Abstract
The main interest of this work was the investigation of the transport mechanisms of salmon calcitonin through the epithelial cell monolayer in the presence and absence of pH-sensititive hydrogel nanospheres composed of poly(methacrylic acid-graftedpoly(ethylene glycol)) (PMAA-g-EG). For this purpose, a gastrointestinal cell culture model, the Caco-2 cell line, was employed. The transport of other macromolecules such as fluorescein sodium, fluorescein isothiocyanate dextran, and 14C-mannitol were also investigated and compared. Transport experiments were conducted in the apical-to-basolateral direction at 37 and 5 °C and from the basolateral-to-apical direction at 37 °C. Results revealed that the presence of P(MAA-g-EG) nanospheres increased the transport of paracellularly transported molecules such as 14C-mannitol and fluorescein isothiocyanate dextran when compared to controls. Fluorescein sodium salt solutions were investigated as an actively transported molecule. The transport of fluorescein was affected by the concentration of PEG chains in the structure. Salmon calcitonin transport was enhanced in the presence of the nanospheres. The comparison of the transport behavior of dextran and calcitonin revealed that the main transport mechanism for salmon calcitonin through epithelial cell monolayers is predominantly paracellular.
Introduction
Oral delivery of peptides and proteins has gathered special attention in recent years as a result of their delicate physicochemical characteristics in aqueous solutions and their susceptibility to degradation by proteolytic enzymes in biological fluids, which presents a tremendous challenge to overcome. The current methods of administration of peptides and proteins consist of intramuscular or intravenous injections. In most cases, the treatment of diseases using peptidic agents possesses less undesirable adverse effects, but it also reduces the quality of life of the patient as a result of the psychological stress caused by frequent injections. As a consequence, low patient compliance is observed, decreasing treatment efficacy. Therefore, the development of noninvasive delivery systems, such as oral systems, is of utmost importance.
This investigation focuses on the peptide salmon calcitonin, a 32-amino-acid polypeptide hormone used in the treatment of bone diseases such as osteoporosis. This disease deserves serious attention because of its impact on the elderly population, especially women.
In this work, we are interested in the transport mechanisms of salmon calcitonin through Caco-2 cell monolayers in the presence and absence of a pH-sensitive hydrogel carrier. Several model fluorescent probes with known transport mechanisms were employed to serve as a basis for comparison. These included fluorescein sodium and fluorescein isothiocyanate dextran (FITC-dextran). Fluorescein sodium is known to be a substrate for the multridrug-resistance-associated protein (MRP) efflux pump (1, 2), known to be expressed in the Caco-2 cell line (3). On the other hand, FITC-dextran is transported through the paracellular route (4, 5), and for this particular investigation its molecular weight (4400) was chosen to be similar to calcitonin.
pH-Sensitive hydrogels are suitable candidates for oral drug delivery of peptides as a result of their ability to respond to their environment. For this purpose, we have developed new grafted hydrogels composed of poly(ethylene glycol) (PEG) grafted on poly(methacrylic acid) (PMAA), henceforth designated as P(MAA-g-EG). P(MAA-g-EG) hydrogels are characterized by their ability to remain collapsed at low pH values, similar to those found in the stomach, and to swell at higher pH’s, such as those encountered in the upper small intestine.
The molecular design of these carriers consisted in the incorporation of poly(ethylene glycol) tethered chains that promote mucosal adhesion and protection to salmon calcitonin, as well as a poly(methacrylic acid) backbone with carboxylic pendant groups (−COOH), which can act as calcium binders leading to epithelial cell junction opening (6). These capabilities allow this system to protect the protein from the undesirable conditions found in the stomach and release it in a more favorable environment such as that encountered in the upper small intestine.
Materials and Methods
Synthesis of P(MAA-g-EG) Nanospheres
Methacrylic acid (MAA, Aldrich Chemical Co., Milwaukee, WI) and methoxy-terminated poly(ethylene glycol) monomethacrylate (PEGMA, Polysciences Inc., Warrington, PA) with a molecular weight of 1000 were used to synthesize P(MAA-g-EG) nanospheres. The monomers were mixed in molar ratios of MAA/(PEG unit), 4:1, 2:1, 1:1, 1:2. Tetra(ethylene glycol) dimethacrylate (TEGDMA, Aldrich Chemical Co., Milwaukee, WI) was incorporated as 0.75% mol of the total monomers and utilized as the cross-linking agent. Irgacure 184 (Ciba-Geigy Corp., Hawthorne, NY) was employed as the photoinitiator, and it was incorporated as approximately 0.1% w/w of the monomer mixture.
The monomers were mixed, and the initiator was dissolved in the monomer mixture. This mixture was then added to deionized water to provide a final concentration of monomer of 0.75% w/w. The container was sealed and bubbled with nitrogen for 15 min to remove any dissolved oxygen. The mixture was placed under a UV source (Spectroline, model SB-125, Westbury, NY) at an intensity of 7 mW/cm2. The solution was allowed to react for 10 min. The resulting nanospheres were washed in deionized water using a dialysis membrane (Spectra/Por, Spectrum Laboratories, Laguna Hills, CA) with a molecular weight cutoff of 300,000. The purpose of this procedure was to minimize the presence of any unreacted monomer or non-crosslinked chains remaining in suspension.
Caco-2 Cell Culture
Caco-2 cells were purchased from the American Tissue Culture Collection (Rockville, MD). The cells were cultivated on 75 cm2 flasks (Beckman Diagnostics, Franklin Lakes, NJ) using Dulbecco’s modified Eagle medium (DMEM) (Sigma, St. Louis, MO) containing 10% fetal bovine serum (Life Technologies, Gaithersburg, MD), 1% nonessential amino acids (Life Technologies, Gaithersburg, MD), 100 units/mL of penicillin, and 100 μg/mL streptomycin (Sigma, St. Louis, MO). Cells were maintained on a controlled atmosphere at 37 °C, 95% relative humidity, and 5% CO2. Culture medium was changed every other day for approximately 5-6 days until cells reached approximately 80-90% confluency. For all experiments cells with passage numbers 60-80 were utilized.
Preparation of Nanosphere Formulations for Transport Experiments
Nanospheres suspensions were prepared by freeze-drying the nanospheres and resuspending in calcium- and magnesium-free Hank’s Balanced Solution (HBSS/CMF) (Sigma, St. Louis, MO). The suspensions were pH equilibrated by placing the suspension on a dialysis membrane (Spectra/Por 0.25 mL/cm, Spectrum Laboratories, Laguna Hills, CA) with a molecular weight cutoff of 15,000 and submerging it on freshly prepared HBSS/CMF. The HBSS/CMF solution was changed several times to obtain an appropriate pH of 7.4. After pH equilibration, nanosphere suspensions were sterilized by γ irradiation with a dose of 2.5 Mrad in a Gammacell 220 (U.S. Nuclear Corp.). Macromolecule solutions were prepared and sterilized by filtration (Nalgene, Rochester, NY) and added to the nanosphere suspension to produce a final nanosphere concentration of 10 mg/mL.
Transport of Model Macromolecules
Fluorescent probes included fluorescein sodium (Sigma, St. Louis, MO) and fluorescein isothiocyanate dextran (FITC-dextran) (Sigma, St. Louis, MO). The final fluorescent probe concentration in the nanosphere formulation was 2 and 180 μg/mL for fluorescein sodium and FITC-dextran, respectively. FITC-dextran solutions also contained 0.5 mg/mL of bovine serum albumin (BSA) (Sigma, St. Louis, MO) to replicate the same experimental conditions as for salmon calcitonin. Fluorescent samples were assayed with a 96-well plate spectrofluorometer (SpectraMax Gemini XS, Molecular Devices, Sunnyvile, CA). Sample solutions of fluorescein sodium and FITC-dextran were independently assayed using the maximum absorbance and emission wavelengths for each molecule. Calibration curves were created for each case, and only the linear range of the curve was employed, which contained approximately 12 points. The original concentrations of fluorescent probes were within the calibration curve.
Salmon cacitonin was employed as the model protein. Nanosphere formulations were prepared to contain a final salmon calcitonin concentration of 80 μg/mL, which also contained 0.5 mg/mL of BSA to minimize protein adhesion to the plastic. Samples were assayed using an ELISA kit (Penninsula Laboratories, San Carlos, CA).
Radiolabeled mannitol (14C-mannitol, American Radiolabeled Chemicals, St. Louis, MO; specific activity 55 μCi/mmol) was utilized as a paracellular marker. Nanosphere formulations were prepared to contain a radioactivity concentration of 0.44 μCi/mL. Samples were diluted with 3 mL of scintillation cocktail (Ultima Gold LX, Packard Instruments, Meriden, CT), and radioactivity was measured with a scintillation counter.
All formulations were prepared in HBSS/CMF, unless otherwise stated, equilibrated overnight for approximately 17 h prior to the experiment in the refrigerator. Controls were prepared by preparing macromolecule solutions without nanospheres at the same macromolecule concentrations.
Transport experiments were conducted on Costar brand 6-well Transwells plates (4.71 cm2/well, pore size 3.0 or 0.45 μm) (Corning Inc., Corning NY). These plates were cultivated as described for 21-25 days and were seeded with a cell density of 5.25 × 104 cells/cm2.
All transport experiments employed HBSS as the experimental medium. Apical chambers always contained HBSS/CMF, whereas the basolateral chambers contained HBSS with a calcium concentration of 200 μmol/L (HBSS/200). Several experiments were conducted for all of the macromolecules investigated. These included transport from the apical-to-basolateral direction and basolateral-to-apical direction at 37 °C and from the apical-to-basolateral direction at 5 °C.
Prior to the beginning of the experiments, membranes were rinsed with HBSS/CMF in the apical chamber and HBSS/200 in the basolateral chamber twice and allowed to equilibrate in the experimental medium for 2 h. This step was important to ensure that a constant transepithelial electrical resistance (TEER) was obtained, particularly for those plates where experiments were conducted at 5 °C. For this purpose TEER values were frequently monitored. After the equilibration period, the corresponding chamber volume was replaced by controls or nanosphere formulations. At certain time intervals (30, 60, 120, 180, and 240 min), the appropriate sample volume was taken and replaced by fresh medium. For all instances, n = 3 for both controls and experimental wells. Samples were appropriately measured and averaged. For all instances, the permeability coefficient was calculated as illustrated elsewhere (7, 8). The 95% confidence interval was calculated for each case and reported as Papp ± CI. A result is considered significant if the confidence interval did not overlap.
Results and Discussion
We investigated the mechanism of transport of salmon calcitonin through the epithelial cell monolayer in the gastrointestinal tract and examined the feasibility of the P(MAA-g-EG) nanosphere system as a transport enhancer. For this purpose, transport experiments for various macromolecules were performed, and the permeability coefficients were calculated.
Results from the transport of fluorescein sodium are depicted in Figure 1 for various nanosphere formulations. The permeability of fluorescein appeared to be directly related to the content of poly(ethylene glycol) in the structure when investigated from the apical-to-basolateral direction. To confirm these findings, further experimentation was conducted by trying to elucidate which component of the hydrogel system affected the transport of fluorescein. For this purpose, cross-linked poly(ethylene glycol) (PEG) and poly(methacrylic acid) (PMAA) nanospheres were prepared following the same experimental procedures employed to prepare P(MAA-g-EG) nanosopheres. The individual effect on the membrane integrity was monitored by the measurement of the transepithelial electrical resistance (TEER) and the transport of 14C-mannitol for each type of nanosphere. TEER values (data not shown) and mannitol’s permeability were not affected by PEG nanospheres, whereas PMAA nanospheres did exhibit a significant reduction in the TEER (data not shown) and an increase in mannitol permeability when compared to controls (see Figures 1 and 4).
Figure 1.
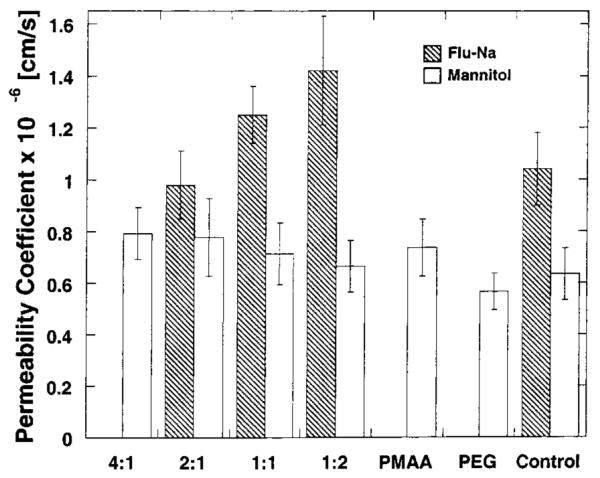
Plot of the permeability coefficients calculated for 14C-mannitol and fluorescein sodium through Caco-2 cell monolayers at 37 °C from the apical-to-basolateral (AB) direction using P(MAA-g-EG) hydrogel nanospheres of composition 4:1, 2:1, 1:1, and 1:2, and cross-linked PMAA and PEG nanospheres. Each bar represents Papp ± 95% CI for n = 3.
Figure 4.
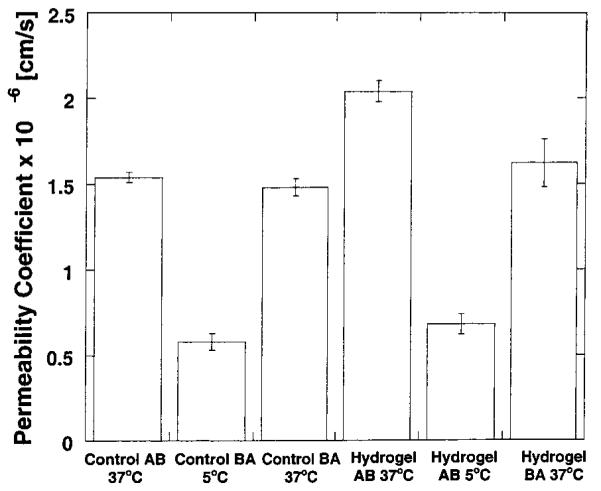
Plot of the permeability coefficients calculated for 14C-mannitol through Caco-2 cell monolayers at 37 and 5 °C from the apical-to-basolateral (AB) direction and basolateral-to-apical direction (BA) using P(MAA-g-EG) hydrogel nanospheres with a composition of the 4:1 ratio. Each bar represents Papp ± 95% CI for n = 3.
Contrary to our expectations, PEG nanospheres demonstrated a dramatic increase in the permeability of fluorescein when experiments were conducted in two directions, as illustrated in Figure 2. It was expected that, given the hydrophilic nature of fluorescein, its transport would be enhanced by the PMAA nanospheres as a result of the opening of the tight junctions, even if there was an active transport mechanism present. However, in a recent work by Batrakova (1, 2, 9, 10), PEG-containing block copolymers known as Pluronic block copolymers revealed that such polymers were capable of increasing the transport of fluorescein and thus were inhibitors for the MRP efflux pump. This behavior may explain the previous findings regarding the slight differences in permeability for nanosphere formulations prepared with different monomer ratios. However, little is known regarding the possible mechanism by which PEG can affect this transport pathway.
Figure 2.
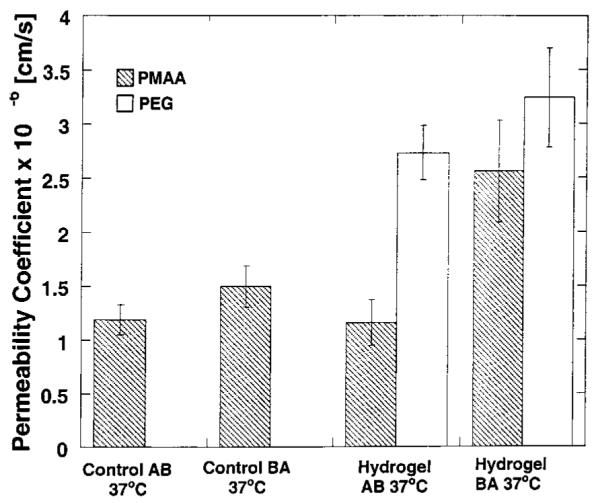
Plot of the permeability coefficients calculated for fluorescein sodium through Caco-2 cell monolayers at 37 °C from the apical-to-basolateral (AB) direction and basolateral-to-apical direction (BA) using PMAA and PEG hydrogel nanospheres. Each bar represents Papp ± 95% CI for n = 3.
It is well-known that the paracellular transport pathway does include a size exclusion component (11). The use of a paracellularly transported molecule with a molecular weight (MW) similar to that of salmon calcitonin can provide information regarding the sieving effects by MW in the transport of hydrophilic macromolecules. Fluorescein isothiocyanate dextran was chosen for this purpose since it possesses a molecular weight similar to that of salmon calcitonin (~4400) but a different structure.
As described, transport experiments with salmon calcitonin and FITC-dextran were conducted under similar conditions to be able to compare results. Figure 3 depicts the results obtained for the transport of the two macromolecules from the apical-to-basolateral direction at two temperatures, 37 and 5 °C, and from the basolateral-to-apical direction. Transport experiments at two temperatures provide information regarding the presence or absence of an active transport mechanism. At low temperatures, active transport mechanisms are decreased significantly. Moreover, transport experiments in two directions provide information regarding the presence of an active mechanism as well.
Figure 3.
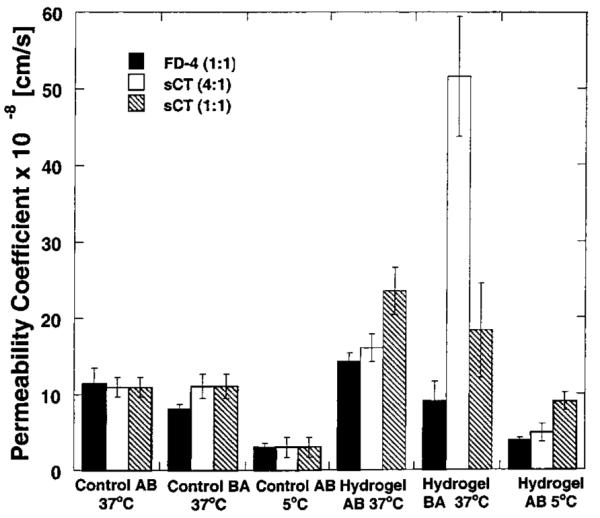
Plot of the permeability coefficients calculated for FITC-dextran and salmon calcitonin through Caco-2 cell monolayers at 37 and 5 °C from the apical-to-basolateral (AB) direction and basolateral-to-apical direction (BA) using P(MAA-g-EG) hydrogel nanospheres. Each bar represents Papp ± 95% CI for n = 3.
The transport of FITC-dextran and salmon calcitonin in the absence of the hydrogel in two directions and temperature revealed that there was no significant difference in the permeability coefficient calculated for both macromolecules (see Figure 3). These similarities in permeability coefficient for both molecules indicated that the sieving component of the paracellular transport dominates when compared to other molecular characteristics, such as composition and structure.
However, a significant decrease in the permeability coefficient at the two temperatures was observed for both macromolecules, which may indicate the presence of an active transport mechanism. Care must be taken in analyzing such results. To ensure the correct analysis of the data, experiments with 14C-mannitol were conducted utilizing the same experimental conditions asfor the transport experiments of FITC-dextran and salmon calcitonin.
Results indicated that a similar reduction in the permeability coefficient is observed (see Figure 4) when mannitol was investigated. These findings confirm that the reduction in permeability coefficient as a consequence of the reduction in experimental temperature is due to changes in membrane and media viscosity and not due to the presence of an active transport mechanism.
The presence of the nanosphere suspension produced an apparent increase in the transport of both macromolecules (see Figure 3) regardless of directionality and temperature. The effect of hydrogel composition in the transport of calcitonin was investigated by performing the experiments with two formulations composed of two monomer ratios (4:1 and 1:1). FITC-dextran experiments were conducted with one ratio (1:1) only. The transport of calcitonin was slightly different in the presence of the formulation composed of the 1:1 ratio nanospheres. However, the comparison cannot be fully performed since the permeability of calcitonin from the 4:1 formulation in the basolateral-to-apical direction was performed under different conditions than the transport with the 1:1 formulation. The protocol difference lies in the fact that the calcitonin solution for the 4:1 ratio was not equilibrated in a solution containing the 200 μmol/L of calcium, rather it was allowed to chelate calcium during the experiment evidencing the importance of the basolateral calcium for the integrity of the membrane.
Conclusions
Transport experiments were conducted by varying the temperature and the direction of transport. P(MAA-g-EG) nanospheres were capable of increasing the transport of the macromolecules fluorescein sodium, fluorescein isothiocyanate dextran, salmon calcitonin, and 14C-mannitol, when compared to controls.
Fluorescein sodium salt solutions were also investigated as an actively transported molecule. The transport of fluorescein exhibited a proportional dependency on the PEG content of P(MAA-g-EG) nanospheres. This was further observed when cross-linked PEG nanospheres were examined. The transport of fluorescein was highly enhanced by the presence of PEG nanospheres when compared to PMAA nanospheres. These observations provided evidence that PEG chains were interacting with the cell monolayer. However, the mechanisms of such interactions are still known.
The comparison of the transport data of FTIC-dextran and salmon calcitonin without the hydrogel nanospheres at the two temperatures and directions revealed that the permeability coefficients were similar. This indicates that the sieving effect of the cell monolayer is an important factor. Furthermore, the comparison of the transport behavior of FITC-dextran and calcitonin indicated that the main transport mechanism for salmon calcitonin was the paracellular route. This route did not appear to be affected by the presence of the hydrogel.
This information is invaluable for the optimization and future design of oral delivery systems not only for calcitonin but for other therapeutic proteins as well.
Acknowledgment
We wish to acknowledge funding by a grant from the National Institutes of Health, GM 43337, and partial support by the National Science Foundation grant DGE 99-72770.
References and Notes
- (1).Batrakova EV, Han H, Alakhov VY, Miller DW, Kabanov AV. Effects of Pluronic Block Copolymers on Drug Absorption in Caco-2 Cell Monolayers. Pharm. Res. 1998;15:850–855. doi: 10.1023/a:1011964213024. [DOI] [PubMed] [Google Scholar]
- (2).Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 Increases Permeability of a Broad Spectrum of Drugs in Polarized BBMEC and Caco-2 Cell Monolayers. Pharm. Res. 1999;16:1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- (3).Gutmann H, Fricker G, Torok M, Michael S, Beglinger C, Drewe J. Evidence for Different ABC-Transporters in Caco-2 Cells Modulating Drug Uptake. Pharm. Res. 1999;16:402–407. doi: 10.1023/a:1018825819249. [DOI] [PubMed] [Google Scholar]
- (4).Borchard G, Luessen HL, de Boer AG, Verhoef JC, Lehr CM, Junginger HE. The Potential of Mucoadhesive Polymers in Enhancing Intestinal Peptide Drug Absorption. III: Effects of Chitosan-glutamate and Carbomer on Epithelial Tight Junctions In Vitro. J. Controlled Release. 1996;39:131–138. [Google Scholar]
- (5).Luessen HL, Lehr CM, Rentel CO, Noach ABJ, de Boer AG, Verhoef JC, Junginger HE. Bioadhesive Polymers for the Peroral Delivery of Peptide Drugs. J. Controlled Release. 1994;29:328–338. [Google Scholar]
- (6).Lehr CM. From Sticky Stuff to Sweet Receptors, Achievements, Limits, and Novel Approaches to Bioadhesion. Eur. J. Drug Metab. Pharmacokinet. 1996;21:139–148. doi: 10.1007/BF03190262. [DOI] [PubMed] [Google Scholar]
- (7).Artursson P. Epithelial Transport of Drugs in Cell Culture. I: A Model for Studying the Passive Diffusion of Drugs over Intestinal Absorptive (Caco-2) Cells. J. Pharm. Sci. 1990;79:476–482. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- (8).Hidalgo IJ, Hillgreen KM, Grass GM, Borchardt RT. Characterization of the Unstirred Water Layer in Caco-2 Cell Monolayers Using Novel Diffusion Apparatus. Pharm. Res. 1991;8:222–227. doi: 10.1023/a:1015848205447. [DOI] [PubMed] [Google Scholar]
- (9).Batrakova EV, Lee S, Li S, Venne A, Alakhov VY, Kabanov AV. Fundamental Relationship between the Composition of Pluronic Block Copolymers and Their Hypersensitization Effect in MDR Cancer Cells. Pharm. Res. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- (10).Miller DW, Batrakova EV, Kabanov AV. Inhibition of Multidrug Resistance Associated Protein (MRP) Functional Activity with Pluronic Block Copolymers. Pharm. Res. 1999;16:396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- (11).Pauletti GM, Okumu FW, Borchardt RT. Effect of Size and Charge on the Passive Diffusion of Peptides Across Caco-2 Cell Monolayers via the Paracellular Pathway. Pharm. Res. 1997;14:164–168. doi: 10.1023/a:1012040425146. [DOI] [PubMed] [Google Scholar]


