Abstract
The purpose of this critical review is to explore the controversy regarding the relationship between radiation dose to the neural progenitor cell (NPC) niches and patient outcomes, in terms of both toxicity and tumor control. NPCs in the subventricular zone (SVZ) and hippocampus are paradoxically associated with long-term neurocognitive sequelae of brain irradiation, as well as resistance to therapy and tumor recurrence. The reconciliation of these somewhat opposing functions is challenging. Current literature suggests that radiation and other treatments against the NPC in the hippocampus and the SVZ may influence patient outcome. As a result, both the SVZ and the hippocampus could have important implications on radiation treatment planning strategies, and future laboratory and clinical evaluations will be critical in designing studies to optimize treatment outcome, effectiveness, and safety.
Introduction
Radiation treatment is important in the management of many pediatric and adult brain tumors. However, radiation to the brain is associated with neurocognitive toxicity.1–6 Recently, controversy has developed regarding the relationship between radiation dose to the neural progenitor cell (NPC) niches and patient outcome, in terms of both toxicity and tumor control.7
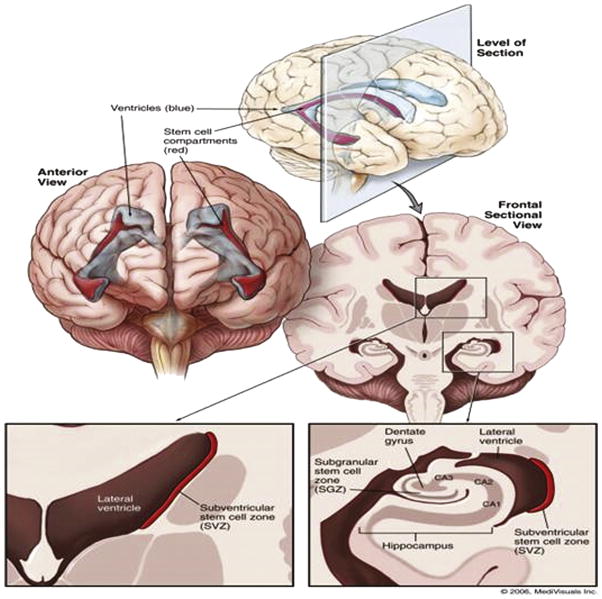
A NPC is a multipotent stem cell with the capacity to differentiate into new neurons and glia.8 Within the mammalian brain, these NPCs are known to reside in 2 areas, the subventricular zones (SVZ) and the subgranular zones (SGZs) (Fig. 1).9 Both are considered the germinal regions of the adult human brain. The SVZ is the largest germinal region in the adult mammalian brain and is located in the lining of the lateral ventricles, whereas the SGZ is located within the dentate gyrus of the hippocampus.10
Figure 1.
Germinal regions of the adult human brain. The subventricular zone (SVZ) is the largest germinal region in the adult mammalian brain, located in the lateral wall of the lateral ventricle. The subgranular zone (SGZ) is located within the dentate gyrus of the hippocampus. The CA1, CA2, and CA3 represent Cornu Annulis fields of hippocampus proper and, along with dentate gyrus, constitute the hippocampal formation, the primary memory center in the brain. (Color version of figure is available online.)
© 2006 MediVisuals Inc. Reprinted with permission from Barani IJ, Benedict SH, Lin PS, “Neural stem cells: implications for the conventional radiotherapy of central nervous system malignancies,” Int J Radiat Oncol Biol Phys 68(2):324-333, 2007.
Although the functional role of NPCs in humans has not been fully defined, animal studies demonstrate that NPCs in the SVZ and the hippocampus are capable of self-renewal, injury repair, and tumor inhibition.11–15 It is hypothesized that injury to NPCs during radiation to the brain may contribute to the long-term sequelae of radiation therapy (RT), most notably in terms of neuropsychological toxicity. Yet, paradoxically, emerging evidence suggests that NPCs may also contribute to cancer recurrence and glioma resistance to chemotherapy and RT.16–19 The purpose of this critical review is to summarize the current literature and explore the controversy regarding RT to the SVZ and hippocampus.
Radiation Sparing of the NPC Niches May Preserve Neurocognitive Functions
Radiation to the brain is associated with neurocognitive toxicity and reduced performance on neuropsychological testing, especially in children (Fig. 2).1–6,20,21 Although the cause of radiation-induced damage to the brain is likely multifactorial, there is a growing body of evidence that suggests that injury to the NPCs may play a role. In this section of the article, we discuss the roles of NPCs and their effects on neurocognitive performances. We also summarize the current literature and discuss how NPC-sparing radiation protocols in both the hippocampus and the SVZ may affect patient outcomes.
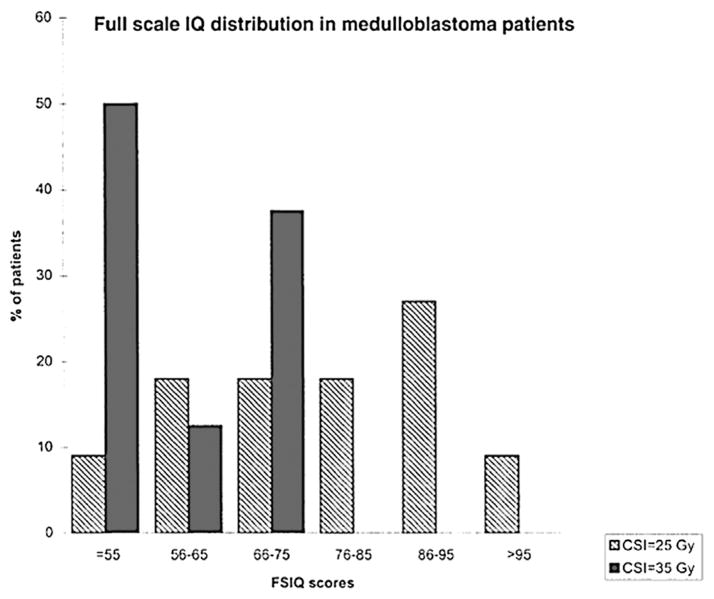
Figure 2.
Full-scale IQ distribution in patients with medulloblastoma from Grill et al.57 Long-term neurocognitive deficits are observed with radiation therapies in a dose-dependent manner. In this study, patients treated with 35 Gy of craniospinal (CSI) radiation demonstrated significantly poorer performance on the full-scale IQ testing than patients treated with 25 Gy of CSI. Number of patients in group CSI = 25 Gy is 11. Number of patients in group CSI = 35 Gy is 8. FSIQ, full-scale IQ.
NPCs are capable of injury repair in both the SVZ of the lateral ventricles and the SGZ of the hippocampus. Previous studies have demonstrated how NPCs migrate to the site of inflammation and replace endogenous cells following cortical injury, stroke, and epilepsy,13,14 that is, NPCs are capable of neuronal renewal and regeneration. Understandingly, radiation-induced damage to these progenitor cells could affect neurocognitive function.22–28 Moreover, radiation doses to the distinct NPC niches seem to have different levels of effect, and previous studies have reported an especially strong association between radiation to the hippocampus and neurocognitive toxicity. For example, animal studies have shown that radiation to the brain significantly reduces the formation of new cells in the hippocampus and is associated with decreased performance of hippocampal-related tasks.23,24,29,30
Similarly, clinical studies have demonstrated that radiation to the hippocampus is associated with neurocognitive deficits.31–37 NPCs are also found in humans, and these germinal areas including the SVZ and the hippocampus have also been associated with the formation of new neurons.8,38 Several studies have linked radiation dose to brain regions, such as the hippocampus to the development of neurocognitive deficits in children.31,36,37 Furthermore, prospective data from a pediatric study suggest a significant association between the mean radiation dose to the hippocampus and temporal lobe and decline in neurocognitive performance (Fig. 3).31
Figure 3.
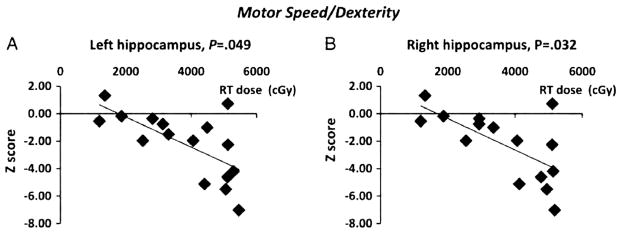
Performance on neuropsychological testing is worse with increasing radiation dose to the hippocampus from Redmond et al.31 The performance on the Purdue Pegboard both hands test (Z-scores), a measure of motor dexterity and speed, at 6 months following completion of RT relative to (A) mean left hippocampal radiation dose, P = 0.049, and (B) mean right hippocampal radiation dose, P = 0.032 is shown. Standardized scores were used in this analysis to account for the effect of age on test performance.
Although studies have shown an association between the RT dose to the hippocampus and neurocognitive function, the relationship between RT dose to the SVZ and neurocognitive function is more controversial.31,39,40 Reductions in neurocognitive performance have been documented following administration of intrathecal chemotherapy in children with leukemia.32 Given that the drug penetrates only a thin layer adjacent to the ventricular system, this suggests that periventricular cells may play an important role in neurocognitive function. However, improved neurocognitive performance in patients with central nervous system germ cell tumors who were treated with whole-ventricle radiation when compared with craniospinal radiation has been reported,40 suggesting that perhaps the cells lining the ventricle may not be as vital as other areas in the brain in neurocognitive sequelae of RT. In addition, only 1 animal study to date has documented a relationship between RT dose to the SVZ and performance on neuropsychological testing,41 and no human studies have confirmed this relationship.
Given the potential relationship between radiation injury to the NPC and neurocognitive deficits, ongoing efforts are evaluating the possibility of hippocampal-sparing RT techniques. Several studies have suggested that sparing of the NPC in the hippocampus and SVZ is possible using modern treatment planning techniques (Fig. 4).10,42–45 In addition, an animal study suggested that these techniques effectively spare the NPCs (Fig. 5).43
Figure 4.
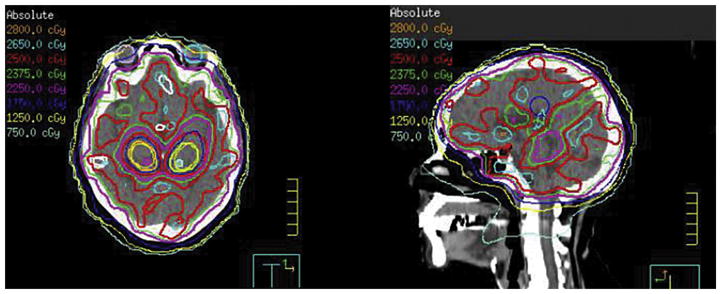
An example hippocampal-sparing prophylactic cranial irradiation (PCI) treatment plan. The prescription dose is 25 Gy in 10 fractions. The mean dose to the hippocampal avoidance region is <8 Gy with >90% of prescription dose covering >90% of whole brain.
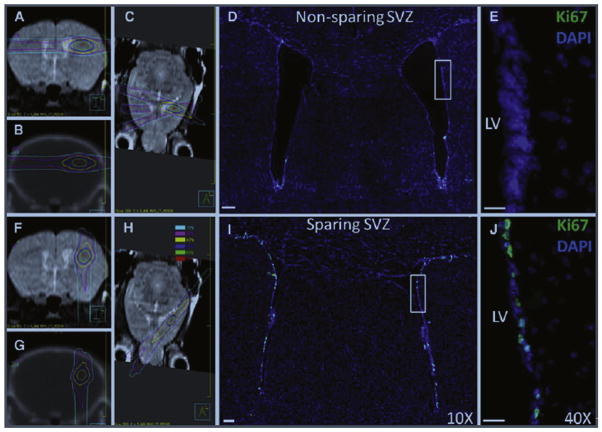
Figure 5.
Mouse radiation treatment plans (left) and microscopy images (right) for the non–NPC-sparing (top) and NPC-sparing (bottom) radiotherapy plans from Redmond et al.43 Left side MR and CT images from the mouse radiation treatment plans showing the radiation dose distribution for the non–NPC-sparing (A–C) and NPC-sparing radiation treatment plans (F–H). It can be noted that for the non–NPC-sparing plan, the region of the SVZ of the ipsilateral lateral ventricle receives a high radiation dose, whereas this region is effectively spared in the NPC-sparing plan. Scans taken are as follows: coronal MRI (A and F), coronal CT (B and G), and axial MRI (C and H). Dose values are shown in the legend. Right side coronal sections showing Ki-67 stains (green) in the SVZ of the lateral ventricles following non–NPC-sparing RT (D and E) and NPC-sparing RT (I and J). Ki-67 is a marker of cellular proliferation and is used in this model as a potential indicator of NPCs. Costaining is performed using DAPI (blue). Images (D and I) were taken with a 109 objective lens and the images (E and J) with 409 objective lens. CT, computed tomography; DAPI, 4′,6-diamidino-2-phenylindole; MRI, magnetic resonance imaging; LV, left ventricle.
Preliminary results from Radiation Therapy Oncology Group (RTOG) 0933 suggest that hippocampal sparing may be associated with reduced short-term memory deficits in patients with brain metastases compared with a historical control treated with conventional whole-brain RT on an earlier RTOG study.42 Specifically, patients treated with hippocampal-sparing whole-brain RT demonstrated a 7% memory loss, compared with 30% in the historical control. Quality-of-life assessments from the same study also demonstrated more favorable results for patients treated with hippocampal-sparing RT when compared with the historical control.42
Given the optimistic phase II data, the concept of hippocampal-sparing RT merits further investigation. An ideal population to further evaluate this hypothesis is in patients with small cell lung cancer (SCLC) who receive prophylactic cranial irradiation, as these patients do not have an intra-parenchymal mass to confound the results, and the chemotherapy that they receive is relatively homogeneous. There is an ongoing single-arm phase II study in patients with limited-stage SCLC that is currently enrolling patients at our institution. The eligibility criteria are based on RTOG 0212, which will be used as a historical control. An intergroup study is currently under development to more definitively evaluate the concept in patients with limited- and extensive-stage SCLC in which patients will be randomized to either hippocampal-sparing prophylactic cranial irradiation or conventional whole-brain radiation.
The Complication: Radiation to NPC Niches May Affect Outcomes in Certain Tumor Types
Although the aforementioned data suggest that limiting RT dose to the NPC regions might be beneficial in reducing neurocognitive sequelae of brain RT, the issue may not be as straightforward as it initially appears. Although NPCs are associated with injury repair, there is also evidence that they may be involved with tumor control as well as tumor recurrence and cancer radioresistant behaviors in certain tumor types. Interestingly, there are data to support both a positive and negative relationship in this setting.
For example, in vitro data from brain tumors suggest that NPCs may transform into cancer stem cells through a series of mutations in the oncogenes and tumor-suppressor genes and that this ultimately may allow them to have protumor behavior.16–18 Oncogenes in NPCs of the SVZ can be activated to induce proliferation, survival, and migration in mice, leading to the formation of gliomas.22,23 Furthermore, NPCs share many properties with cancer stem cells, including their ability to migrate in brain.40 Glioma cells have been shown to release factors that actively recruit nearby NPCs and induce their malignant transformation into cancer stem cells.46
Previous studies have also suggested that radiation might encourage the induction of NPCs into cancer stem cells and that the consequent malignant transformation of NPCs may potentially contribute to glioblastoma resistance to chemotherapy and RT. Irradiated cancer cells showed enrichment in CD133-expressing cells, a marker that is specific for both NPCs and cancer stem cells. These CD133+ cells survive radiation much more effectively by activating DNA damage repair mechanisms.47 It has been postulated that CD133+ cancer stem cells become radio-resistant by promoting the translocation of L1CAM intracellular domains from the cytoplasm into the nucleus, which activates checkpoint responses and subsequent DNA damage repair.47,48 Overall, these studies provide evidence that CD133+ cancer cells survive radiation more effectively and contribute to tumor growth through preferential activation of the DNA repair mechanisms. We can hypothesize from these studies that it is possible for CD133+ NPCs to transform into CD133+ cancer stem cells, especially as radiation specifically to the murine SVZ and the hippocampus has been linked to the formation of tumor satellites, as well as an increased migration of NPCs to the tumor core and to the satellite sites.49
Clinical data also support the hypothesis that NPCs contribute to local recurrences or treatment resistance or both. Retrospective human studies have suggested worse patient outcomes with glioblastoma located immediately adjacent to lateral ventricles.19,47,50–53 The explanation for this finding is unclear, but emerging data suggest a potential relationship between radiation dose to the SVZ and patient outcome.9,52–54 In a recent study, Chen et al9 retrospectively examined adult patients with GBM and reported improved progression-free survival (PFS) and overall survival (OS) in the subgroup of patients who underwent gross total resection, when they received an ipsilateral SVZ dose of ≥40 Gy compared with the same subgroup who received an ipsilateral SVZ dose <40 Gy (Fig. 6). Similarly, Lee et al54 retrospectively studied adult patients with GBM and reported improvements in both PFS and OS among the patients who received high ipsilateral SVZ doses, more than 59.4 Gy. These results are important and suggest that higher radiation dose to the SVZ may be beneficial to patient outcomes. Furthermore, these studies suggest that acute toxicity of delivery of a high radiation dose to the SVZ may be acceptable as there was no statistically significant difference in the Karnofsky Performance Status for patients receiving low dose to SVZ (<40 Gy) vs high dose (>60 Gy) to the SVZ.9,54
Figure 6.
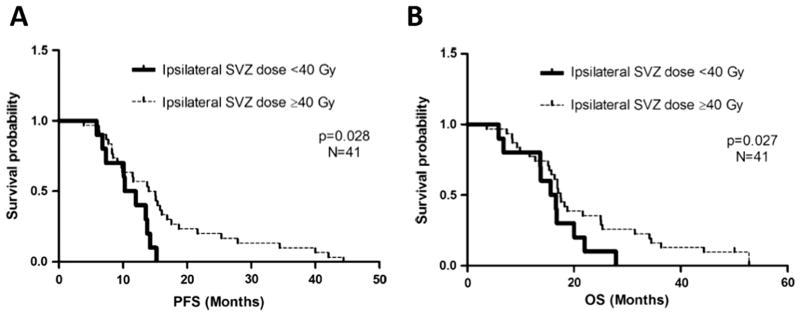
Progression-free survival (PFS) and overall survival (OS) improve in the subgroup of patients that underwent gross total resection and received increased dose to SVZ in Chen et al.9 (A) PFS by ipsilateral subventricular dose in gross total resection patients (n = 41). PFS in patients whose ipsilateral subventricular zone (SVZ) received less than 40 Gy was significantly different from that in those who received a dose of 40 Gy or greater as measured by median PFS of 10.3 vs 15.1 months (95% CI: 7.4–13.2 months) and log-rank test (PZ.023), as well as adjusted hazard ratio for PFS (2.60) (PZ.028). (B) Overall survival (OS) by ipsilateral subventricular dose in gross total resection patients (n = 41). OS in patients whose ipsilateral subventricular zone (SVZ) received less than 40 Gy was significantly different from those who received a dose of 40 Gy or greater as measured by median overall survival of 15.6 vs 17.5 months (95% CI: 11.3–19.9 months) and adjusted hazard ratio for progression-free survival (2.60) (PZ.027).
Nevertheless, although the aforementioned retrospective series are thought provoking and hypothesis generating, they could be confounded. For example, outcomes could be affected by differences in upfront bevacizumab administration or the salvage therapies used at the time of progression. Similarly, in these retrospective series, the dose received by the SVZ was likely directly affected by tumor location and geometry as tumors that reside close to the SVZ likely received higher SVZ radiation dose. Furthermore, methylation status of the O6-methylguanine-methyltransferase (MGMT) promoter was not accounted for in these retrospective series but could affect results.9,54 As a result, prospective evaluation of the relationship between radiation dose to the SVZ and outcomes in patients with brain cancer will be critical to better understanding the relationship.
To address this question, a prospective single-arm phase II study in patients undergoing RT plus temozolomide chemotherapy for newly diagnosed GBM at our institution limited the radiation dose to the NPC niches as much as possible without compromising coverage of the tumor. The primary end point is local recurrence in the spared NPC niches. This study recently closed to accrual, with results forthcoming.54 Another study open to accrual at our institution in the same patient population will examine potential benefits in tumor outcomes with the delivery of higher doses of RT to the SVZ. Other interesting possibilities for future investigation might include therapeutic agents that target the SVZ. For example, recent findings have attributed dopamine signaling mechanisms of the D3 subtype receptor to the migratory behavior of NPCs toward the tumor site and have suggested that D3 blocking drugs to the SVZ could also prolong patient survival.55
How to Reconcile the Opposing Viewpoints?
This review has explored the potential role of NPCs in the SVZ and hippocampus in both the long-term neurocognitive sequelae of brain irradiation and in resistance to therapy and tumor recurrence. The reconciliation of these somewhat opposing functions is challenging and is a critical avenue for future laboratory studies and prospective clinical trials. One possibility is that the role of the NPCs varies with both tumor pathology and location in the brain. For example, given the vast heterogeneity of cancers, it is likely that the role of the NPCs varies dramatically between different pathologies as well as within the same tumor type and even the same tumor. In addition, it is possible that NPCs in the SVZ may play a different role than NPCs in the hippocampus, and perhaps they should be given different considerations during radiation treatment planning.
An improved understanding of this complex relationship will be critical to allowing us to apply it effectively in the clinic. Importantly, to date we remain uncertain of the precise radiation tolerance of NPC and cancer stem cells to fractionated RT. This detail is critical both in designing future studies to spare the NPC and in designing trials to intentionally deliver radiation dose to these areas in certain types of tumors.
Similarly, although we hypothesize that NPCs may transform into cancer stem cells and are associated with tumor recurrence and cancer radioresistant behaviors, there are directly contradictory animal studies as well. For example, NPCs have been shown to migrate to the site of tumors with greater NPC attraction being associated with decreased tumor size and improved OS.11,12,15,56 This paradox once again highlights the need for additional studies, both in the laboratory and in the clinic, to more fully address this fascinating and seemingly vital question.
In summary, the relationship between NPC in the hippocampus and SVZ remains an interesting academic question and one that likely will have important implications for radiation treatment planning strategies in the future. However, additional laboratory and clinical studies will be critical in allowing us to explore this topic further and ultimately apply it to our patients with maximum effectiveness and safety.
Footnotes
This work is completed at Johns Hopkins School of Medicine.
The authors declare no conflicts of interest.
References
- 1.Horská A, LaClair A, Mohamed M, et al. Low cerebellar vermis volumes and impaired neuropsychologic performance in children treated for brain tumors and leukemia. Am J Neuroradiol. 2010;31:1430–1437. doi: 10.3174/ajnr.A2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis LM, Delattre J-Y, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 4.Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Physi. 2008;72:1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Seiferheld W, Schultz C, et al. Secondary analysis of Radiation Therapy Oncology Group study (RTOG) 9310: An intergroup phase II combined modality treatment of primary central nervous system lymphoma. J Neurooncol. 2005;74:201–205. doi: 10.1007/s11060-004-6596-9. [DOI] [PubMed] [Google Scholar]
- 6.Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs IC, Haas-Kogan D, Terezakis S, et al. The subventricular zone neural progenitor cell hypothesis in glioblastoma: Epiphany, Trojan Horse, or Cheshire fact? Int J Radiat Oncol Biol Phys. 2013;86:606–608. doi: 10.1016/j.ijrobp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013;86:616–622. doi: 10.1016/j.ijrobp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barani IJ, Cuttino LW, Benedict SH, et al. Neural stem cell-preserving external-beam radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2007;68:978–985. doi: 10.1016/j.ijrobp.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 11.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staflin K, Lindvall M, Zuchner T, et al. Instructive cross-talk between neural progenitor cells and gliomas. J Neurosc Res. 2007;85:2147–2159. doi: 10.1002/jnr.21344. [DOI] [PubMed] [Google Scholar]
- 13.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 14.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Jeon JY, An JH, Kim SU, et al. Migration of human neural stem cells toward an intracranial glioma. Exp Mol Med. 2008;40:84–91. doi: 10.3858/emm.2008.40.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lantos P, Cox D. The origin of experimental brain tumours: A sequential study. Experientia. 1976;32:1467–1468. doi: 10.1007/BF01937439. [DOI] [PubMed] [Google Scholar]
- 17.Lantos P, Pilkington G. The development of experimental brain tumours a sequential light and electron microscope study of the subependymal plate. Acta Neuropathol. 1979;45:167–175. doi: 10.1007/BF00702668. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Ayuso-Sacido A, Moliterno JA, Kratovac S, et al. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neurooncol. 2010;97:323–337. doi: 10.1007/s11060-009-0035-x. [DOI] [PubMed] [Google Scholar]
- 20.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 21.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 22.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin North Am. 2007;18:115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Winocur G, Wojtowicz JM, Sekeres M, et al. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 25.Tada E, Parent J, Lowenstein D, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 26.Tada E, Yang C, Gobbel GT, et al. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol. 1999;160:66–77. doi: 10.1006/exnr.1999.7172. [DOI] [PubMed] [Google Scholar]
- 27.Mizumatsu S, Monje ML, Morhardt DR, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 28.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 29.Madsen TM, Kristjansen P, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 30.Saxe MD, Battaglia F, Wang J-W, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redmond KJ, Mahone EM, Terezakis S, et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: A prospective study. Neurooncology. 2013;15:360–369. doi: 10.1093/neuonc/nos303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iuvone L, Mariotti P, Colosimo C, et al. Long-term cognitive outcome, brain computed tomography scan, and magnetic resonance imaging in children cured for acute lymphoblastic leukemia. Cancer. 2002;95:2562–2570. doi: 10.1002/cncr.10999. [DOI] [PubMed] [Google Scholar]
- 33.Lesnik PG, Ciesielski KT, Hart BL, et al. Evidence for cerebellar-frontal subsystem changes in children treated with intrathecal chemotherapy for leukemia: Enhanced data analysis using an effect size model. Arch Neurol. 1998;55:1561. doi: 10.1001/archneur.55.12.1561. [DOI] [PubMed] [Google Scholar]
- 34.Waber DP, Tarbell NJ, Kahn CM, et al. The relationship of sex and treatment modality to neuropsychologic outcome in childhood acute lymphoblastic leukemia. J Clin Oncol. 1992;10:810–817. doi: 10.1200/JCO.1992.10.5.810. [DOI] [PubMed] [Google Scholar]
- 35.Bakke SJ, Fossen A, Storm-Mathiesen I, et al. Long-term cerebral effects of CNS chemotherapy in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1993;10:267–270. doi: 10.3109/08880019309029495. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neurooncology. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalali R, Mallick I, Dutta D, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:974–979. doi: 10.1016/j.ijrobp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Sanai N, Tramontin AD, Quiñones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 39.Khatua S, Dhall G, O’Neil S, et al. Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr Blood Cancer. 2010;55:42–46. doi: 10.1002/pbc.22468. [DOI] [PubMed] [Google Scholar]
- 40.Mabbott DJ, Monsalves E, Spiegler BJ, et al. Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 2011;117:5402–5411. doi: 10.1002/cncr.26127. [DOI] [PubMed] [Google Scholar]
- 41.Lazarini F, Mouthon M-A, Gheusi G, et al. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gondi V, Mehta MP, Pugh S, et al. Memory preservation with conformal avoidance of the hippocampus during whole-brain radiotherapy for patients with brain metastases: Primary endpoint results of RTOG 0933. ASTRO. 2013 [Google Scholar]
- 43.Redmond KJ, Achanta P, Grossman SA, et al. A radiotherapy technique to limit dose to neural progenitor cell niches without compromising tumor coverage. J Neurooncol. 2011;104:579–587. doi: 10.1007/s11060-011-0530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator–based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutiérrez AN, Westerly DC, Tomé WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: A planning study. Int J Radiat Oncol Biol Phys. 2007;69:589–597. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fomchenko EI, Holland EC. Platelet-derived growth factor–mediated gliomagenesis and brain tumor recruitment. Neurosurg Clin North Am. 2007;18:39–58. doi: 10.1016/j.nec.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 48.Cheng L, Wu Q, Huang Z, et al. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011;30:800–813. doi: 10.1038/emboj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabatabai G, Frank B, Möhle R, et al. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-β-dependent HIF-1α-mediated induction of CXCL12. Brain. 2006;129:2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 50.Chaichana KL, McGirt MJ, Frazier J, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 51.Jafri NF, Clarke JL, Weinberg V, et al. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neurooncology. 2013;15:91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evers P, Lee P, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta T, Nair V, Paul SN, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109:195–203. doi: 10.1007/s11060-012-0887-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee P, Eppinga W, Lagerwaard F, et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: A pooled analysis. Int J Radiat Oncol Biol Phys. 2013;86:609–615. doi: 10.1016/j.ijrobp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Kast R, Ellingson B, Marosi C, et al. Glioblastoma treatment using perphenazine to block the subventricular zone’s tumor trophic functions. J Neurooncol. 2014;116:207–212. doi: 10.1007/s11060-013-1308-y. [DOI] [PubMed] [Google Scholar]
- 56.Glass R, Synowitz M, Kronenberg G, et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]