Abstract
Insulin-loaded polymer microparticles (ILP) composed of crosslinked poly(methacrylic acid) and poly(ethylene glycol) are multi-functional carriers showing high insulin incorporation efficiency, a rapid insulin release in the intestine based on their pH-dependent complexation properties, enzyme-inhibiting effects and mucoadhesive characteristics. Thus, they are potential carriers for insulin delivery via an oral route. Recent studies suggest that the polymer composition and particle size of ILP strongly influenced insulin bioavailability. Therefore, the present study aimed at finding an optimal formulation and designing carriers for oral insulin delivery using in vivo experiments. Various types of ILPs were prepared and administered orally to healthy and type 1 and 2 diabetic rats. The most promising formulation was subsequently used for in vivo multiple oral administration studies using diabetic rats. The microparticles of diameters of <53 µm (SS-ILP) composed of a 1:1 molar ratio of methacrylic acid/ethylene glycol units showed the most pronounced hypoglycaemic effects following oral administration to healthy rats, achieving a 9.5% pharmacological availability compared to subcutaneous insulin injection. Their usefulness was also confirmed with both type 1 and 2 diabetic rat groups. In a multiple administration study, SS-ILP significantly suppressed the postprandial rise in blood glucose and showed continuous hypoglycaemic effects following 3 times/day oral administration to both diabetic rat groups in the presence of foods. These results indicate that the blood glucose levels of diabetic rats can be effectively controlled by oral SS-ILP administration, and thus SS-ILP would be a promising delivery carrier of insulin via the oral route.
Keywords: Complexation polymers, Delivery, Insulin, Multiple administration, Oral
1. Introduction
Over the past several decades, biotechnology has developed extensively and led to a significant increase in the number of bioengineered products. The progress of these fields has had a great impact on the therapeutic agents used to treat many diseases, such as diabetes, etc., which are often difficult to treat with current medicines. Most of these products are peptides and proteins, which are generally administered via the parenteral route. However, oral administration is the most desirable route for taking these drugs because of ease of administration, thus resulting in good patient compliance.
Insulin remains the most effective drug for a diabetic patient to control their blood glucose levels. The route for insulin delivery is restricted to subcutaneous injections, as opposed to the oral route, due to insulin inactivation by proteolytic enzymes in the gastrointestinal tract and low permeability through the intestinal membrane [1,2]. However, the subcutaneous injection of insulin has various disadvantages such as hyperinsulinemia, pain, allergic reactions and low patient compliance. Another important issue with insulin is that parenteral administration does not replicate the normal dynamics of endogenous insulin release, resulting in a failure to achieve a lasting glycaemic control in patients [3,4]. The portal delivery of insulin, which mimics endogenous insulin release, can be achieved via intestinal administration. Obviously, from this perspective, the development of an oral delivery system providing adequate bioavailability of insulin would revolutionize the treatment of diabetes. Moreover, it can be anticipated that such a technique could be applied to delivery of other proteinic drugs.
To date, various strategies have been developed to try and achieve an oral insulin delivery system, including co-administration with absorption enhancers [5,6] or enzyme inhibitors [6,7], chemical modification [8], polymeric carriers [9–11], lipid-based carriers as liposomes [12] and solid lipid nanoparticles [13]. Nevertheless, these approaches show low bioavailability and also some of them exhibit several negative effects such as irritation of the intestinal mucosal membrane and impairment of the membrane barrier. We have previously reported that copolymer hydrogel networks of poly(methacrylic acid) grafted with poly(ethylene glycol) (P(MAA-g-EG)) is a promising class for oral carriers of insulin [14–17]. Insulin-loaded P(MAA-g-EG) microparticles (henceforth designated as ILP) (with a molar ratio of MAA:EG = 1:1 and particle diameters of 100 to 150 µm) successfully enhanced oral insulin absorption in healthy and streptozotocin-induced type 1 diabetic rats, with up to 4.2% bioavailability [14]. The safety of ILP to the mucosal membrane has also been reported [16,17]. However, our recent study suggests that the molar ratio of MAA:EG is an important parameter as it affects the insulin release behavior from microparticles, the ability of the carrier to protect insulin from enzymes, and the adhesive characteristics on the mucus membrane [15]. In addition, our previous in situ absorption study showed that the ILP particle size influenced strongly the intestinal insulin absorption with the smaller ILP size greatly enhancing insulin absorption, leading to a 12.8% bioavailability [16]. Based on observations obtained from the in vitro and in situ experiments, the present study aimed to identify an optimal formulation and prove the ability of the prepared particles to act as carriers for oral insulin delivery in in vivo experiments. The most promising formulation would be used for in vivo single and multiple oral administration studies using type 1 and 2 diabetic rats.
2. Materials and methods
2.1. Materials
Methacrylic acid (MAA), dimethoxy propyl acetophenone (DMPA) and streptozotocin were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Tetraethylene glycol dimethacrylate (TEGDMA) and poly(ethylene glycol) monomethylether monomethacrylate (PEGMA, with nominal PEG molecular weight of 1000, corresponding to 23 repeating units) were purchased from Polysciences Inc. (Warrington, PA, USA). Crystalline recombinant human insulin (26.0 IU/mg) was obtained from Wako Pure Chemical Industries Co., Ltd. (Osaka, Japan). All other chemicals were of reagent grade and were used without further purification.
2.2. Synthesis of complexation hydrogels
P(MAA-g-EG) microparticles were synthesized by a UV-initiated free radical solution polymerization of MAA and PEGMA. The monomers were mixed together with molar feed ratio of solutions with 1:0, 4:1 and 1:1 MAA:EG units. TEGDMA was used as a crosslinking agent and was added in the amount of 0.75 mol% of the total monomers. The monomer mixture was diluted with a 1:1 by weight mixture of ethanol and water. Nitrogen was bubbled through the well-mixed solutions for 30 min to remove dissolved oxygen, which could act as a free radical scavenger. The photoinitiator DMPA was added in the amount of 1 wt.% of the monomers in a nitrogen atmosphere. The reaction mixture was pipetted between glass plates separated by Teflon spacers of 0.9 mm thickness and exposed to UV light (Ultracure 100, Efos Inc., Buffalo, NU, USA) with an intensity of 1 mW/cm2 at 365 nm for 30 min. The ensuing hydrogel films were removed and rinsed for 5 days in deionized water (changed daily) to remove the unreacted monomers and sol fraction. Then, the copolymers were dried under vacuum at room temperature for 2 days and crushed and sieved to powders with diameters of <53 (Super-Small size: SS size) and 212–300 (Large size: L size) µm.
2.3. Insulin incorporation
Insulin incorporation was performed by equilibrium partitioning into complexation hydrogels as described previously [15]. Briefly, crystalline human insulin (10 mg) was dissolved in 200 µL of 0.1 M HCl. The insulin solution was diluted with 19.6 mL of phosphate-buffered saline (PBS, pH = 7.4) and pH was adjusted to 7.4 with 200 µL of 0.1 M NaOH. Loading was accomplished by imbibing 140 mg of dried microparticles for 2 h in the insulin solution. The particles were collapsed with 20 mL of 0.1 M HCl and filtered using cellulose acetate/cellulose nitrate filter paper with 1.0 µm pores. The insulin-loaded polymers were dried under vacuum and stored at 4 °C prior to use in further studies. Insulin incorporation efficiency was determined by HPLC as described previously [15].
2.4. In vivo absorption study
This research complied with the regulations of the Committee on Ethics in the Care and Use of Laboratory Animals of Hoshi University. Male Wistar rats (180–200 g) were purchased from Sankyo Lab Service Co., Ltd. (Tokyo, Japan). Animals were housed in rooms controlled between 23 ± 1 °C and 55 ± 5% relative humidity and allowed free access to water and food during acclimatization. Type 1 diabetes was induced by intravenous injection of streptozotocin (55 mg/kg body weight) dissolved in a citrate buffer at pH 4.5. As a spontaneous model for type 2 diabetes [18], Goto-Kakizaki (GK) rats were used because GK rats display malfunctional insulin secretion against glucose and mild hyperglycaemia. GK rats were purchased from Sankyo Lab Service Co., Ltd. (Tokyo, Japan). The average pre-dose blood glucose levels of the healthy, type 1 and type 2 diabetic rats used in this study were 99, 319 and 149 mg/dL, respectively.
During the experimental period, rats were housed in cages and given water ad libutum. In this study, drug administration and blood sampling were conducted without anesthesia. Gelatin capsules (Qualicaps® capsule, Shionogi Qualicaps Co., Ltd, Nara, Japan) containing ILP (approximately 1, 3, and 6 mg at the insulin doses 10, 25, and 50 IU/kg, respectively) or insulin solutions were orally administered using a sonde needle.
In a single administration study in healthy rats, the insulin doses were 10, 25, and 50 IU/kg. In the case of type 1 and 2 diabetic rats, one insulin dose of 25 IU/kg was used. In order to calculate the efficacy of oral insulin administration relative to subcutaneous insulin, insulin solutions (1.0 IU/kg) were administered subcutaneously to healthy rats. Insulin solutions were prepared by dissolving an appropriate amount of crystalline human insulin in PBS. Rats were fasted for 48 h prior to the experiment. During the experiment, a 0.2 mL aliquot of blood was collected from the jugular vein.
In the multiple administration study, type 1 and 2 diabetic rats were fasted for 12 h prior to the study, and administered with ILP 3 times/day. The insulin dose was fixed at 25 IU/kg. In the case of the multiple administration study, ILP (25 IU/kg) was also administered in combination with subcutaneous insulin (0.1 IU/kg), but only at the first dosing. The diabetic rats were fed for 30 min, 30 min after each drug administration and allowed free access to water. The hypoglycaemic effects of SS-ILP were influenced by the fasting period of rats. Although the hypoglycaemic effects were decreased by shortening the fasting period, sufficient insulin’s effects were observed following oral administration of SS-ILP to the non-fasted rats (data not shown in this contribution). These results suggest that SS-ILP may have the ability to reduce the blood glucose levels sufficiently under a feeding condition. In this study, therefore, the diabetic rats were fed for 30 min after each drug administration in the multiple oral administration study.
After blood sampling, the plasma was separated by centrifugation at 13,000 rpm for 1 min and kept at 4 °C until insulin concentration analysis. The plasma insulin concentrations were determined the same day of the absorption study by an immuno-chemiluminometric assay (MLT Research Limited, Wales, UK) using a microplate luminometer (Mithras LB940, Beltold Japan Co., Ltd., Osaka, Japan). Blood glucose levels were determined using a glucose meter (Novo Assist Plus, Novo Nordisk Pharma Ltd., Tokyo, Japan). Post-dose blood glucose levels were expressed as a percentage of pre-dose blood glucose levels.
The extent of hypoglycaemic response was calculated as the area above the blood glucose level-time curve ([AAC]p.o. and [AAC]s.c. (% glu.reduc. · h) for oral and subcutaneous insulin to healthy rats, respectively) for 0–8 h. The relative pharmacological availability (PA(%)) of the orally administered insulin was calculated according to Eq. (1) in which [AAC]s.c. was determined after subcutaneous administration of 1.0 IU/kg of insulin.
| (1) |
2.5. Statistical analysis
The results were expressed as the mean ± S.E. For group comparison, an analysis of variance (ANOVA) with a one-way layout was applied. Significant differences in mean values were evaluated by a Student’s t-test. A difference was considered to be statistically significant when the P value was less than 0.05.
3. Results
3.1. Influence of the MAA/EG molar composition of the carrier hydrogel on oral insulin absorption
P(MAA-g-EG) microparticles were synthesized by a UV-initiated free radical solution polymerization of MAA and PEGMA. The copolymer microparticles were sieved to powders with diameters of <53 (SS size) and 212–300 (L size) µm. The incorporation efficiency was unaffected by the size of ILP, and 86.4 ± 1.0% and 88.4 ± 1.0% of the insulin in the initial solution was loaded into the L-ILP and SS-ILP, respectively. All in vivo studies utilized insulin doses that were 10, 25, and 50 IU/kg. In the case of type 1 and 2 diabetic rats, one insulin dose of 25 IU/kg was used. In order to calculate the efficacy of oral insulin administration relative to subcutaneous insulin, insulin solutions (1.0 IU/kg) were administered subcutaneously to healthy rats.
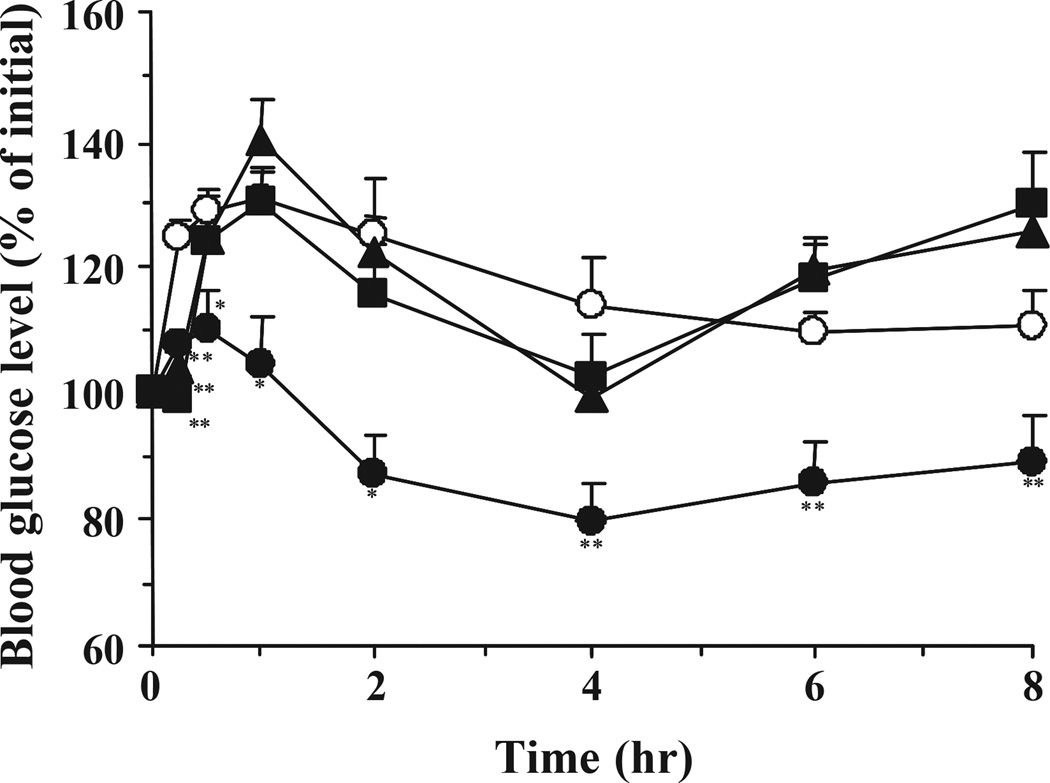
Fig. 1 shows the blood glucose levels versus time profiles following oral administration of ILPs (<53 µm) with different molar ratios of EG to MAA at a dose of 25 IU/kg to healthy rats. First, it is clear that no hypoglycaemic effect was observed during administration of pure insulin solution, demonstrating the well known absence of insulin absorption via the oral route in the absence of a suitable carrier. Rather, blood glucose levels rapidly increased due to the physical stress during oral administration and blood sampling. Over the course of the study period, blood glucose levels did not return to the initial levels. However, the oral administration of insulin-loaded microparticles prepared with a 1:1 molar ratio of MAA/EG units suppressed the initial rise in blood glucose levels as compared with other formulations. In the case of the microparticles prepared from 4:1 and 1:0 molar ratios of MAA/EG, a stress-induced increase in blood glucose levels occurred, as with the insulin solution, and there were no significant differences in blood glucose level-time profiles between control and both microparticle groups. The blood glucose levels of the rats decreased remarkably, achieving a maximum hypoglycaemic effect at 4 h, and with effects continuing up to 8 h.
Fig. 1.
Changes in blood glucose level versus time profiles following oral administration of P(MAA-g-EG) microparticles containing different ratios of MAA:EG in healthy male Wistar rats fasted for 48 h. MAA:EG = 1:0 (closed squares, n = 6), 4:1 (closed triangles, n = 6), and 1:1 (closed circles, n = 12). Insulin solution was used as control (open circles, n = 12). The dose of insulin was 25 IU/kg body weight. Each value represents mean ± S.E. Statistically significant difference from control: p <0.01, **; p <0.05, *.
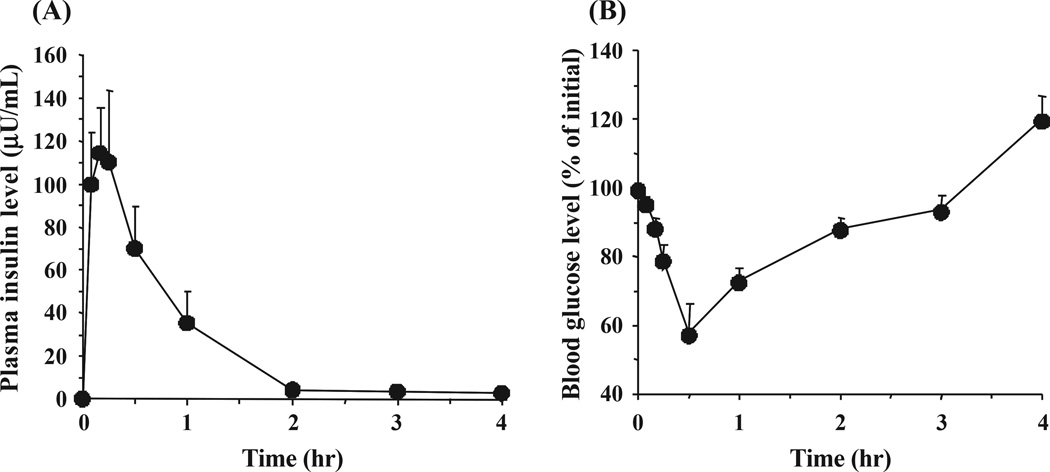
Fig. 2 shows insulin and blood glucose level-time profiles following subcutaneous injection of an insulin solution to healthy rats at a dose of 1.0 IU/kg. Table 1 shows the values of the hypoglycaemic response, the AAC, and the relative pharmacological availability of each formulation calculated from the comparison of the AAC values to that of s.c. injection. While there is no significant improvement of the values of PA in the in vivo studies with administration of pure insulin solution, or insulin-loaded microparticles prepared from 1:0 and 4:1 molar ratios of MAA/EG, a remarkably higher PA was observed when using formulations based on ILPs with a 1:1 molar ratio of MAA/EG.
Fig. 2.
(A) Plasma insulin and (B) blood glucose level versus time profiles in healthy male Wistar rats following subcutaneous injection of insulin solution. The dose of insulin was 1.0 IU/kg body weight. Each value represents mean ± S.E. (n = 3).
Table 1.
Pharmacological availability of various ILPs administered orally to Wistar rats
| Preparation | AAC (% glu. reduc. · h) | PA (%) |
|---|---|---|
| Insulin solution | 1.3 ± 0.8 | 0 |
| P(MAA-g-EG) microparticles | ||
| MAA:EG = 1:0 | 2.8 ± 1.7 | 0.2 ± 0.1 |
| MAA:EG = 4:1 | 2.5 ± 1.9 | 0.2 ± 0.1 |
| MAA:EG = 1:1 | 106.1 ± 47.3** | 7.4 ± 3.3** |
AAC denotes area above the curve.
PA denotes pharmacological availability.
Each value represents mean ± S.E.
Statistically significant difference from insulin solution: p <0.01, **.
3.2. Influence of ILP particle size on oral insulin absorption
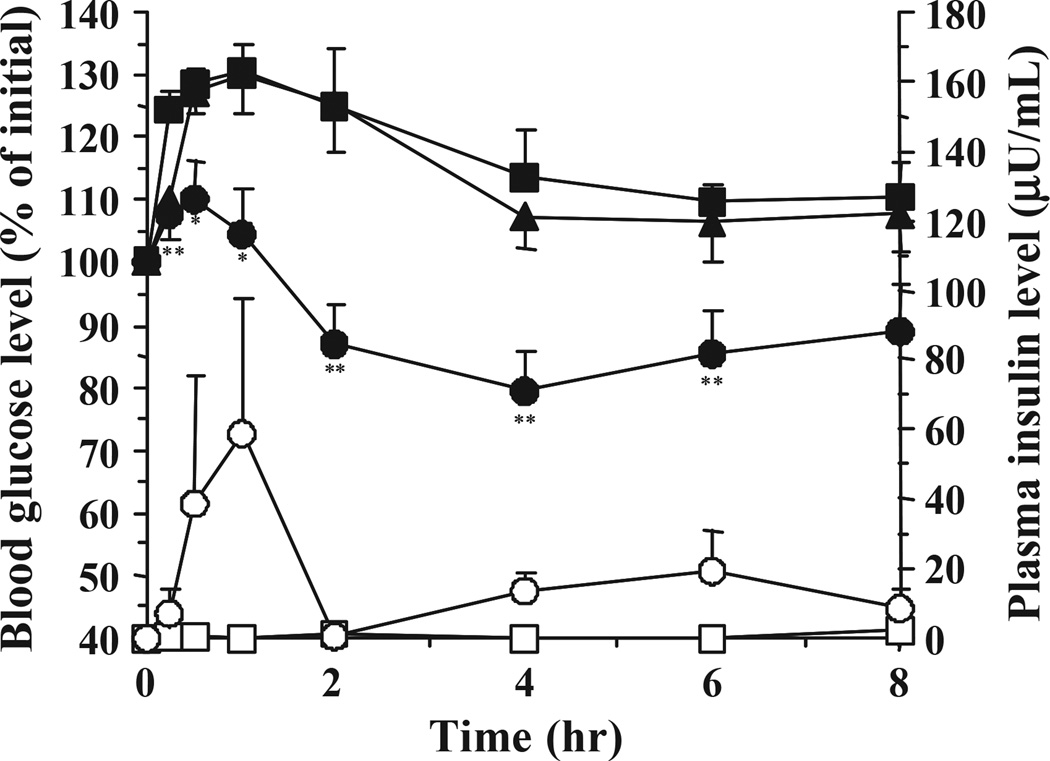
Fig. 3 shows the insulin and blood glucose level profiles following oral administration of the 1:1 molar ratio of MAA/EG microparticles with diameters of <53 (SS-ILP) and 212–300 (L-ILP) µm and with a dose of 25 IU/kg. The L-ILP samples did not induce any hypoglycaemic effects and their profiles were close to those observed in the control group. In contrast, the SS-ILP induced insulin absorption from the gastrointestinal tract, and resulted in significant hypoglycaemic effects. This observation was consistent with our previous results [16]. In our previous study, conducted by the in situ absorption method, we found that the smaller sized microparticles induced larger hypoglycaemic effects. This was due to a higher mucoadhesive capacity [16] of the smaller sized particles and a rapid insulin release, which are characteristics that may be highly advantageous to increasing insulin absorption from the gastrointestinal tract.
Fig. 3.
Changes in plasma insulin (open symbol) and blood glucose level (closed symbol) versus time profiles following oral administration of L-ILP (triangles, n = 6), and SS-ILP (circles, n = 12) in healthy male Wistar rats fasted for 48 h. Insulin solution was used as control (square, n = 12). The dose of insulin was 25 IU/kg body weight. Each value represents mean ± S.E. Statistically significant difference from control: p <0.01, **; p <0.05, *.
A dose-dependent insulin study was performed and the PA values following oral administration of ILPs at doses of 10, 25 and 50 IU/kg are summarized in Table 2. No pharmacological responses were observed following administration of an insulin solution even at the highest dose, 50 IU/kg. On the other hand, the ILPs exhibited high PA values. Although the PA values of ILPs decreased as insulin doses increased, the PA values were greater in SS-ILPs than L-ILPs at all insulin doses. Therefore, the SS-ILP formulation was selected as the most promising one and used for the in vivo single and multiple administration studies using diabetic rats.
Table 2.
Pharmacological availability of various ILPs administered orally to Wistar rats
| Dose | Preparation | AAC | PA | ||||
|---|---|---|---|---|---|---|---|
| (IU/kg) | (% glu. reduc. · h) | (%) | |||||
| 10 | Insulin solution |
* |
0.7 ± 0.6 |
** |
* |
0 |
** |
| L-ILP | 19.0 ± 16.6 | 3.3 ± 2.9 | |||||
| SS-ILP | 54.6 ± 11.6 | 9.5 ± 2.0 | |||||
| 25 | Insulin solution |
* |
1.3 ± 0.8 |
** |
* |
0 |
** |
| L-ILP | 4.7 ± 3.2 | 0.3 ± 0.2 | |||||
| SS-ILP | 106.1 ± 47.3 | 7.4 ± 3.3 | |||||
| 50 | Insulin solution |
* |
1.7 ± 1.2 |
** |
* |
0 |
** |
| L-ILP | 14.3 ± 9.9 | 0.5 ± 0.3 | |||||
| SS-ILP | 51.5 ± 10.3 | 1.8 ± 0.3 | |||||
AAC denotes area above the curve.
PA denotes relative pharmacological availability.
Each value represents mean ± S.E.
Statistically significant difference between the preparation: p <0.01, **;
p <0.05, *.
3.3. Hypoglycaemic effects following the single oral administration of SS-ILP to diabetic rats
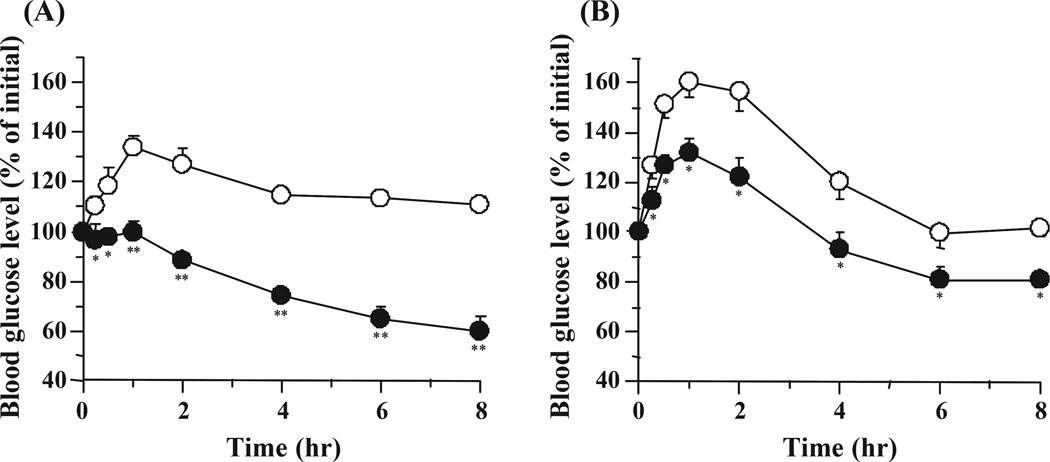
Fig. 4 shows the hypoglycaemic effect following a single oral administration of SS-ILP to diabetic type 1 and 2 rats at a dose of 25 IU/kg. The oral administration of an insulin solution to type 1 and 2 diabetic rats did not decrease the blood glucose levels, however, a significant glucose reduction was observed in both diabetic rat groups following oral administration of SS-ILP. The strong hypoglycaemic effect was observed during the entire study period in both rat groups and blood glucose levels did not return to the initial levels. These observations clearly demonstrate that the SS-ILP is an effective formulation for oral insulin delivery to diabetic rats, irrespective of the diabetes type.
Fig. 4.
Changes in blood glucose level versus time profiles following oral administration of SS-ILP (closed circles, n = 5) in (A) type 1 and (B) type 2 diabetic rats fasted for 24 h. Insulin solution was used as control (open circles, n = 5). The dose of insulin was 25 IU/kg body weight. Each value represents mean ± S.E. Statistically significant difference from control: p <0.01, **; p <0.05, *.
3.4. Hypoglycaemic effects following the multiple oral administration of SS-ILP to diabetic rats
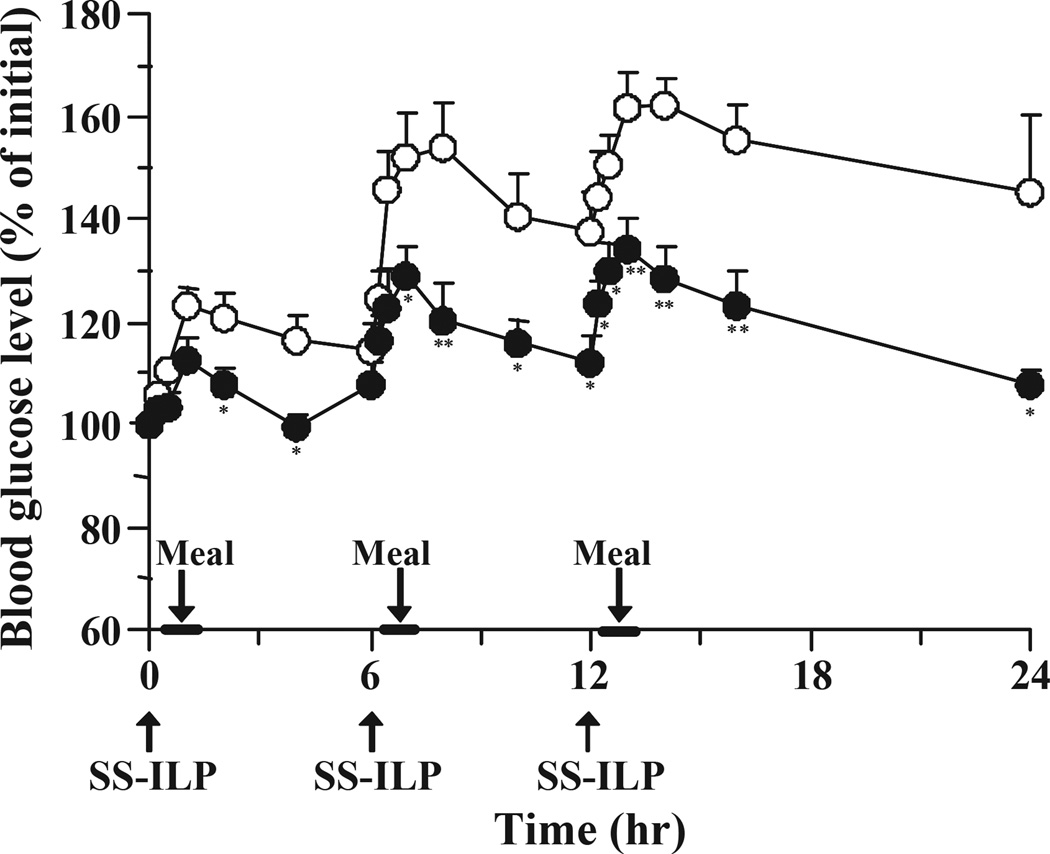
In order to ensure the usefulness of the SS-ILP formulations we carried out a study in multiple oral dosing, as would be needed for a diabetes regimen. The SS-ILP was administered 3 times/day to type 1 and 2 diabetic rats. Fig. 5 shows the blood glucose level-time profiles following multiple oral administration of SS-ILP to type 1 diabetic rats. As shown in Fig. 5, SS-ILP significantly suppressed the postprandial rise in blood glucose and showed continuous hypoglycaemic effects. In addition, the blood glucose levels prior to each meal could be kept almost the same as the pre-dose values. These results suggest that the multiple oral administration of SS-ILP was highly effective for controlling the glucose level after meals. In contrast, in the insulin solution administration group, glucose levels increased after the meal and did not return to the initial values. Therefore, the pre-dose value prior to the second and the third oral administration of insulin solution was substantially higher than the baseline values.
Fig. 5.
Changes in blood glucose level versus time profiles in type 1 diabetic rats following multiple oral administration of SS-ILP (closed circles). Insulin solution was used as control (open circles). Each dose of insulin was 25 IU/kg body weight. Each value represents mean ± S.E. (n = 5 – 10). Statistically significant difference from control: p <0.01, **; p <0.05, *.
In the type 2 diabetic rat group, the overall blood glucose levels tended to fluctuate more than those observed in the streptozotocin-induced type 1 diabetic rat group (Fig. 6). However, SS-ILP significantly suppressed the postprandial rise in blood glucose levels in the type 2 diabetic rat group, similarly to the type 1 diabetic rat group.
Fig. 6.
Changes in blood glucose level versus time profiles in type 2 diabetic rats following multiple oral administration of SS-ILP (closed circles). Insulin solution was used as control (open circles). Each dose of insulin was 25 IU/kg body weight. Each value represents mean ± S.E. (n = 6). Statistically significant difference from control: p <0.01, **; p <0.05, *.
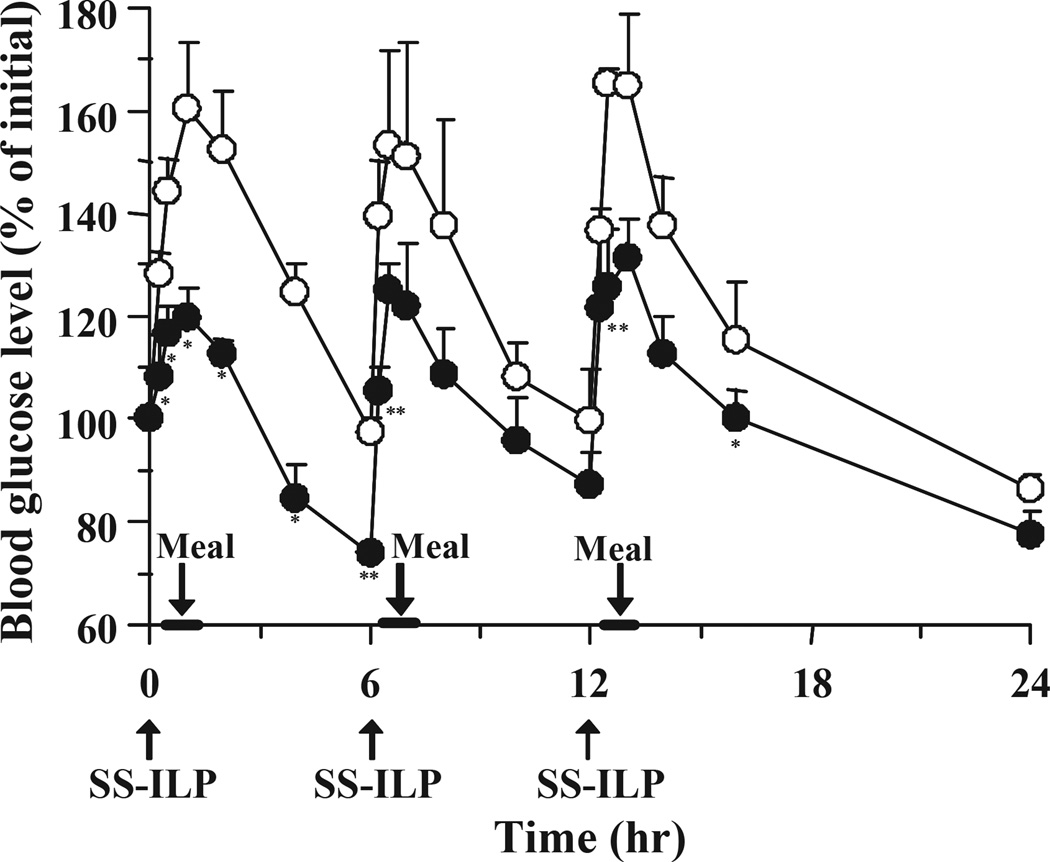
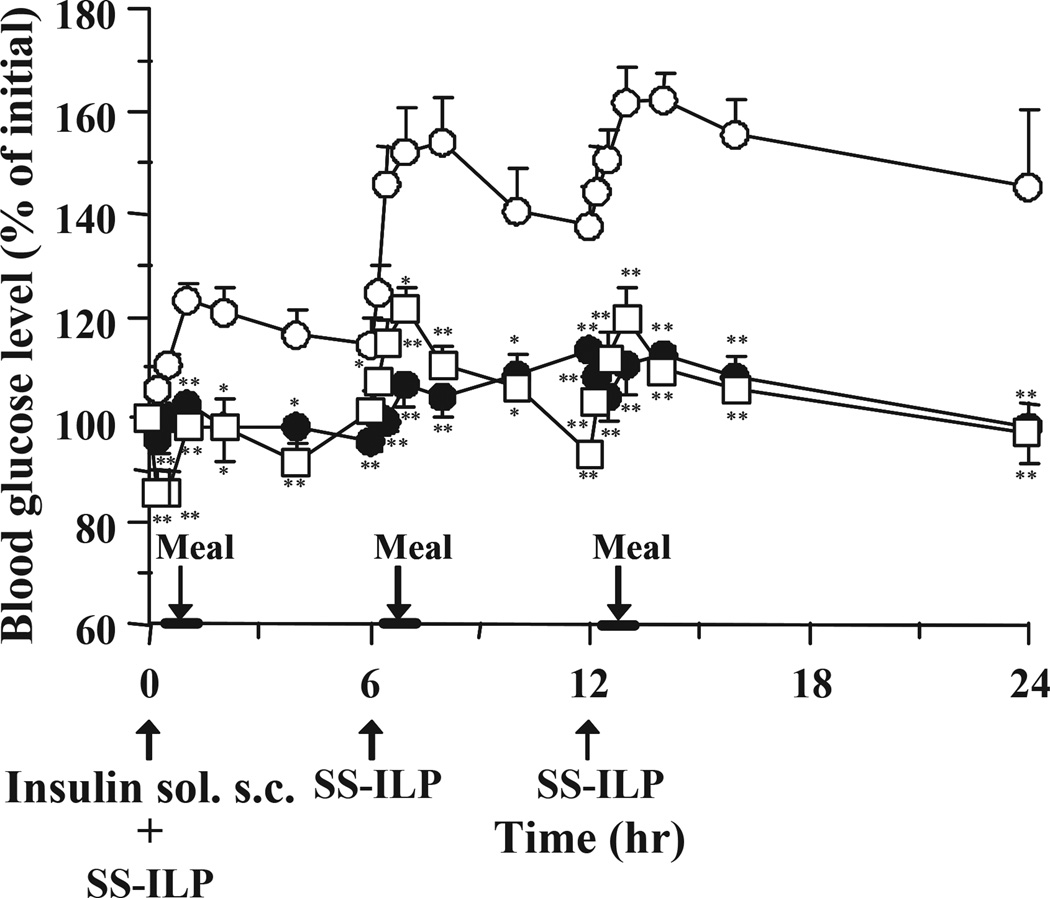
As shown in Fig. 5, multiple oral administration of SS-ILP caused a significant decrease of glucose levels compared with control, however the glucose levels after a 24-h administration were slightly higher than the baseline levels. Therefore, in order to avoid increasing the pre-dose glucose levels, an insulin s.c. injection was administered to type 1 diabetic rats together with the oral SS-ILP at the first dosing (Fig. 7). As shown in Fig. 7, co-administration of oral ILP and an insulin s.c. injection strongly suppressed the initial rises of glucose levels after the first meal. In addition, throughout the study period, blood glucose levels could be kept low by only one s.c. injection along with multiple oral SS-ILP administrations.
Fig. 7.
Changes in blood glucose level versus time profiles in type 1 diabetic rats following multiple oral administration of SS-ILP and subcutaneous insulin (only at the first dosing) (open square), insulin solution (open circles) and subcutaneous administration of insulin solution (closed circles). Insulin solution was used as control (open circles). The dose of insulin was 25 IU/kg (oral) and 0.1 IU/kg (subcutaneous) body weight. Each value represents mean ± S.E. (n = 5 – 10). Statistically significant difference from control: p <0.01, **; p <0.05, *.
4. Discussion
Peptides and proteins are often administered parenterally for systemic treatment because of their inherent instability in the gastrointestinal tract and their low permeability across biological membranes, which is a result of their high molecular weight and hydrophilic nature. Thus, the preparation of oral delivery systems for peptide and protein drugs may be the most difficult in the field of drug development. Despite numerous studies, oral bioavailability of insulin is quite low and normally insufficient for producing an effective systemic effect [19,20]. Therefore, it was essential to develop effective and reliable oral delivery systems for this class of drugs. For such a development to succeed, oral peptide and protein drug delivery systems should contain at least two essential requirements. First, the system should protect the drugs from enzymatic degradation. Second, the system should increase the drug permeability within in the intestinal membrane. A formulation that satisfies these requirements is encapsulation or incorporation, which is the most widely used formulation strategy for peptide and protein delivery systems. To date, various polymeric carriers have been used to develop such drug delivery systems, however, the carriers have not shown sufficient bioavailability when administered by the oral route. In the case of insulin, an oral delivery system which can successfully control blood glucose levels following multiple oral administrations under feeding conditions has not yet been reported.
In order to develop therapeutically effective oral insulin systems, we prepared complexation hydrogels and studied their usefulness. These hydrogels were shown to exhibit pH-dependent swelling behavior due to the formation/dissociation of interpolymer complexes [21–23]. In the acidic environment of the stomach, these copolymers form interpolymer complex due to hydrogen bonding between etheric groups of the graft PEG chain and the acidic protons of the MAA network [21,22]. Under these conditions, the network mesh size is significantly decreased and diffusion of insulin through the network is prevented [15,23,24]. Hence, insulin loaded into the hydrogels can be protected from proteolytic degradation by gastric enzymes such as pepsin. In the basic/neutral environment of the small intestine, the complexes immediately dissociate due to repulsion of ionized pendant acid groups and the network’s pore size rapidly increases, leading to the release of insulin. Furthermore, P(MAA-g-EG) was shown to protect insulin from proteolytic degradation by inhibiting calcium dependent proteases such as trypsin and alpha-chymotrypsin [25]. In addition, the network exhibits mucoadhesive characteristics due to the graft PEG chains which act as anchors for the mucus layer [26]. These observations suggest that P(MAA-g-EG) will have a promising effect in an in vivo situation. Therefore, in this study we attempted to prove their potential as an oral insulin carrier.
As shown in Fig. 1, an increase of the EG molar ratio of P(MAA-g-EG) significantly increased the hypoglycaemic effect, with administration using the 1:1 molar ratio microparticles of MAA/EG showing the highest PA values (Table 1). These results suggest that the PEG chains in the hydrogels play a critical role in oral insulin delivery. Polyethylene glycol chains are the essential component for exhibiting pH-dependent swelling, since the interpolymer complexes are formed by hydrogen bonding between the carboxylic acid on MAA and the ether group on grafted PEG chains. We previously reported that PEG chains strongly influence the mesh size of the hydrogel networks [21]. As the EG molar ratio increased, the mesh size significantly decreased. Therefore, an increase in the EG molar ratio provided a network having a small enough mesh size to limit insulin diffusion in low pH environments, while in neutral pH environments it provided a large enough mesh size for insulin to diffuse in and out of the network. In fact, both 4:1 and 1:0 ratios of MAA/EG microparticles released more than 50% of incorporated insulin in the acidic environment [15], thereby large amount of insulin may then be degraded by proteolytic enzymes in the stomach in this study. In contrast, a 1:1 ratio of MAA/EG microparticles strongly suppressed insulin release in the acidic environment, which avoided the proteolytic degradation of insulin in the stomach. Insulin could then rapidly be released in the small intestine. This resulted in higher PA values of the 1:1 ratio of MAA/EG microparticles.
Another important factor of PEG chains is the enhancement effect of mucoadhesion of the hydrogels. It was reported that the tethered PEG chains enhanced adhesion to the mucus layers due to interdiffusion and entanglements with mucus layers [27]. Therefore, the mucoadhesive effects of a 1:1 ratio of MAA/EG microparticles would be stronger than that of a 1:0 or 4:1 ratio of MAA/EG microparticles. Insulin degradation by pancreatic proteases, such as trypsin and chymotrypsin, occurs in both intestinal fluid and the mucus/glycocalyx layers [1,2]. Interpenetration of the carriers into the mucus/glycocalyx layers might lead to a reduction in proteolytic degradation because insulin is released more closely to the surface of the intestinal epithelial cells.
The bioadhesive capacity of the polymer would also be dependent on particle size. Therefore, we hypothesized that a smaller size of ILP would cause a greater hypoglycaemic effect as compared with larger sized ILPs. As expected, the SS-ILP, having a particle size less than 53 µm, showed higher mucoadhesion than L-ILP having a particle size between 180–230 µm [16]. In addition, insulin was more rapidly released from SS-ILP than from L-ILP [16]. This implies that the smaller particle size can provide for near instant insulin release at the absorption site resulting in a higher local concentration, thereby allowing more insulin absorption. This greatly contributes to the high PA values of SS-ILP in single oral administration studies. The strong hypoglycaemic effects of SS-ILP were also demonstrated in a multiple oral administration study. In fact, this is the first demonstration that oral insulin decreases blood glucose concentration following multiple oral administrations in the presence of foods.
Another factor affecting the insulin absorption is the dosing amount of L- and SS-ILP. As shown in Table 2, the PA values following oral administration decreased with an increase in insulin dose, and the highest PA values were obtained at a dose of 10 IU/kg following oral administration of L- and SS-ILP. In this study, insulin dose was adjusted by the amount of ILP. Therefore, rats were administered with more amount of the polymer at higher insulin dose. Since the volume of releasing medium strongly influenced the insulin release amount from the microparticles [24], the small intestinal fluid volume might not be enough for completely releasing insulin at higher dose.
5. Conclusions
This study clearly demonstrated that the oral application of SS-ILP in healthy and type 1 and 2 diabetic rats caused significant hypoglycaemic effects. In a multiple administration study, SS-ILP significantly suppressed the postprandial rise in blood glucose and showed continuous hypoglycaemic effects following 3 times/day oral administration to both diabetic rat groups in the presence of foods. These findings suggest that blood glucose levels of diabetic rats can be effectively controlled by oral SS-ILP administration, and complexation polymer hydrogels may be useful carriers for the oral delivery of peptides and proteins, specially insulin.
Acknowledgments
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology, of Japan and the National Institutes of Health of the USA through grant EB000246-13A2.
References
- 1.Morishita M, Aoki Y, Sakagami M, Nagai T, Takayama K. In situ ileal absorption of insulin in rats: effects of hyaluronidase pretreatment diminishing the mucous/glycocalyx layers. Pharm. Res. 2004;21:309–316. doi: 10.1023/b:pham.0000016244.88820.28. [DOI] [PubMed] [Google Scholar]
- 2.Aoki Y, Morishita M, Asai K, Akikusa B, Hosoda S, Takayama K. Regional dependent role of the mucous/glycocalyx layers in insulin permeation across rat small intestinal membrane. Pharm. Res. 2005;22:1854–1862. doi: 10.1007/s11095-005-6137-z. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin. Pharmacokinet. 1997;33:285–301. doi: 10.2165/00003088-199733040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal V, Khan MA. Current status of the oral delivery of insulin. Pharm. Technol. 2001;25:76–90. [Google Scholar]
- 5.Mesiha M, Plakogiannis F, Vejosoth S. Enhanced oral absorption of insulin from desolvated fatty acid-sodium glycocholate emulsions. Int. J. Pharm. 1994;111:213–216. [Google Scholar]
- 6.Radwant MA, Aboul-Enein HY. The effect of oral absorption enhancers on the in vivo performance of insulin-loaded poly(ethylcyanoacrylate) nanospheres in diabetic rats. J. Microencapsul. 2002;19:225–235. doi: 10.1080/02652040110081406. [DOI] [PubMed] [Google Scholar]
- 7.Morishita I, Morishita M, Takayama K, Machida Y, Nagai T. Hypoglycemic effect of novel oral microspheres of insulin with protease inhibitor in normal and diabetic rats. Int. J. Pharm. 1992;78:9–16. [Google Scholar]
- 8.Asada H, Douen T, Waki M, Adachi S, Fujita T, Yamamoto A, Muranishi S. Absorption characteristics of chemically modified-insulin derivatives with various fatty acids in the small and large intestine. J. Pharm. Sci. 1995;84:682–687. doi: 10.1002/jps.2600840604. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Sato K, Sugimoto K, Tao R, Murakami T, Kurosaki Y, Nakayama T. Oral administration of insulin as poly(vinyl alcohol)-gel spheres in diabetic rats. Biol. Pharm. Bull. 1996;19:897–900. doi: 10.1248/bpb.19.897. [DOI] [PubMed] [Google Scholar]
- 10.Damge C, Vranckx H, Balschmidt P, Couvreur P. Poly(alkyl cyanoacrylate) nanospheres for oral administration of insulin. J. Pharm. Sci. 1997;86:1403–1409. doi: 10.1021/js970124i. [DOI] [PubMed] [Google Scholar]
- 11.Marschutz MK, Caliceti P, Bernkop-Schnurch A. Design and in vivo evaluation of an oral delivery system for insulin. Pharm. Res. 2000;17:1468–1474. doi: 10.1023/a:1007696723125. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y. Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm. Res. 1996;13:896–901. doi: 10.1023/a:1016009313548. [DOI] [PubMed] [Google Scholar]
- 13.Prego C, Garcia M, Torres D, Alonso MJ. Transmucosal macromolecular drug delivery. J. Control. Release. 2005;101:151–162. doi: 10.1016/j.jconrel.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 15.Morishita M, Lowman AM, Takayama K, Nagai T, Peppas NA. Elucidation of the mechanism of incorporation of insulin in controlled release systems based on complexation polymers. J. Control. Release. 2002;81:25–32. doi: 10.1016/s0168-3659(02)00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. J. Control. Release. 2004;97:115–124. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with Caco-2 cultures. Eur. J. Pharm. Biopharm. 2004;57:447–455. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Portha B, Serradas P, Bailbe D, Suzuki K, Goto Y, Giroix MH. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes. 1991;40:486–491. doi: 10.2337/diab.40.4.486. [DOI] [PubMed] [Google Scholar]
- 19.Carino GP, Mathiowits E. Oral insulin delivery. Adv. Drug Deliv. Rev. 1999;35:249–257. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- 20.Owens DR, Zinman B, Bollit G. Alternative routes of insulin delivery. Diabetes Med. 2003;20:886–898. doi: 10.1046/j.1464-5491.2003.01076.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowman AM, Peppas NA. Analysis of the complexation/decomplexation phenomena in graft copolymer networks. Macromolecules. 1997;30:4959–4965. [Google Scholar]
- 22.Lowman AM, Cowans BA, Peppas NA. Investigation of interpolymer complexation in swollen polyelectrolyte networks by solid state NMR spectroscopy. J. Polym. Sci. Polym. Phys. 2000;38:2823–2831. [Google Scholar]
- 23.Peppas NA. Devices based on intelligent biopolymers for oral protein delivery. Int. J. Pharm. 2004;277:11–17. doi: 10.1016/j.ijpharm.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J. Control. Release. 2004;95:589–599. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20:1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J. Control. Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 27.Sahlin JJ, Peppas NA. Enhanced hydrogel adhesion by polymer interdiffusion: use of linear poly(ethylene glycol) as an adhesion promoter. J. Biomater. Sci. Polym. Ed. 1997;8:421–436. doi: 10.1163/156856297x00362. [DOI] [PubMed] [Google Scholar]