrity of the tight junctions in the Caco-2 monolayers became weak. Most probably, complexation or binding of cellular Ca2+ with swollen P(MAA-g-EG) is responsible for this effect.41-46
It has been reported that the normal cellular Ca2+ level in Caco-2 cells is maintained by the basolateral buffer solution, and the integrity of the tight junctions is not affected by removing Ca2+ from the apical side.14 For example, Kriwet and Kissel 4 reported that the application of poly(acrylic acid) microparticles with calcium chelating ability to apical side of Caco-2 monolayers led to a modest drop of the TEER by 17% of the initial value, whereas the addition of poly-(acrylic acid) to the basolateral side induced significant TEER reduction of almost 70%. In contrast, the apically applied P(MAA-g-EG) in calcium-deficient media caused significant reduction of TEER, suggesting that the P(MAA-g-EG) could somehow affect the basolateral Ca2+ concentration level. Basolateral-to-apical Ca2+ transport through the paracellular process seems to be related to this behavior.47 Chirayath et al.47 reported that Ca2+ flux in the basolateral-to-apical direction was 14.3 ± 1.11 nmol/h/well. This flux was not so high, but it might be increased in the presence of the apical P(MAA-g-EG) because concentration gradient of Ca2+ across the cell monolayers is probably kept maximum by the chelation of Ca2+ with the P(MAA-g-EG). This may cause the Ca2+ depletion or decreased Ca2+ concentration in the basolateral side, leading to the significant decrease in TEER.
In the calcium-rich medium, the TEER-reducing effect of P(MAA-g-EG) became weaker, in contrast to that in the calcium-deficient media (Figs. 3 and 4). It has been demonstrated that the integrity of the tight junctions is dependent on the basolateral Ca2+ concentration. Therefore, the effect of chelating agents for Ca2+ on the integrity of the tight junctions would be related to their chelation capacity. The calcium binding capacity of P(MAA-g-EG) with MAA-to-EG molar ratio of 1:1 and EG repeating unit of 23 was 66.1 mg Ca2+/g polymer.12 The cumulative amount of Ca2+ to P(MAA-g-EG) supplied from the media throughout prewashing is ~300 mg Ca2+/g polymer. Assuming that the binding constant of Ca2+ to P(MAA-g-EG) in the present case is comparable to the previous case, the P(MAA-g-EG) hydrogels in calcium-rich media should be presaturated with Ca2+. Interestingly, however, the reduction in TEER was still statistically significant when compared with the control group.
For certain absorption enhancers, the TEER-reducing effect has been linked to epithelial damage. However, this is unlikely in the case of P(MAA-g-EG) because cytotoxicity tests show no toxicological damage of the P(MAA-g-EG) at the dose used in the TEER experiments (Figs. 1 and 2). It seems that another “unknown” mechanisms regulating the integrity of the junctional complex may contribute to the weak TEER-reducing effect observed in the calcium-rich media, though it cannot negate a possible effect by calcium-chelating as a consequence of our underestimation for the binding constant of Ca2+ to P(MAA-g-EG) in the present work.
At present, it is unclear that the weak TEER-reducing effect observed in the calcium-rich media can be ascribed to our underestimation for the binding constant of Ca2+ to P(MAA-g-EG) in the present work or to other yet unknown mechanisms contributing to regulation for the integrity of the junctional complex. Further investigations will have to clarify the predominant factors affecting this behavior.
Our results clearly demonstrate that insulin transport across the cell monolayers is also enhanced by the application of P(MAA-g-EG) hydrogels to the apical side in a calcium-concentration dependent manner (Figs. 5 and 6). In the calcium-deficient conditions, the Papp of insulin in the presence of P(MAA-g-EG) was 21 times higher than that in the absence of P(MAA-g-EG), whereas the significant enhancement of insulin transport by the apically applied P(MAA-g-EG) was not observed in the calcium-rich media (Table I). There may be two major pathways, i.e., transcellular and paracellular routes, for insulin transport through the Caco-2 cell monolayers. It has been suggested that insulin can be absorbed into the cells through insulin receptors present in the cellular membrane of Caco-2 cells.48 However, the activity of insulin-degrading enzyme (IED), a thiol metalloprotease degrading insulin in many insulin target cells and localized in the cytosol of Caco-2 cells, has to be minimized in order to improve transepithelial transport of insulin.49,50 Several chelating agents including EDTA and 1,10-phenanthroline were shown to strongly inhibit IDE activity and thus cytosolic insulin degradation.50 This inhibiting effect was not expected for the P(MAA-g-EG) because the absorption of P(MAA-g-EG) gels by the cells, which may be a requisite for the enzyme inhibitory effect, probably did not occur.
In contrast, paracellular transport of insulin is plausible in the Caco-2 cell monolayers as discussed in several reports.3,51 Indeed, the increase in insulin transport-enhancing effect observed in the calcium-deficient media was accompanied by a corresponding decrease in TEER, suggesting that the increased permeability in paracellular route is possibly responsible for this effect. Regulation of paracellular permeability has been shown to occur even by the addition of highly purified insulin to the basolateral side in certain cultured intestinal epithelial cell monolayers including Caco-2 cell line.52 In the case of Caco-2 cells, the TEER was shown to be significantly reduced when the monolayers were treated with 3 μg/mL purified bovine insulin.52 The insulin concentration in the basolateral compartment at 4 h was ~1 μg/mL (Fig. 6), which was three times lower than the required insulin concentration. In addition, the TEER-reducing effect by the insulin treatment required long-term (4 days) exposure to insulin,52 whereas the significant reduction of the TEER and the increased insulin-permeation were observed within 4 h in the present study. Therefore, it is reasonable to consider that the significantly enhanced transport of insulin shown in Figure 6 would be as a consequence of the application of P(MAA-g-EG) to the apical cell monolayers and thereby the loosening of the tight junctions of cell monolayers.
Incubation of the monolayers with P(MAA-g-EG) in the calcium-deficient condition resulted in a relatively high permeability of insulin in comparison to that without P(MAA-g-EG) (Table I). The estimated Papp value of insulin with P(MAA-g-EG) was 2.48 × 10−7 cm/sec. Even this Papp value seems to be low however and indeed much smaller compared with the Papp of insulin obtained with the everted rat small intestine from the literature (the corresponding Papp values for the duodenum, jejunum, and ileum were reported as 9.39 × 10−8, 9.08 × 10−7, and 7.01 × 10−7 cm/sec).53
At first glance, the implication of this comparison casts some doubt on the insulin transport-enhancing effect exerted by P(MAA-g-EG) in vivo. Several studies, however, suggested that transport studies of hydrophilic compounds across Caco-2 cells could potentially underestimate transport across small intestinal tissue. For example, Tanaka et al.54 demonstrated that the permeability of FITC-dextran with MW 4000 across Caco-2 monolayers was one order of magnitude lower than in rat jejunum and five times lower than in rat colon. An analogous tendency was observed in the present study, though the difference between the Papp value of insulin in the Caco-2 cells and that in the rat small intestine reached 1 to 2 orders of magnitude. From these consideration, the P(MAA-g-EG) can be expected to have a potential to demonstrate the insulin transport-enhancing effect even in vivo conditions.
CONCLUSIONS
The cytotoxic, electrophysiological, and insulin-transport enhancing effects of P(MAA-g-EG) hydrogels on intestinal epithelial cell monolayers were evaluated using the Caco-2 cell line. It was confirmed that P(MAA-g-EG) hydrogels possessed low cytotoxic effects independent of hydrogel composition, i.e., monomer molar ratio and the length of PEG graft chains. The application of P(MAA-g-EG) microparticles with 1:1 molar ratio of MAA/EG units and EG repeating units of 23 to the apical side of the cell monolayers gave rise to reduction of the cell monolayer integrity as evidenced by the changes in TEER values, indicating opening of the tight junctions. These effects were highly depended on the Ca2+ concentration in the culture media. The Ca2+-chelating capabilities of P(MAA-g-EG) hydrogels play an important role in determining the ability and effectiveness of the hydrogels to open the tight junctions, which regulates permeation through the paracellular transport pathway. As a result, the apical-to-basolateral transport of insulin across the cell monolayers was also enhanced by the apical application of P(MAA-g-EG) hydrogels in a Ca2+-concentration-dependent manner. In conclusion, the present study demonstrates that P(MAA-g-EG) hydrogel microparticles can be a cytocompatible carrier possessing the transport-enhancing effect of insulin in the intestinal epithelial cells.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: EB 00246
References
- 1.Saffran M, Sundesh KG, Savariar C, Burnham JC, Williams F, Neckers DC. A new approach to the oral administration of insulin and other peptide drugs. Science. 1986;233:1081–1084. doi: 10.1126/science.3526553. [DOI] [PubMed] [Google Scholar]
- 2.Damgé C, Michel C, Aprahamian M, Couvreur P. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes. 1988;37:246–251. doi: 10.2337/diab.37.2.246. [DOI] [PubMed] [Google Scholar]
- 3.Kotzé AF, de Leeuw BJ, Lueßen HL, de Boer AG, Verhoef JC, Junginger HE. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: in vitro evaluation in Caco-2 cell monolayers. Int J Pharm. 1997;159:243–253. [Google Scholar]
- 4.Kriwet B, Kissel T. Poly(acrylic acid) microparticles widen the intercellular spaces of Caco-2 cell monolayers: An examination by confocal laser scanning microscopy. Eur J Pharm Biopharm. 1996;42:233–240. [Google Scholar]
- 5.Lueßen HL, de Leeuw BJ, Pérard D, Lehr C-M, de Boer AG, Verhoef JC, Junginger HE. Mucoadhesive excipients in peroral peptide drug delivery. I. Influence of mucoadhesive excipients on the proteolytic activity of intestinal enzymes. Eur J Pharm Sci. 1996;4:117–128. [Google Scholar]
- 6.Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, Santos CA, Vijayaraghavan K, Montgomry S, Bassett M, Morrell C. Biologically erodible microspheres as potential oral drug delivery systems. Nature. 1997;386:410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 7.Sakuma S, Suzuki N, Kikuchi H, Hiwatari K, Arikawa K, Kishida A, Akashi M. Oral peptide delivery using nanoparticles composed of novel graft copolymers having hydrophobic backbone and hydrophilic branches. Int J Pharm. 1997;149:93–106. [Google Scholar]
- 8.Klier J, Scranton AB, Peppas NA. Self-associating networks of poly(methacrylic aid-g-ethylene glycol) Macromolecules. 1990;23:4944–4949. [Google Scholar]
- 9.Lowman AM, Peppas NA. Analysis of the complexation/de-complexation phenomena in graft copolymer networks. Macromolecules. 1997;30:4959–4965. [Google Scholar]
- 10.Lowman AM, Morishita M, Peppas NA, Nagai T. Novel bioadhesive complexation networks for oral protein drug delivery. In: McCulloch I, Shalaby SW, editors. Materials for controlled release applications. American Chemical Society; Washington, DC: 1998. pp. 156–164. [Google Scholar]
- 11.Peppas NA, Lowman AM. Protein delivery from novel bioadhesive complexation hydrogels. In: Frokjaer S, Christup L, Krogsgaard-Larsen P, editors. Protein and peptide drug research. Munksgaard; Copenhagen: 1998. pp. 206–216. [Google Scholar]
- 12.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20:1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 13.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 14.Nicklin PL, Irwin WJ, Hassan IF, Mackay M. Development of a minimum-calcium Caco-2 monolayer model: calcium and magnesium ions retard the transport of pamidronate. Int J Pharm. 1995;123:187–197. [Google Scholar]
- 15.Ma TY, Tran D, Hoa N, Nguyen D, Merryfield M, Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: Role of cytoskeletal involvment. Microscopy Res Tech. 2000;51:156–168. doi: 10.1002/1097-0029(20001015)51:2<156::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Collares-Buzato CB, McEwan GTA, Jepson MA, Simmons NL, Hirst BH. Paracellular barrier and junctional protein distribution depend on basolateral extracellular Ca2+ in cultured epithelia. Biochim Biophys Acta. 1994;1222:147–158. doi: 10.1016/0167-4889(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 17.Muranishi S. Absorption enhancers. CRC Crit Rev Ther Drug Carrier Syst. 1990;7:1–33. [PubMed] [Google Scholar]
- 18.Lee VHL, Yamamoto A, Kompella UB. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. CRC Crit Rev Ther Drug Carrier Syst. 1991;8:91–192. [PubMed] [Google Scholar]
- 19.Sakai M, Imai T, Ohtake H, Otagiri M. Cytotoxicity of absorption enhancers in Caco-2 cell monolayers. J Pharm Pharmacol. 1998;50:1101–1108. doi: 10.1111/j.2042-7158.1998.tb03319.x. [DOI] [PubMed] [Google Scholar]
- 20.Quan Y-S, Hattori K, Lundborg E, Fujita T, Murakami M, Muranishi S, Yamamoto A. Effectiveness and toxicity screening of various absorption enhancers using Caco-2 cell monolayers. Biol Pharm Bull. 1998;21:615–620. doi: 10.1248/bpb.21.615. [DOI] [PubMed] [Google Scholar]
- 21.Tomita M, Hayashi M, Awazu S. Absorption-enhancing mechanism of sodium caprate and decanoylcarnitine in Caco-2 cells. J Pharmcol Exp Ther. 1995;272:739–743. [PubMed] [Google Scholar]
- 22.Schipper NGM, Vårum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1. Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res. 1996;13:1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- 23.Park K, Shalaby WSW, Park H. Biodegradable hydrogels for drug delivery. Technomic Publishing Company, Inc.; Lancaster: PA: 1993. pp. 1–12. [Google Scholar]
- 24.Peppas NA, Ichikawa H, Torres-Lugo M. Cytotoxicity and transport enhancement of proteins through cell monolayers using novel pH-sensitive hydrogels. Proceedings: World Meeting APV/APGI. 2000;3:201–202. [Google Scholar]
- 25.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 26.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 27.Kim B, La Flamme K, Peppas NA. Dynamic swelling behavior of pH-sensitive anionic hydrogels used for protein delivery. J Appl Polym Sci. 2003;89:1606–1613. [Google Scholar]
- 28.López JE, Peppas NA. Cellular evaluation of insulin transmucosal delivery. Pharm Res. 2003 doi: 10.1163/156856204323005262. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 29.López JE, Peppas NA. Effect of PEG molecular weight and microparticle size on oral insulin delivery from P(MAA-g-EG) microparticles. Eur J Pharm Biopharm. 2003 doi: 10.1081/ddc-120037480. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 30.Foss AC, Peppas NA. Acrylic-based copolymers for oral insulin delivery systems. Polym Prepr. 2001;42(2):94–95. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 31.Peppas NA, Kim BS, Donini C, Sipahigil O, Leobandung W. Stimuli-sensitive polymers for oral and parenteral administration. In: Barratt G, Duchêne D, Fattal F, Legendre JY, editors. New trends in polymers for oral and parenteral administration: from design to receptors. Editions de Santé; Paris: 2001. pp. 32–41. [Google Scholar]
- 32.Garcia M, Torres-Lugo M, Alonso MJ, Peppas NA. Biointeractions of pH-sensitive poly(methacrylic acid-g-ethylene glycol) hydrogel microspheres with the caco-2 model cell line. In: Barratt G, Duchêne D, Fattal F, Legendre JY, editors. New trends in polymers for oral and parenteral administration: from design to receptors. Editions de Santé; Paris: 2001. pp. 386–389. [Google Scholar]
- 33.Morishita M, Lowman AM, Takayama K, Nagai T, Peppas NA. Elucidation of the mechanism of incorporation of insulin in controlled release systems based on complexation polymers. J Control Release. 2002;81:25–32. doi: 10.1016/s0168-3659(02)00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres-Lugo M, Garcia M, Record R, Peppas NA. Physicochemical behavior and cytotoxic effects of P(MAA-g-EG) nanospheres for oral delivery of proteins. J Control Release. 2002;80:197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 35.Torres-Lugo M, García M, Record R, Peppas NA. pH-sensitive hydrogels as gastrointestinal tract absorption enhancers: transport mechanisms of salmon calcitonin and other model molecules using the caco-2 cell model. Biotechnol Prog. 2002;18:612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichikawa H, Peppas NA. Synthesis and characterization of pH-responsive nanosized hydrogels of poly(methacrylic acid-g-ethylene glycol) for oral peptide delivery. In: Barratt G, Duchêne D, Fattal F, Legendre JY, editors. New trends in polymers for oral and parenteral administration: from design to receptors. Editions de Santé; Paris: 2001. pp. 261–264. [Google Scholar]
- 37.Goto T, Morishita M, Foss A, Peppas NA, Lowman AM, Takayarma K. In vivo studies of oral insulin delivery systems based on complexation hydrogels; Proceedings Control Rel Society; 2002.p. 29. [Google Scholar]
- 38.Robinson DN, Peppas NA. Preparation and characterization of pH-responsive poly(methacrylic acid-g-poly(ethylene glycol) nanospheres. Macromolecules. 2002;35:3668–3674. [Google Scholar]
- 39.Torres-Lugo M, Peppas NA. Preparation and characterization of P(MAA-g-EG) nanospheres for protein delivery applications. J Nanoparticle Res. 2002;4:73–81. [Google Scholar]
- 40.Sipahigil O, Gürsoy A, Cakalagaoglu F, Peppas NA. Histopathological evaluation of drug loaded P(MAA-g-EG) hydrogels. Proceed Int Pharm Technol Symp. 2002;11:71–72. [Google Scholar]
- 41.Donini C, Robinson DN, Colombo P, Giordano F, Peppas NA. Preparation of P(MAA-g-EG) nanospheres for pharmaceutical applications. Int J Pharmacol. 2002;245:83–91. doi: 10.1016/s0378-5173(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 42.Sipahigil O, Torres-Lugo M, Peppas NA. FTIR spectroscopic analysis of protein/carrier Interactions in novel protein delivery systems. STP Pharma. 2002;12:345–350. [Google Scholar]
- 43.Peppas NA, Argade A, Bhargava S. Preparation and properties of poly(ethylene oxide) star polymers. J Appl Polym Sci. 2003;87:322–327. [Google Scholar]
- 44.Leobandung W, Ichikawa H, Fukumori Y, Peppas NA. Monodisperse nanoparticles of poly(ethylene glycol) macromers and N-isopropyl acrylamide for biomedical applications. J Appl Polym Sci. 2003;87:1678–1684. [Google Scholar]
- 45.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with caco-2 cultures. J Biomed Mater Res. 2003 doi: 10.1016/j.ejpb.2004.02.008. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 46.Kim B, Peppas NA. Analysis of molecular interactions in P(MAA-g-EG) hydrogels. Polymer. 2003;44:3701–3707. [Google Scholar]
- 47.Chirayath MV, Gajdzik L, Hulla W, Graf J, Cross HS, Peterlik M. Vitamin D increases tight-junction conductance and paracellular Ca2+ transport in Caco-2 cell cultures. Am J Physiol. 1998;274:G389–G396. doi: 10.1152/ajpgi.1998.274.2.G389. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald RS, Thornton WH, Jr, Bean TL. Insulin and IGF-1 receptors in a human intestinal adenocarcinoma cell line (Caco-2): Regulation of Na+ glucose transport across the brush-border. J Receptor Res. 1993;13:1093–1113. doi: 10.3109/10799899309063266. [DOI] [PubMed] [Google Scholar]
- 49.Bai JPF, Chang LL. Transepithelial transport of insulin: 1. Insulin degradation by insulin-degrading enzyme in small intestinal epithelium. Pharm Res. 1995;12:1171–1175. doi: 10.1023/a:1016263926946. [DOI] [PubMed] [Google Scholar]
- 50.Bai JPF, Hsu MJP, Shier WT. Insulin-degrading enzyme in a human colon adenocarcinoma cell line (Caco-2) Pharm Res. 1995;12:513–517. doi: 10.1023/a:1016241610649. [DOI] [PubMed] [Google Scholar]
- 51.Björk E, Isaksson U, Edman P, Artursson P. Starch microspheres induce pulsatile delivery of drugs and pepties across the epithelial barrier by reversible separation of the tight junctions. J Drug Target. 1995;2:501–507. doi: 10.3109/10611869509015920. [DOI] [PubMed] [Google Scholar]
- 52.McRoberts JA, Aranda R, Riley N, Kang H. Insulin regulates the paracellular permeability of cultured intestinal epithelial cell monolayers. J Clin Invest. 1990;85:1127–1134. doi: 10.1172/JCI114544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schilling RJ, Mitra AK. Intestinal mucosal transport of insulin. Int J Pharm. 1990;62:53–64. [Google Scholar]
- 54.Tanaka Y, Taki T, Sakame T, Nadai T, Sezaki H, Yamashita S. Characterization of drug transport through tight-junctional pathway in Caco-2 monolayer: Comparison with isolated rat jejunum and colon. Pharm Res. 1995;12:523–528. doi: 10.1023/a:1016245711557. [DOI] [PubMed] [Google Scholar]