Abstract
Objective
This paper provides an overview of research on the neurobiological correlates of childhood adversity and a selective review of treatment implications.
Method
Findings from a broad array of human and animal studies of early adversity were reviewed.
Results
Topics reviewed include neuroendocrine, neurotrophic, neuroimaging, and cognitive effects of adversity, as well as genetic and epigenetic influences. Effects of early life stress on treatment outcome are considered, and development of treatments designed to address the neurobiological abnormalities is discussed.
Conclusion
Early adversity is associated with abnormalities of several neurobiological systems that are implicated in the development of psychopathology and other medical conditions. Early life stress negatively impacts treatment outcome and individuals may require treatments that are specific to this condition.
Keywords: Early life stress, childhood abuse, neurobiology, treatment
Introduction
Childhood maltreatment, a well-known risk factor for psychopathology, including major depressive disorder (MDD) and anxiety disorders, is increasingly understood to be an environmental exposure that acts much like a toxin, with the potential to influence several neurobiological systems implicated in the pathophysiology of these disorders. Childhood adversity, such as abuse, neglect, parental loss, and other stressful experiences, has been estimated to account for 45% of the variance in childhood-onset psychopathology and 26-32% of the risk for later-onset psychiatric disorders (1). The consequences of stress exposures occurring in early development are especially profound because 1) young children are entirely dependent on caregivers for their basic physical, social and emotional needs, and 2) this period involves rapid and large-scale developmental changes in neural pathways that regulate emotion and behavior (2, 3). Work with rodents and non-human primates demonstrates that early caregiving behaviors play a critical role in the normal development of brain circuits involved in the regulation of stress reactivity, learning and memory, neuroplasticity, and behavior (4). Early adversity in humans also results in long-lasting alterations in these neurobiological, behavioral, and social systems (5).
Investigations of early stress in humans are complicated by the fact that several important factors can modify the effects of stress exposure, including the type, frequency, and nature of the stress, the co-occurrence of multiple stressors, and the developmental period when the stress occurs; these are difficult to measure in humans. Adverse experiences can have heritable influences, such as abuse due to parental psychopathology, so that genetic and environmental contributions to the correlates of early life stress can be difficult to disentangle. Finally, the assessment of neurobiological influences involves indirect measures of peripheral markers or neuroimaging techniques which may not adequately reflect neuronal signaling in relevant brain circuits thought to underlie risk for psychopathology. In contrast, animal models do allow causal inferences because they involve selected stressors, control of potentially confounding variables, and direct examination of behavior and brain tissue. However, the utility of these models depends upon the degree to which they parallel human processes.
Aims of the study
Here we provide a selective review of the burgeoning literature on the neurobiological correlates of early-life stress in humans, with inclusion of important relevant findings from animal models. Where available, we identify studies that may hold particular promise for influencing treatment development and clinical decision-making, particularly in relation to risk for depressive and anxiety disorders.
Material and methods
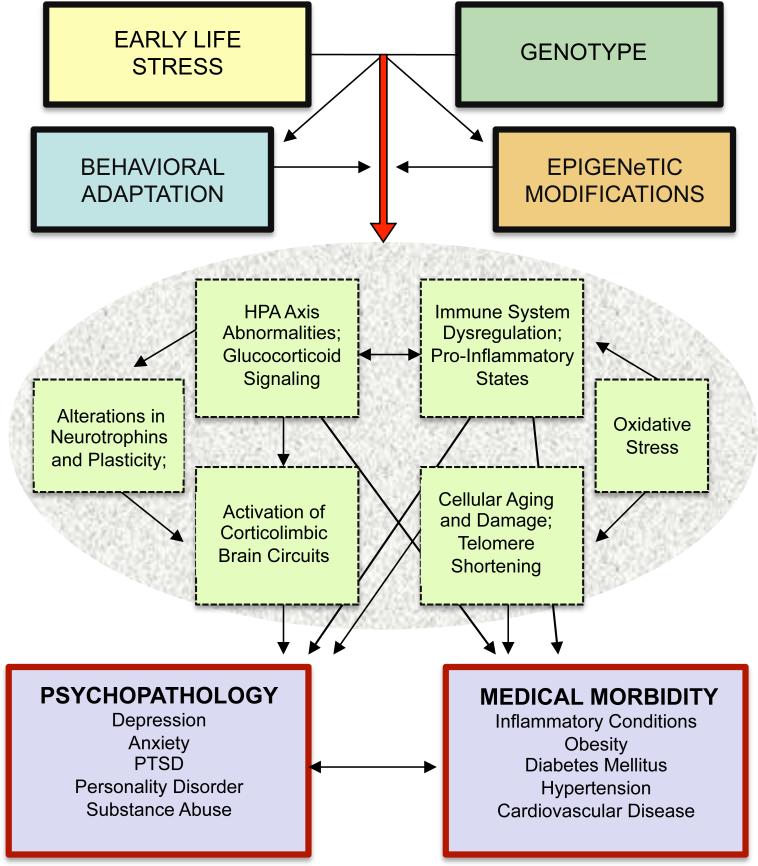
Findings from a broad array of human and animal studies of early adversity are reviewed. Topics include neuroendocrine, neurotrophic, neuroimaging, and cognitive effects of adversity, as well as genetic and epigenetic influences. Studies examining effects of early life stress on treatment outcome are reviewed, and investigations of treatments designed to address the neurobiological abnormalities associated with stress exposure are discussed. The Figure provides a model depicting the mediators and moderators of the neurobiological correlates of childhood adversity reviewed in this paper.
Figure 1.
Model: Neurobiological effects of childhood adversity. Childhood adversity engenders risk for psychopathology and medical morbidity through a number of inter-related pathways. Genetic sensitivity to environmental factors is an important determinant of risk, and epigenetic effects of early adversity influence the biological impact of stress exposure. Behavioral adaptation may exaccerbate or ameliorate these effects. Ensuing alterations of neuroendocrine, neuroimmune, and neurotrophin pathways are thought to be mechanisms of the development of psychiatric disorders and related medical conditions.
Results
Neuroendocrine Response to Stress
Stress exposure activates the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system, which play a major role in coordinating the neural and behavioral response to stressors. Following stress exposure, corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) are released from the hypothalamus. CRH serves as a neurotransmitter and modulates sympathetic arousal; it also acts in concert with AVP to stimulate secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH stimulates the adrenal cortex to synthesize and release glucocorticoids; cortisol is the most abundant and potent glucocorticoid in humans. In addition, the sympathetic nervous system secretes catecholamines. In the context of normative acute stress experiences, this activation results in the alteration of several physiological systems to allow adaptive responses to stressors. Appetite, gastrointestinal activity, sexual function, and fuel storage are inhibited in order to prioritize metabolism for rapid cognitive and muscular action. Cardiovascular activation increases blood pressure, heart rate, and cardiac output, and blood is diverted to muscles and brain (6). This system is regulated homeostatically, with negative feedback from cortisol acting at hypothalamic and pituitary glucocorticoid receptors to terminate the acute stress response. However, when stressors are excessive or prolonged, attempts at maintaining homeostasis can lead to alterations of basal and provoked HPA axis activity and associated functioning of autonomic, metabolic, and immune systems (7, 8).
Childhood Stress, Neuroendocrine Function, and Psychopathology
Early-life stress is linked to altered neuroendocrine function in adults with psychiatric disorders such as MDD, post-traumatic stress disorder (PTSD), and borderline personality disorder (9, 10). While several studies in adults and children with MDD documented increases in basal and provoked HPA axis activity, attenuated HPA axis function has also been associated with MDD and depressive symptoms (11). PTSD has been associated with decreased basal or stress-induced cortisol concentrations in several studies, but increased ACTH and/or cortisol concentrations in others (12). HPA axis dysfunction may be more proximally linked to the experience of childhood adversity, which often precedes the onset of depression and PTSD. It is now well established that early adversity alters HPA axis function, with evidence of both exaggerated and attenuated cortisol and ACTH levels (3, 13-15). The precise mechanism underlying diminished cortisol responses is not known, but a trajectory of initial hyperactivation of the HPA system in response to excessive and prolonged stress exposure, progressing to a state of chronic adrenal stress hyporeactivity, has been proposed (16, 17).
Severe and Chronic Stress: Neurotoxicity and Anxiogenesis
Glucocorticoid receptors are widely distributed throughout the limbic system, including the hypothalamus, hippocampus, amygdala and prefrontal cortex (PFC). In rodents, chronic stress and glucocorticoid administration result in remodeling and inhibition of cell proliferation in the PFC and hippocampus (17). In the amygdala, a brain region that regulates fear and anxiety, glucocorticoid exposure results in neuronal proliferation and dendritic growth (17). CRH mediates anxiety and fear responding in the amygdala, and in contrast to the negative feedback effect of cortisol on CRH secretion from the hypothalamus, in the amygdala cortisol enhances CRH activity. Administration of CRH to the brain in rodents and monkeys produces anxiety-like behavior, including neophobia, suppression of exploratory, social, reproductive, and appetitive behavior, and increased physiological reactivity to novel or stressful stimuli. Glucocorticoids, CRH, AVP, ACTH, cortisol, and epinephrine enhance unconditioned fear responses and learning in aversive conditioning paradigms (18). This may provide important clues to the development and treatment of fear and anxiety following trauma exposure, as occurs in PTSD, as discussed further below.
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that plays a key role in the growth, differentiation, maintenance, and survival of neurons (19). BDNF is decreased in rodent stress models and in patients with MDD, and may play a critical role in glucocorticoid-induced neurotoxicity. Rodent models involving early stress or the administration of glucocorticoids decrease BDNF expression and lead to hippocampal and cortical atrophy, as well as behavioral changes associated with depression. Importantly, chronic treatment with exercise, antidepressants, and electroconvulsive shock have all been shown to increase BDNF levels and hippocampal neurogenesis in rodents (19). Peripheral BDNF administration produced behavioral antidepressant-like effects and increased hippocampal neuronal survival in rodents (20).
In humans, evidence is mounting that BDNF may be involved in the pathogenesis of stress-related disorders such as MDD. Postmortem brains of suicide victims show reduced BDNF expression in hippocampal and cortical brain regions (21), and this corresponds to neuroimaging findings of decreased volumes of hippocampus and PFC in adults with a history of childhood maltreatment, MDD, or PTSD (4). BDNF is also reduced in the blood of medication-naïve patients with MDD and is increased in patients with MDD who are on antidepressants (22). A few recent studies have shown that early stress exposure is linked to peripheral BDNF levels in subjects with a lifetime history of MDD (e.g. (23-25)). These findings, combined with data from animal studies showing reduced BDNF and behavioral changes associated with depression, suggest that neurotoxic effects of stress may be causally involved in major depression.
Childhood Adversity: Neuroimaging and Cognitive Correlates
As mentioned above, several neuroimaging studies of children and adults have examined effects of early adversity. Structural imaging studies examine the volume of brain regions and functional studies evaluate activity of brain regions during tasks or resting states. Much of this work has focused on frontal and limbic systems involved in emotional processing, motivation, working memory, and behavioral regulation. In particular, the PFC, amygdala and hippocampus represent key nodes in the brain stress circuitry, and are strongly implicated in the pathophysiology of mood and anxiety disorders. These regions have a high density of glucocorticoid receptors and therefore may be more susceptible to the neurotoxic effects of excessive glucocorticoid exposure during key developmental periods. The body of literature on this topic is growing and many of the findings are mixed, but overall, structural and functional neuroimaging results indicate that childhood maltreatment is associated with abnormalities in the PFC, corpus callosum, amygdala, and hippocampus (for reviews see (26, 27)).
In addition to their role in affective and cognitive processing, some of these same regions function together in the “default mode network,” which is active during resting states and may be involved in self-reflection and readiness for future events. Decreased connectivity within this network has been linked to early life stress and PTSD, suggesting a link between stress exposure and disrupted processing of internal experiences, which may contribute to the pathophysiology of stress-related illnesses (28, 29).
Taken together, these neuroimaging findings suggest that childhood maltreatment may impair frontal brain regions that are necessary for inhibitory control of limbic responses. For example, loss of “top-down” control by the PFC may lead to anxiety symptoms, as the ascending signals from the amygdala are not sufficiently modulated (30). As discussed above, animal models demonstrate that stress, glucocorticoids, and alteration of neurotrophic factors lead to structural changes in cortical and limbic brain regions. While studies of this effect in healthy adults have yielded mixed findings, cortisol has been linked to smaller volumes of PFC (31), hippocampus (32) and anterior cingulate (33) in adults and children with early adversity. Increased cortisol concentrations have also been associated with diminished functional connectivity between the PFC and amygdala (34).
Consistent with neuroimaging findings, children with early adversity may have impaired cognitive function. For example, children institutionalized before adoption had poorer cognitive performance that was associated with longer durations of institutionalization. Deficits include overall decrements in intelligence quotient (IQ) as well as specific problems with working memory, language, attention, and executive function (27, 35) that are present even after controlling for IQ (36). In terms of other cognitive and affective faculties, younger maltreated children, particularly neglected children, have difficulty discriminating facial expressions of emotion, and physically abused children may have enhanced sensitivity to angry and threatening emotional expressions (27). Academic performance may be impaired in adults with a history of childhood maltreatment, but IQ deficits have not been documented in this group. Methodological differences between studies of adults and children may account divergent findings regarding IQ (discussed below), or some deficits may resolve over time, perhaps in response to environmental enrichment (27, 37). Consistent with findings with children, however, adults with a history of childhood maltreatment show increased sensitivity to negative emotional expressions (38).
Genetic Influences on the Effects of Early Stress
Although early stress increases risk for the development of psychopathology, some individuals are resilient and do not suffer many of the detrimental effects of adverse childhood exposures. Interactions of risk or protective genes and environmental exposures (G × E interactions) have long been proposed to account for these divergent outcomes, and recent studies have identified genes involved in monoaminergic, neuroendocrine, and neurotrophin systems as likely candidates. Much of this work has focused on genes that regulate the serotonin system, in particular 5-HTTLPR, a functional polymorphism in the promoter region of the serotonin transporter gene. In 2003, Caspi and colleagues (39) published a groundbreaking prospective study of a representative birth cohort in Dunedin, New Zealand in which they reported a G × E interaction of 5-HTTLPR with both early adversity and adult stressful life events. Stress exposure significantly increased the risk of developing depression, and individuals with the short (s) variant of the gene who were exposed to stress had the greatest risk. The finding of a stress × 5-HTTLPR interaction has been replicated in numerous studies. Substantial controversy was generated by negative findings from two meta-analyses of a subset of these studies (40, 41). However, a broader view of this literature (42) and a more inclusive meta-analysis (43) support an interaction effect of this gene with psychosocial stress in the development of depression, and indicate that the most robust findings are from studies of childhood maltreatment as the stressor.
Additional work has demonstrated interactions of early adversity with other serotonin genes as well as genes that regulate other systems, such as the HPA axis and neurotrophin systems, as reviewed in Nugent et al (44). For example, the gene for the type I CRH receptor (CRHR1) interacts with childhood maltreatment to increase risk for depressive symptoms or diagnoses (44-46), as well as abnormal diurnal or provoked cortisol concentrations in children and adults (47, 48). FKBP5, a gene that regulates sensitivity of the glucocorticoid receptor, has been shown to interact with childhood maltreatment to predict PTSD and depression (49). As discussed above, BDNF is a nerve growth factor that is reduced in response to stress in animal studies and in patients with MDD. The Met allele of a functional variant of the BDNF gene (Val66Met) results in abnormal intracellular packaging and secretion of BDNF. Several studies have now shown that this allele interacts with early-life stress to increase risk for depression in children and adults (44, 50).
It is important to acknowledge that the efforts to identify risk genes for major depression and other psychiatric conditions have rarely yielded robust findings. Among other reasons for this, it is likely that many genes are involved, and, given the heterogeneous nature of our diagnostic categories, different genes may be critical for different individuals. Similarly, genes that are sensitive to the influences of stress exposure may only be relevant for those with a significant history of adversity. As with other studies of childhood adversity, methodological considerations are paramount in interpreting the findings of investigations of gene-environment interactions (see below for a discussion).
Epigenetic Effects of Early-Life Stress
Gene variants such as those discussed above arise from differences in the sequence of DNA, whereas epigenetic alterations to DNA do not influence DNA sequence, but alter the likelihood that genes will be expressed. Animal work and a few human studies implicate epigenetic changes to the glucocorticoid receptor gene as a mechanism underlying the neurobiological effects of early adversity. Animal models of early stress have also found epigenetic changes to other genes that regulate neuroendocrine function (e.g., 51). Methylation is a stable form of epigenetic modification that can block binding of transcription factors and reduce gene expression. In rodents, low levels of maternal care (licking, grooming, and arched-back nursing) result in greater methylation of the promoter region of the glucocorticoid receptor gene.. In rodents, low levels of maternal care (licking, grooming, and arched-back nursing) result in greater methylation of the promoter region of the glucocorticoid receptor gene. As discussed above, the glucocorticoid receptor serves an important regulatory function in modulating stress responses, and this animal model leads to reduced numbers of glucocorticoid receptors and abnormal hormonal and behavioral responses to stress (52).
Very few human studies of epigenetic changes in relation to early-life stress have been conducted thus far, but early results are consistent with the animal findings. Suicide victims with childhood maltreatment had greater methylation of the glucocorticoid receptor gene promoter in the hippocampus (53). Maternal depressed/anxious mood in the third trimester was associated with greater methylation of this gene in a study of infant cord blood, and methylation was positively associated with salivary cortisol responses to stimulation of the infants at age three months (54). A small study found that maternal exposure to intimate partner violence was associated with greater methylation of this gene in adolescent offspring (55). Our group recently found greater methylation of the promoter region of this gene in peripheral blood of adults with a history of early adversity, and methylation was linked to altered cortisol response to a standardized endocrine challenge test (56). Other genes that regulate stress-responses in humans, such as genes in the BDNF, serotonin, and dopamine systems, have also been examined for stress-related epigenetic changes with encouraging preliminary results. One study showed that lifetime stress predicted epigenetic changes to the gene for catechol-O-methyltransferase (COMT), an enzyme that degrades catecholamines. Stress and COMT methylation were linked to alterations in working memory and prefrontal activity (57). Overall, these initial epigenetic findings are consistent with what is known about the neurobiology of these systems and early-life stress exposure, and suggest that epigenetic modulation of neuroendocrine, neurotrophic, and monoaminergic systems could be a key mechanism underlying the neurobiological effects of childhood adversity.
Allostatic Load: Metabolic Syndrome, Immune, and Cellular Aging Effects
Stress and Disease
A recent study found that adults with six or more adverse childhood experiences died on average nearly 20 years earlier than those without such experiences (58). Excessive glucocorticoid activity due to chronic stress can lead to failure of multiple physiologic regulatory systems, termed allostatic load (17). Consistent with this, a history of early-life stress increases risk for several medical conditions, including obesity, cardiovascular disease, diabetes, fibromyalgia, and chronic fatigue (59). Activation of the HPA axis and sympathetic nervous system alters physiologic systems involved in the metabolic syndrome, which consists of central obesity, hypercholesterolemia, and increases in blood pressure and insulin resistance (60, 61). The metabolic syndrome has been associated with HPA axis dysregulation, and our group recently found that indices of the metabolic syndrome were linked to blunted cortisol responses to the dexamethasone (Dex)/CRH test, consistent with the allostatic load hypothesis (62).
Stress and the Immune System
Glucocorticoids have both permissive and suppressive effects on immunity and inflammation. The immunosuppressive actions of exogenous glucocorticoids are well known, and glucocorticoid deficiency results in excessive activation of inflammatory and immune responses (6). Acute stress initiates the inflammatory response, involving increases in pro-inflammatory cytokines, as well as chemokines, adhesion molecules, and acute phase reactants (63). Pro-inflammatory cytokines and deficient cellular immunity are involved in a variety of stress-related disorders, including MDD (64), and findings from several studies indicate that early stress may partially account for this association. In a sample of healthy adults without psychopathology, our group found that a history of childhood maltreatment was associated with exaggerated interleukin (IL)-6 responses to psychosocial challenge (65). Childhood adversity has emerged as an independent risk factor for systemic inflammation later in life in large epidemiological samples (66).
Stress and Telomere Shortening
Excessive glucocorticoid and immune activation resulting from chronic or early stress exposure may lead to telomere shortening, a cellular marker of aging that has been linked to morbidity and mortality in cardiovascular disease, diabetes, and cancer, in addition to MDD and bipolar disorder (67). Telomeres are “caps” at the ends of linear chromosomes comprised of DNA repeats that serve to promote chromosomal stability. In replicating somatic cells, chromosome ends shorten with each cell division, and telomeres serve as buffers against loss of genetic information and chromosomal fusion or recombination. Telomeres shorten with each cell division and when they become critically short, the cell undergoes replicative senescence or programmed cell death. Exposure to radiation or toxins has long been known to influence telomere length; more recent work identifies psychosocial stress as an important factor as well. In a study of female caregivers, Epel and colleagues (68) found that psychological stress was linked to leukocyte telomere shortening. Several studies have confirmed this effect in a variety of contexts. Because rates of telomere shortening are fastest during infancy and early childhood and regulation of telomere length may be programmed in early development, childhood maltreatment may have a particularly profound effect on telomere attrition. In a study of healthy adults with no current or past psychiatric disorder, our group found that those with a history of childhood maltreatment had shorter telomeres than adults with no such history (48). The association of shorter telomere length with childhood adversity has been replicated in several studies involving a variety of measures of early adversity in samples of adults and children (67).
In summary, early-life stress has been associated with several medical conditions including obesity, cardiovascular disease, diabetes, fibromyalgia, and chronic fatigue, in addition to major depression and related psychiatric disorders. Psychosocial stress may induce telomere shortening through effects on glucocorticoid and inflammatory activation. These processes may therefore represent a mechanism of the effect of early-life stress on risk for psychiatric and other medical conditions.
Methodological Influences on the Study of Early-Life Stress
The literature reviewed above identifies neurobiological effects of early adversity seen in both animal models and human studies that are associated with psychiatric and medical conditions, and these effects may represent mechanisms of risk for these disorders. However, this broad overview does not allow for a detailed examination of individual findings, where considerable variability can be found. Animal models allow for rigorous experimental control, and therefore provide some of the strongest evidence for neurobiological effects of early stress; however, findings are specific to the species and strain of animal as well as the stress paradigm used. In humans there are wide variations in the nature of stress exposure, such as type of adversity, intensity, and duration, as well as mitigating and ameliorating influences. Adverse experiences commonly co-occur, and are often associated with other risk factors, such as parental psychopathology and poverty. Individual differences, including risk and protective genes, as well as buffering environmental experiences, are also critical, yet difficult to study adequately. Timing of exposure to adversity is important, because different brain regions and circuits differ in the timing of development, and attachment and other buffering influences differ across development as well. Effects may resolve or worsen over time, depending on exposure to subsequent risk and protective influences.
It is difficult to comprehensively assess early-life stress in research. Structured interviews may be ideal but are very labor intensive, and in fact, it is possible that the anonymity afforded by questionnaires may yield some benefit. Questionnaires are used most commonly, but it is difficult in this format to accurately measure all important characteristics. These include the precise nature, developmental timing, duration and frequency of occurrences over time, and the subjective level of stress. Studies of general stressors have commonly used stressor checklists which vary between studies and often do not have objective stressor characteristics or subjective stress ratings. Studies of children often use child protective service records to identify maltreatment, which does not capture all experiences of abuse or neglect in maltreatment or control groups. A number of studies have also examined neurobiological systems in children raised in orphanages who experienced neglect. Only a few studies have begun to investigate these complexities in the nature of stress exposure and other individual differences, and no conclusions can yet be drawn regarding specific effects of these influences.
Importantly, most investigations include participants with trauma-related psychiatric illnesses, such as PTSD, MDD, or borderline personality disorder, and many include those on psychotropic medication. Insofar as abnormalities may be specific to particular disorders or to medication treatment, findings of studies that do not account for effects of these disorders will be more difficult to interpret. On the other hand, samples that exclude individuals with trauma-related disorders may be heterogeneous and include both at-risk and resilient individuals. Studies of children with recent trauma exposure can focus on risk, but typically involve more severe maltreatment because such children are identified through social services. In addition, non-maltreated control groups may include children with stress or trauma that has not been measured or detected. Adults, by contrast, self-report their experiences, and these may cover the full range of maltreatment. Such reports may be subject to reporting and memory biases, although false negatives are more common than false positives (69). Adults and children also differ with respect to types of protocols and procedures that they can tolerate. For example, drawing blood from young children, perhaps especially those who have been maltreated, can be traumatic, so studies of neurobiological correlates of stress exposure are often limited to substances that can be measured in saliva. In neuroimaging studies, young children may not be able to remain still in the scanner or participate in complex protocols.
Treatment Approaches
Treatment of Maltreated Children
A history of childhood maltreatment portends a more unfavorable course of depressive illness and response to conventional pharmacotherapy and psychotherapy in adolescents and adults (70); for example, standard cognitive behavioral therapy (CBT) may not be effective in this population (e.g., (71, 72). On the other hand, trauma-focused CBT (TF-CBT) teaches coping skills combined with gradual exposure to trauma narratives to allow the child to become more tolerant of trauma-reminders, which is associated with reductions in psychological and physical stress-reactivity. In comparison with supportive therapy, TF-CBT is more effective for symptoms of PTSD and depression (73). Another approach, Trauma Systems Therapy (TST), assesses emotional and behavioral dysregulation in the environmental context to develop a team-based treatment plan involving home-and office-based work, and advocacy for social and educational services. A recent uncontrolled study found improvements over 15 months of treatment (74), but controlled studies have yet to be conducted.
Treatment of Adults with a History of Childhood Adversity
Only a few studies have examined whether some treatments are more efficacious than others for adults with a history of childhood maltreatment. A study of treatment of chronic depression found that patients with a history of childhood trauma had better responses to psychotherapy, with or without the antidepressant nefazodone, compared to nefazodone alone (75). A study of inpatients with major depression found that for those with a history of childhood maltreatment, the combination of interpersonal therapy and antidepressants was significantly more effective than antidepressants without psychotherapy (76). Thus, psychotherapy appears to be an important component of treatment for patients with a history of childhood trauma.
Psychotherapeutic approaches that specifically target emotional and behavioral sequelae of childhood maltreatment have been developed for adults. Dialectical behavioral therapy (DBT) utilizes CBT and mindfulness approaches to address problems with emotion regulation, distress tolerance, and interpersonal effectiveness. DBT has demonstrated efficacy for borderline personality disorder and associated problems (77), and for PTSD related to childhood abuse (78). Behavioral approaches that aim to normalize responses to fear-provoking stimuli have also proven successful in treating anxiety disorders, including PTSD. Exposure therapy extinguishes inappropriate fear responding by coupling exposure to the feared situation with a safe environment. Exposure therapy is an effective treatment for PTSD (79). However, exposure therapy does not address difficulties with affect regulation and interpersonal relationships that often result from childhood maltreatment. A treatment that combines skills training in affect and interpersonal regulation (adapted from DBT) followed by exposure therapy has demonstrated efficacy in a randomized controlled trial of child-abuse-related PTSD (80).
Development of Treatment Approaches that Target Stress Neurobiology
A number of treatment approaches that are based on an understanding of stress neurobiology are currently in development. Memories undergo consolidation and re-consolidation over time, and memory retrieval can render a fixed memory unstable and vulnerable to change, requiring reconsolidation to be re-established (81). This process has been examined in rodents and humans using fear conditioning and extinction training paradigms, which involve behavioral and learning/memory processes that are similar to those of exposure therapy. For example, a recent fear conditioning experiment in mice found that the combination of fluoxetine plus extinction training, but neither treatment alone, produced an enduring loss of conditioned fear memory (82). Further experiments revealed that fluoxetine, through the actions of BDNF, converted the fear memory circuitry to a more immature state, increasing synaptic plasticity and allowing extinction-guided remodeling of this circuitry.
In humans, pharmacologic treatments have recently been added to exposure therapy in an effort to maximize the therapeutic benefits of behavior therapy. D-cycloserine, a partial agonist at n-methyl-d-aspartate (NMDA) receptors which are involved in synaptic remodeling and memory function, is known to enhance cognition and has been found to facilitate both fear extinction in animals and exposure therapy in humans with some anxiety disorders (83). However, two recent studies did not find an overall augmentation effect of D-cycloserine on exposure therapy for PTSD (84, 85). Drawing on evidence from animal studies that beta-adrenergic blockers abolish stress-induced fear learning and attenuate reconsolidation of fear memories, the beta-blocker propranolol has been tested for prevention and treatment of PTSD. Post-exposure propranolol administration has received only limited support as a preventative treatment for the development of PTSD (86). However, recent evidence suggests that propranolol can reduce the strength of trauma memories and PTSD symptoms when administered following memory reactivation via traumatic script-driven imagery exposure (87). Further treatment development in this area is ongoing in an effort to “erase” fear memories by modulating or re-programming the brain's fear circuitry.
A number of novel pharmacologic treatments that target the neurobiological sequelae of excessive stress exposure are also in development. Several companies have developed CRH receptor antagonists as a potential therapy for MDD, anxiety, and substance use disorders. Clinical trials for depression have not been successful or have found problems with safety or efficacy (88), but studies of other disorders, including PTSD, are ongoing. Antagonists of AVP, which interacts with CRH to activate the HPA axis, have shown potential in animal models of stress exposure (e.g., 51). However, results of four recent double-blind, placebo-controlled trials of a vasopressin V1B receptor antagonist in MDD and generalized anxiety disorder have been disappointing in terms of efficacy, although reassuringly no adverse effects related to the role of AVP as a regulator of fluid and electrolyte balance were observed (89). There are no other such drugs in advanced clinical development (90). Mifepristone, a glucocorticoid receptor antagonist with anti-progesterone activity (also known as the abortifacient RU-486), has been found in preclinical trials to prevent stress-induced depressive-like behaviors (91) and reconsolidation of cue-conditioned fear, which is thought to be a mechanism of PTSD symptoms (92). Clinical trials have suggested that short-term treatment with mifepristone might be effective and is well-tolerated in psychotic depression, but findings are mixed (93-95). Thus, results from studies of drugs that modulate HPA axis function have been disappointing; it remains unknown whether these treatments might be more effective in the subset of such patients with a history of significant stress exposure.
As discussed above, a variety of antidepressant treatments increase BDNF and subsequent hippocampal neurogenesis in rodents. Depressed patients taking antidepressant medications also show enhanced peripheral BNDF levels, and some evidence documents increases in peripheral BDNF in response to exercise in humans (96). While the relevance of changes in peripheral BDNF to effects in the brain is unknown, a recent study in rodents showed antidepressant-like effects of peripherally administered BDNF (20). This is not likely to be a viable treatment for humans due to the pharmacokinetics and adverse side effects, but collectively this research suggests that BDNF may be a good indicator of antidepressant efficacy and that drugs targeting BDNF could have therapeutic potential.
Treatment implications of genetic research
Investigations of risk and protective genes aim to identify individuals at greatest risk for stress-induced disorders. This work is complicated by the fact that such pathways are governed by multiple sets of genes, and a particular gene may only account for a small percent of the risk for these disorders. Genetic research on early-life stress also aims to further elucidate the neurobiological pathways involved in the pathogenesis of these disorders, and such research may inform the development of novel treatments.
Another avenue of genetic research aims to determine whether genes that regulate the pathways involved in depression and anxiety confer a differential response to pharmacologic treatment. Although initial reports suggested that several gene variants may predict response to antidepressants, thus far findings have not been consistently replicated (49). However, such studies have only recently examined whether environmental influences might account for variability in these outcomes. There is some early promising evidence that gene variants identified in G × E studies of risk for depression and anxiety may also confer differential response to treatment among individuals who have been exposed to stressful life events. These include 5-HTTLPR and variants in the CRHR1 and FKBP5 genes. Here, too, some of the findings have been inconsistent, and studies have usually focused on adult stress exposure and have not examined the effects of childhood adversity (49). Other evidence suggests that some “risk” genotypes may be better conceptualized as “environmental sensitivity” genotypes. Results of a recent meta-analysis indicate that the s “risk” allele of 5-HTTLPR actually confers sensitivity to social support and protection from risk in enriched environments (97). Building on this work, Eley and colleagues (98) recently conducted the first study to examine genetic predictors of response to CBT, and found that individuals with two copies of the 5-HTTLPR s allele have a better response to treatment.
Epigenetic marks have long been thought to be fixed in post-mitotic cells, but rodent studies indicate that DNA methylation can be reversed in adult brain. Thus, it is possible that drugs or even psychotherapeutic approaches could reverse epigenetic effects of early-life stress. Drugs that have shown this effect in animal models are available (e.g., valproic acid and S-adenosyl-methionine, a putative antidepressant) or are in development (e.g., histone deacetylase (HDAC) inhibitors) for the treatment of cancer and other disorders (99, 100). In a rodent model, exercise induced epigenetic changes to a promoter of the BDNF gene in the hippocampus (101). Such studies raise the possibility for future research to determine whether modifying the social and environmental experiences of maltreated children might reverse epigenetic effects of early-life stress.
Treatment implications of metabolic, immune, and cellular aging findings
The association of early and chronic stress with inflammation and other health problems in adulthood underscores the importance of preventing early trauma, reducing additional risk factors, and enhancing protective mechanisms. Research on interventions to prevent or alter the biological trajectory from stress to disease pathology is currently lacking. Human biomarker studies have not yet identified a particular diagnostic test or even a consistently “high” or “low” pattern of candidate peripheral cytokine concentrations predictive of specific mental illness. However, a growing body of preclinical evidence, as well as findings from cross-sectional clinical studies, suggests there may eventually be an identifiable subpopulation of depressed patients for whom the role of cytokines is closely linked to psychiatric symptomatology. Agents that target inflammatory processes are being considered for the treatment of depression, including nonsteroidal anti-inflammatory drugs (NSAIDS), TNF-alpha antagonists, and antibiotics (102-104). Other drugs, such as thiazolidinediones with anti-inflammatory effects, are being re-examined for their potential role in the treatment of mood disorders (105). Several nonpharmacological strategies also appear promising. Reduction of adiposity and dietary modifications, such as lowering processed food intake or supplementation with 3-omega fatty acid supplements (106), may be useful to reduce depressive symptoms. Exercise, yoga, meditation, and therapeutic massage have all shown neuroimmune modulatory effects, and as such may offer some therapeutic potential for preventing or reducing mood symptoms, as well as promoting healthy function of other somatic tissues and organ systems.
Of note, there is evidence that enrichment of the environment later in life may reverse some, but not all, of the biological consequences associated with exposure to early life adversity. Additionally, it should be pointed out that not all exposure to stress is deleterious, and that exposure to psychological stress challenge under controlled conditions may eventually be useful as a novel therapeutic or prophylactic treatment approach in psychiatry. For example, “behavioral immunization” has been proposed as a potential mechanism for promoting resilience to psychological stressors (107). This paradigm involves exposure to acute, psychologically stressful experiences of mild intensity, which serves to “innoculate” an individual and enhances emotional resilience by inducing immunological memory, much as pathogen exposure activates the innate immune response to antigen and leads to formation of immunological memory and t-cell function for clearance of an infection. Further research will be needed to determine the efficacy and critical features of strategies employing combinations of novel behavioral and pharmacological tools to build resilience early in development, maintain adaptive capacity across the lifespan, and diminish the proinflammatory effects of stress on physical and emotional well being.
Discussion
It is clear that early-life stress and, most dramatically, childhood maltreatment confer risk for depressive and anxiety disorders. The findings reviewed in this paper broadly indicate that 1) the neuroendocrine, neurotrophic, and other physiological systems that are activated in response to extreme or prolonged stress may be the mechanisms by which risk for these disorders is elevated in relation to early-life stress, 2) risk and protective genes influence sensitivity to stress and risk for mood and anxiety disorders, 3) Early-life stress influences treatment outcome, consistent with clinical insights that psychotherapy may be particularly valuable for these patients, and new treatments that target stress-related neurobiological abnormalities are in development.
Much of the research in humans and animals that identifies abnormal function in stress-sensitive brain systems is focused on early-life stress, due to the fact that this period is characterized by dependence on caregivers and other environmental influences, as well as major developmental changes in the neural systems that coordinate stress responding, mood, fear/anxiety, and behavior. Future treatment research will likely benefit from continued refinement in the type and treatment protocols of pharmacological and behavioral strategies used, as well as attention to patient characteristics that may influence outcome. Treatment trials that target the neural and behavioral systems identified in human and animal models of stress exposure have usually focused on depression or PTSD, rather than specifically on those with a history of early stress. Future work should test novel treatments with this population, which may receive the greatest benefit from approaches that target the neurobiologic effects of early stress exposure.
Clinical Recommendations.
Early adversity negatively impacts treatment outcome; psychotherapeutic approaches that target cognitive, emotional, and interpersonal difficulties associated with childhood stress and trauma have demonstrated efficacy.
Several key neurobiological systems that are altered in response to early trauma may be involved in the pathogenesis of psychiatric and other medical conditions. Pharmacologic approaches that target the identified neurobiological abnormalities are currently in development.
Additional Comments.
Risks including poverty, parental mental illness, substance abuse, maltreatment, and other traumatic or stressful life events often co-occur, complicating both research and treatment efforts.
Studies of neurobiological correlates of childhood adversity are also limited by co-occurring disorders and treatments that may influence the systems of interest.
More research on effective treatments for the effects of childhood maltreatment and other forms of early adversity is needed.
Acknowledgments
Supported by National Institute of Mental Health grants R01 MH083704 (ART), R21 MH091508 (ART), and R01 MH068767-01 (LLC).
Drs. Tyrka, Carpenter, and Price report having received research funding from the National Institute of Health, NeoSync, Neuronetics, Medtronic, and Cyberonics. Dr. Tyrka also received honoraria for continuing medical education from Lundbeck and Takeda. Darcy Burgers reported no biomedical financial interests or potential conflicts of interest. Dr. Philip reports research funding from NeoSync and Neuronetics. Dr. Price received research funding from HRSA, served on advisory panels for Abbott and AstraZeneca, and served as a paid consultant to Gerson Lehrman, Wiley, Springer, Qatar National Research Fund, Alberta Heritage Foundation for Medical Research, Abbott, and AstraZeneca. Dr. Carpenter received consulting fees from Abbott, Cyberonics, Novartis, Wyeth, AstraZenica and Neuronetics, served on the speakers’ bureau for Neuronetics, and received honoraria for continuing medical education from AstraZenica.
Footnotes
Declaration of Interest
The authors identify no conflicts of interest.
References
- 1.GREEN JG, MCLAUGHLIN KA, BERGLUND PA, GRUBER MJ, SAMPSON NA, ZASLAVSKY AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DOZIER M, ALBUS K, FISHER PA, SEPULVEDA S. Interventions for foster parents: implications for developmental theory. Dev Psychopathol. 2002;14:843–860. doi: 10.1017/s0954579402004091. [DOI] [PubMed] [Google Scholar]
- 3.LUPIEN SJ, MCEWEN BS, GUNNAR MR, HEIM C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 4.MCCRORY E, DE BRITO SA, VIDING E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 5.KAFFMAN A, MEANEY MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 6.SAPOLSKY RM, ROMERO LM, MUNCK AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 7.DALLMAN MF, LA FLEUR SE, PECORARO NC, GOMEZ F, HOUSHYAR H, AKANA SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 8.MCEWEN BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HEIM C, NEMEROFF CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 10.RINNE T, DE KLOET ER, WOUTERS L, GOEKOOP JG, DERIJK RH, VAN DEN BRINK W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52:1102–1112. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- 11.KUNUGI H, HORI H, ADACHI N, NUMAKAWA T. Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry Clin Neurosci. 2010;64:447–459. doi: 10.1111/j.1440-1819.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- 12.YEHUDA R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 13.HEIM C, SHUGART M, CRAIGHEAD WE, NEMEROFF CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 14.CARPENTER LL, TYRKA AR, ROSS NS, KHOURY L, ANDERSON GM, PRICE LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TYRKA AR, WIER L, PRICE LH, ROSS N, ANDERSON GM, WILKINSON CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HEIM C, NEWPORT DJ, MILLER AH, NEMEROFF CB. Long-term neuroendocrine effects of childhood maltreatment. Jama. 2000;284:2321. [PubMed] [Google Scholar]
- 17.MCEWEN BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 18.SCHULKIN J. Angst and the amygdala. Dialogues Clin Neurosci. 2006;8:407–416. doi: 10.31887/DCNS.2006.8.4/jschulkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DUMAN CH, SCHLESINGER L, TERWILLIGER R, RUSSELL DS, NEWTON SS, DUMAN RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198:366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SCHMIDT HD, DUMAN RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DWIVEDI Y. Brain-derived neurotrophic factor and suicide pathogenesis. Ann Med. 2010;42:87–96. doi: 10.3109/07853890903485730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SEN S, DUMAN R, SANACORA G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ELZINGA BM, MOLENDIJK ML, OUDE VOSHAAR RC, BUS BA, PRICKAERTS J, SPINHOVEN P, et al. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology (Berl) 2011;214:319–328. doi: 10.1007/s00213-010-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.JEON HJ, KANG ES, LEE EH, JEONG EG, JEON JR, MISCHOULON D, et al. Childhood trauma and platelet brain-derived neurotrophic factor (BDNF) after a three month follow-up in patients with major depressive disorder. J Psychiatr Res. 2012;46:966–972. doi: 10.1016/j.jpsychires.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.MONDELLI V, CATTANEO A, BELVEDERI MURRI M, DI FORTI M, HANDLEY R, HEPGUL N, et al. Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72:1677–1684. doi: 10.4088/JCP.10m06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MCCRORY E, DE BRITO SA, VIDING E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.HART H, RUBIA K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LANIUS RA, BLUHM RL, FREWEN PA. How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatr Scand. 2011;124:331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- 29.PHILIP NS, SWEET LH, TYRKA AR, PRICE LH, BLOOM RF, CARPENTER LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2012.10.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.KOENIGS M, GRAFMAN J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CARRION VG, WEEMS CF, RICHERT K, HOFFMAN BC, REISS AL. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biol Psychiatry. 2010;68:491–493. doi: 10.1016/j.biopsych.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CARRION VG, WEEMS CF, REISS AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 33.TREADWAY MT, GRANT MM, DING Z, HOLLON SD, GORE JC, SHELTON RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One. 2009;4:e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VEER IM, OEI NY, SPINHOVEN P, VAN BUCHEM MA, ELZINGA BM, ROMBOUTS SA. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 35.PECHTEL P, PIZZAGALLI DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DE BELLIS MD, HOOPER SR, SPRATT EG, WOOLLEY DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J Int Neuropsychol Soc. 2009;15:868–878. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ENGERT V, EFANOV SI, DEDOVIC K, DUCHESNE A, DAGHER A, PRUESSNER JC. Perceived early-life maternal care and the cortisol response to repeated psychosocial stress. J Psychiatry Neurosci. 2010;35:370–377. doi: 10.1503/jpn.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DANNLOWSKI U, STUHRMANN A, BEUTELMANN V, ZWANZGER P, LENZEN T, GROTEGERD D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 39.CASPI A, SUGDEN K, MOFFITT TE, TAYLOR A, CRAIG IW, HARRINGTON H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 40.MUNAFO MR, DURRANT C, LEWIS G, FLINT J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 41.RISCH N, HERRELL R, LEHNER T, LIANG KY, EAVES L, HOH J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CASPI A, HARIRI AR, HOLMES A, UHER R, MOFFITT TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.KARG K, BURMEISTER M, SHEDDEN K, SEN S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NUGENT NR, TYRKA AR, CARPENTER LL, PRICE LH. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl) 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.KRANZLER HR, FEINN R, NELSON EC, COVAULT J, ANTON RF, FARRER L, et al. A CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:960–968. doi: 10.1002/ajmg.b.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.GRABE HJ, SCHWAHN C, APPEL K, MAHLER J, SCHULZ A, SPITZER C, et al. Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1483–1493. doi: 10.1002/ajmg.b.31131. [DOI] [PubMed] [Google Scholar]
- 47.CICCHETTI D, ROGOSCH FA, OSHRI A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Dev Psychopathol. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.TYRKA AR, PRICE LH, GELERNTER J, SCHEPKER C, ANDERSON GM, CARPENTER LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.KEERS R, UHER R. Gene-environment interaction in major depression and antidepressant treatment response. Curr Psychiatry Rep. 2012;14:129–137. doi: 10.1007/s11920-011-0251-x. [DOI] [PubMed] [Google Scholar]
- 50.KEERS R, UHER R, HUEZO-DIAZ P, SMITH R, JAFFEE S, RIETSCHEL M, et al. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenomics J. 2011;11:138–145. doi: 10.1038/tpj.2010.14. [DOI] [PubMed] [Google Scholar]
- 51.MURGATROYD C, PATCHEV AV, WU Y, MICALE V, BOCKMUHL Y, FISCHER D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 52.WEAVER IC, CERVONI N, CHAMPAGNE FA, D'ALESSIO AC, SHARMA S, SECKL JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 53.MCGOWAN PO, SASAKI A, D'ALESSIO AC, DYMOV S, LABONTE B, SZYF M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.OBERLANDER TF, WEINBERG J, PAPSDORF M, GRUNAU R, MISRI S, DEVLIN AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 55.RADTKE KM, RUF M, GUNTER HM, DOHRMANN K, SCHAUER M, MEYER A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.TYRKA AR, PRICE LH, MARSIT C, WALTERS OC, CARPENTER LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.URSINI G, BOLLATI V, FAZIO L, PORCELLI A, IACOVELLI L, CATALANI A, et al. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011;31:6692–6698. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.BROWN DW, ANDA RF, TIEMEIER H, FELITTI VJ, EDWARDS VJ, CROFT JB, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37:389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 59.JONES GT, POWER C, MACFARLANE GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 60.EPEL ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 61.ANAGNOSTIS P, ATHYROS VG, TZIOMALOS K, KARAGIANNIS A, MIKHAILIDIS DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94:2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 62.TYRKA AR, WALTERS OC, PRICE LH, ANDERSON GM, CARPENTER LL. Altered Response to Neuroendocrine Challenge Linked to Indices of the Metabolic Syndrome in Healthy Adults. Horm Metab Res. 2012 doi: 10.1055/s-0032-1306342. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MILLER GE, CHEN E, SZE J, MARIN T, AREVALO JM, DOLL R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.HAROON E, RAISON CL, MILLER AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CARPENTER LL, GAWUGA CE, TYRKA AR, LEE JK, ANDERSON GM, PRICE LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DANESE A, MOFFITT TE, HARRINGTON H, MILNE BJ, POLANCZYK G, PARIANTE CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.PRICE LH, KAO HT, BURGERS DE, CARPENTER LL, TYRKA AR. Telomeres and Early-Life Stress: An Overview. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.EPEL ES, BLACKBURN EH, LIN J, DHABHAR FS, ADLER NE, MORROW JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.HARDT J, RUTTER M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 70.NANNI V, UHER R, DANESE A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 71.BARBE RP, BRIDGE JA, BIRMAHER B, KOLKO DJ, BRENT DA. Lifetime history of sexual abuse, clinical presentation, and outcome in a clinical trial for adolescent depression. J Clin Psychiatry. 2004;65:77–83. doi: 10.4088/jcp.v65n0113. [DOI] [PubMed] [Google Scholar]
- 72.SHAMSEDDEEN W, ASARNOW JR, CLARKE G, VITIELLO B, WAGNER KD, BIRMAHER B, et al. Impact of physical and sexual abuse on treatment response in the Treatment of Resistant Depression in Adolescent Study (TORDIA). J Am Acad Child Adolesc Psychiatry. 2011;50:293–301. doi: 10.1016/j.jaac.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.ROBERTS NP, KITCHINER NJ, KENARDY J, BISSON JI. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database Syst Rev. 2010:CD007944. doi: 10.1002/14651858.CD007944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ELLIS B, FOGLER J, HANSEN S, FORBES P, NAVALTA C, SAXE G. Trauma Systems Therapy: 15-Month Outcomes and the Importance of Effecting Environmental Change. Psychological Trauma: Theory, Research, Practice, and Policy. 2011 epub. [Google Scholar]
- 75.NEMEROFF CB, HEIM CM, THASE ME, KLEIN DN, RUSH AJ, SCHATZBERG AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ZOBEL I, KECH S, VAN CALKER D, DYKIEREK P, BERGER M, SCHNEIBEL R, et al. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand. 2011;123:276–282. doi: 10.1111/j.1600-0447.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- 77.LYNCH TR, TROST WT, SALSMAN N, LINEHAN MM. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181–205. doi: 10.1146/annurev.clinpsy.2.022305.095229. [DOI] [PubMed] [Google Scholar]
- 78.STEIL R, DYER A, PRIEBE K, KLEINDIENST N, BOHUS M. Dialectical behavior therapy for posttraumatic stress disorder related to childhood sexual abuse: a pilot study of an intensive residential treatment program. J Trauma Stress. 2011;24:102–106. doi: 10.1002/jts.20617. [DOI] [PubMed] [Google Scholar]
- 79.FOA EB. Prolonged exposure therapy: past, present, and future. Depress Anxiety. 2011;28:1043–1047. doi: 10.1002/da.20907. [DOI] [PubMed] [Google Scholar]
- 80.CLOITRE M, STOVALL-MCCLOUGH KC, NOONER K, ZORBAS P, CHERRY S, JACKSON CL, et al. Treatment for PTSD related to childhood abuse: a randomized controlled trial. Am J Psychiatry. 2010;167:915–924. doi: 10.1176/appi.ajp.2010.09081247. [DOI] [PubMed] [Google Scholar]
- 81.NADER K, EINARSSON EO. Memory reconsolidation: an update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- 82.KARPOVA NN, PICKENHAGEN A, LINDHOLM J, TIRABOSCHI E, KULESSKAYA N, AGUSTSDOTTIR A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DAVIS M. NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci. 2011;13:463–474. doi: 10.31887/DCNS.2011.13.4/mdavis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.KLEINE RA, HENDRIKS GJ, KUSTERS WJ, BROEKMAN TG, VAN MINNEN A. A Randomized Placebo-Controlled Trial of d-Cycloserine to Enhance Exposure Therapy for Posttraumatic Stress Disorder. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 85.LITZ BT, SALTERS-PEDNEAULT K, STEENKAMP MM, HERMOS JA, BRYANT RA, OTTO MW, et al. A randomized placebo-controlled trial of d-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 86.HOGE EA, WORTHINGTON JJ, NAGURNEY JT, CHANG Y, KAY EB, FETEROWSKI CM, et al. Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neurosci Ther. 2012;18:21–27. doi: 10.1111/j.1755-5949.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.BRUNET A, POUNDJA J, TREMBLAY J, BUI E, THOMAS E, ORR SP, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31:547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- 88.ZORRILLA EP, KOOB GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.GRIEBEL G, BEESKE S, STAHL SM. The vasopressin V1b receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry. 2012 doi: 10.4088/JCP.12m07804. [DOI] [PubMed] [Google Scholar]
- 90.GRIEBEL G, HOLSBOER F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012;11:462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- 91.WULSIN AC, HERMAN JP, SOLOMON MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.PITMAN RK, MILAD MR, IGOE SA, VANGEL MG, ORR SP, TSAREVA A, et al. Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behav Neurosci. 2011;125:632–638. doi: 10.1037/a0024364. [DOI] [PubMed] [Google Scholar]
- 93.DEBATTISTA C, BELANOFF J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 94.BLASEY CM, BLOCK TS, BELANOFF JK, ROE RL. Efficacy and safety of mifepristone for the treatment of psychotic depression. J Clin Psychopharmacol. 2011;31:436–440. doi: 10.1097/JCP.0b013e3182239191. [DOI] [PubMed] [Google Scholar]
- 95.KLING MA, COLEMAN VH, SCHULKIN J. Glucocorticoid inhibition in the treatment of depression: can we think outside the endocrine hypothalamus? Depress Anxiety. 2009;26:641–649. doi: 10.1002/da.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.ZOLADZ JA, PILC A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 97.VAN IJZENDOORN MH, CASPERS K, BAKERMANS-KRANENBURG MJ, BEACH SR, PHILIBERT R. Methylation matters: interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biol Psychiatry. 2010;68:405–407. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ELEY TC, HUDSON JL, CRESWELL C, TROPEANO M, LESTER KJ, COOPER P, et al. Therapygenetics: the 5HTTLPR and response to psychological therapy. Mol Psychiatry. 2012;17:236–237. doi: 10.1038/mp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.BOHACEK J, MANSUY IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology. 2012;38:220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.MCGOWAN PO, SZYF M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis. 2010;39:66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 101.GOMEZ-PINILLA F, ZHUANG Y, FENG J, YING Z, FAN G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.MIYAOKA T, WAKE R, FURUYA M, LIAURY K, IEDA M, KAWAKAMI K, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:222–226. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 103.LOTRICH FE, SEARS B, MCNAMARA RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: Relationship with interleukin-6. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.RAISON CL, RUTHERFORD RE, WOOLWINE BJ, SHUO C, SCHETTLER P, DRAKE DF, et al. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. Arch Gen Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.SEPANJNIA K, MODABBERNIA A, ASHRAFI M, MODABBERNIA MJ, AKHONDZADEH S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:2093–2100. doi: 10.1038/npp.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MARTINS JG, BENTSEN H, PURI BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144–1149. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- 107.LEWITUS GM, SCHWARTZ M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532–536. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]