Abstract
The objective of this study was to investigate the insulin incorporation and release properties of poly(methacrylic acid-g-ethylene glycol) P(MAA-g-EG) microparticles as a function of copolymer composition. These microparticles exhibited unique pH-responsive characteristics in which interpolymer complexes were formed in acidic media and dissociated in neutral / basic environments. The microparticles containing equimolar amounts of MAA and PEG were capable of efficient insulin loading using equilibrium partitioning (>90%). Additionally, insulin release from the gel was significantly retarded in acidic media while rapid release occurred under neutral / basic conditions. In contrast, as the amount of MAA of the polymer was increased, the entrapment efficiency of insulin within the gel greatly reduced and the insulin was readily released from the polymer network in the acidic and neutral / basic media. In addition, in order to evaluate the potential application of the microparticles to other drugs, theophylline, vancomycin, fluorescein-isothiocyanate-labeled dextrans (FITC-Ds) with average molecular weights of 4400 (FITC-D-4), 12,000 (FITC-D-10) and 19,500 (FITC-D-20) were utilized as model hydrophilic drugs. The incorporation profiles showed that the uptake of theophylline and vancomycin to the microparticles was lower than that of insulin. Additionally, polymer microparticles loaded with theophylline and vancomycin exhibited pH-sensitive release behavior, however, the oscillatory behavior is less pronounced than those of insulin. The values of drug incorporation ratio showed that the microparticles were capable of incorporating almost 90% of insulin and 15% of vancomycin from solution. On the other hand, the other hydrophilic drugs showed very low incorporation efficiency to the microparticles. These data suggest that gels containing equimolar amounts of MAA:EG have the potential to be used as an oral carrier of peptide drugs, especially for insulin.
Keywords: Insulin, Controlled release, Complexation polymers
1. Introduction
Hydrogels are crosslinked polymeric networks that can be swollen in water, buffered or physiological solutions. In recent years, there has been considerable work performed in the development of cross-linked polymeric networks which are sensitive to their surrounding physiological environment and therefore would be desirable systems for site-specific drug delivery [1–6]. Possible applications of these copolymers are intranasal, intraocular, buccal, enteric and colonic delivery. A particularly interesting application of this technology is mucosal delivery systems for bioactive proteins and peptides. It has been reported that the bioadhesive poly(acrylates) like Carbopol® and polycarbophil have a potential to protect peptides from proteolytic degradation by inhibiting proteases from the GI tract [7,8]. Similarly, recent study has shown that graft copolymer networks of poly(methacrylic acid) grafted with poly(ethylene glycol) (henceforth designated P(MAA-g-EG)) have a potential to protect peptides from proteolytic degradation by inhibiting proteases from the GI tract [6]. These polymers seemed to be useful in minimizing proteolysis of peptide / protein drugs, therefore, have been shown to be promising candidates as such a system.
Carriers consisting of networks of P(MAA-g-EG) function because the structure of the copolymers exhibited pH sensitive swelling behavior due to the reversible formation of interpolymer complexes stabilized by hydrogen bonding between the etheric groups of the grafted PEG chains and the carboxylic acid protons of the PMAA network [5]. The complex formation in the insoluble copolymers is sensitive to the nature and pH of the surrounding fluid as well as the copolymer composition and graft chain length. In the acidic environment of the stomach, the gels are in the complexed state. Under these conditions drug cannot readily diffuse through the membrane because of the small mesh size [4,5]. As the polymer passes the stomach into the intestine, the environmental pH increases above the transition pH of the gel. The complexes immediately dissociate and the network pore size rapidly increases leading to the release of drug [4,5]. Because of their nature, these materials may be ideal for the delivery of drugs at rates specified by the pH of the environmental fluid.
We previously reported that the insulin incorporated P(MAA-g-EG) particles successfully enhanced insulin absorption in both diabetic and non-diabetic rats, achieving 4.2% bioavailability (relative to subcutaneous administration) with significant hypoglycemic effects [9]. These results strongly indicate that P(MAA-g-EG) particles may be potential carriers for insulin via the oral route. The objective of this study was to further examine the insulin incorporation and release properties of P(MAA-g-EG) particles as a function of copolymer composition. In addition, we evaluated the potential application of the particles to a water-soluble drug using theophylline and vancomycin hydrochloride as the model drugs. Furthermore, fluorescein-isothiocyanate-labeled dextrans (FITC-Ds) with different molecular weights were used in order to evaluate the relationship between molecular weight and the incorporation efficiency to the microparticles.
2. Materials and methods
2.1. Materials
Methacrylic acid (MAA), dimethoxy propyl acetophenone (DMPA) and fluorescein-isothiocyanate-labeled dextrans (FTIC) with Mw of 4400, 12,000 and 19,500 (FITC-D-4, FITC-D-10 and FITC-D-20, respectively) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Poly-(ethylene glycol) (PEG) monomethacrylate (PEGMA, with PEG of molecular weight 1000) and tetraethylene glycol dimethacrylate (TEGDMA) were obtained from Polysciences Inc. (Warrington, PA, USA). Crystalline porcine insulin (26.9 IU / mg) was kindly supplied by Shimizu Pharmaceutical Co. (Shizuoka, Japan). Theophylline and vancomycin hydrochloride were purchased from Wako Pure Chemical (Osaka, Japan). All references to water imply the use of MilliQ water previously filtered through a 0.2 μm cellulose nitrate membrane. All other chemicals were at least reagent grade and were used without further purification.
2.2. Hydrogel synthesis
Microparticles of P(MAA-g-EG) were prepared by a free-radical solution polymerization of MAA and PEGMA with PEG of molecular weight 1000. The monomers were mixed in appropriate molar ratios to yield solutions with 4:1 and 1:1 ratios of MAA:EG in the gels, henceforth designated P(MAA-g-EG)(4:1) and P(MAA-g-EG)(1:1). Solutions of pure MAA were also prepared. TEGDMA was added as a crosslinking agent in the amount of X=0.075 moles TEGDMA per mole MAA. Following complete dissolution of the monomer, nitrogen was bubbled through the well-mixed solutions for 30 min to remove dissolved oxygen, a free radical scavenger, which would act as an inhibitor.
The initiator, DMPA, was added in the amount of 1% weight of the monomers in a nitrogen atmosphere. The reaction mixtures were pipetted between flat plates to form films of 0.9 mm thickness. The monomer films were exposed to UV light (Ultracure 100, Efos Inc., Buffalo, NY, USA) at 1 mW/ cm2 at 365 nm and allowed to react for 30 min. The ensuing hydrogels were removed from the plates and rinsed for 5 days in DI water (changed daily) to remove unreacted monomers and the sol fraction. The co-polymers were then dried and pulverized into microparticles with diameters of 100–150 μm.
2.3. Drug incorporation
Drug incorporation experiments were performed using a range of hydrophilic drugs having different molecular weights. Each drug (10 mg) was dissolved in pH 7.4 phosphate buffered saline (PBS; 20 ml) and incorporated into the microparticles by equilibrium partitioning. In the case of insulin, crystalline porcine insulin was first dissolved in 100 μl of 0.1 N HCl. The insulin solution was diluted with 19.8 ml of PBS and normalized with 100 μl of 0.1 N NaOH. Incorporation was accomplished by soaking 140 mg of each set of the dried microparticles in the drug solution. At specified time points, 0.2-ml samples were withdrawn from the solutions using filtered syringes (pore size: 10 μm; Ishikawa Manufactory Co., Ltd., Tochigi, Japan).
Following drug incorporation, the microparticles were then filtered using cellulose acetate / cellulose nitrate filter paper (pore size: 1.0 μm) and washed with 100 ml 0.1 N HCl to collapse the microparticles and exude the remaining buffer solution. The drug-incorporated microparticles were dried under vacuum and stored at 4 °C prior to use in further studies.
The drug incorporation ratio was determined that a 10-mg sample of drug-incorporated microparticles was placed in 10 ml of PBS. At 6 h after the polymer swelling, 0.2-ml samples were taken from the solutions using filtered syringes (pore size: 10 μm; Ishikawa Manufactory). The drug incorporation ratio was then determined as the ratio of the assayed drug amount to the theoretical amount.
2.4. In vitro release studies
Drug release experiments were performed following the Japanese Pharmacopoeia (JP) paddle method. The first (pH 1.2) and the second (pH 6.8) fluids of JP were used for the release experiments, and the solution (37 °C) was stirred with a paddle at 100 rev. / min. Dry, drug loaded polymer microparticles (100 mg) were treated for 2 h with the first fluid (200 ml), the polymer samples were then collected by filtration and transferred to the second fluid (200 ml). Samples (0.3 ml) were taken at discrete intervals using filtered syringes (pore size: 10 μm; Ishikawa Manufactory).
HPLC was used to determine the concentration of insulin [10], vancomycin [11] or theophylline [12] in each sample. The HPLC conditions used for each drug are described in Table 1. Each sample was directly injected into a HPLC system composed of a pump (LC-10AS, Shimadzu Co. Ltd., Kyoto, Japan), a UV detector (SPD-10AV, Shimadzu), an integrator (C-R3A, Shimadzu) and a GL-PACK Nucleosil 100-5C18 column (150×4.6 mm i.d.). The concentration of FITC-Ds was determined using a fluorescence spectrometer. The excitation and emission wavelengths were set at 495 and 520 nm, respectively.
Table 1.
Chromatographic conditions
| Drug | Mobile phase | Fr (mL/min) | WL (nm) | Ref. no. |
|---|---|---|---|---|
| Insulin | Acetonitrile–0.1% trifluoroacetic acid–sodium chloride (31:69:0.58, v/v/w) | 1.2 | 220 | [10] |
| Vancomycin | Acetonitrile–5 mM potassium dihydrogenphosphate (10:90, v/v), pH 2.8 with phosphoric acid | 1.2 | 282 | [11] |
| Theophylline | Methanol–isopropanol–perchloric acid–water (2.5:10:0.1/87.4, v/v/v/v) | 1.2 | 254 | [12] |
Fr, flow rate, WL, wavelength.
3. Results and discussion
3.1. Insulin incorporation and release experiments
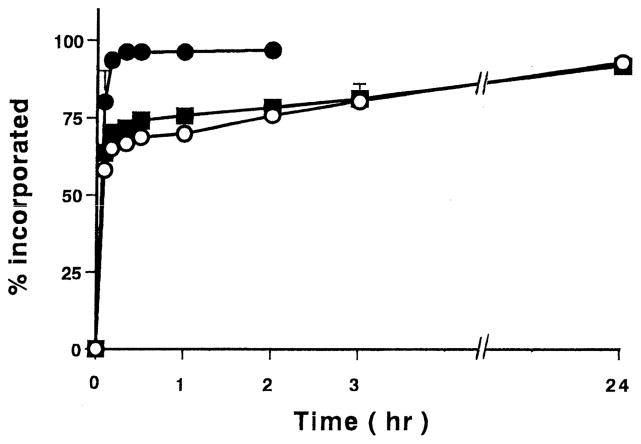
The incorporation profiles of insulin into initially dry microparticles of PMAA or P(MAA-g-EG) microparticles are shown in Fig. 1. Clearly, all of the formulations were able to incorporate a large amount of insulin from the solution. The overall rate of insulin uptake was significantly faster for P(MAA-g-EG)(1:1) than P(MAA-g-EG)(4:1) and PMAA. For the case of the P(MAA-g-EG)(1:1), nearly all of the insulin partitioned into the gels within the first 20 min of incubation. Both the P(MAA-g-EG)(4:1) and PMAA showed rapid insulin incorporation behavior with the insulin becoming almost completely partitioned into the polymers after 24 h incubation.
Fig. 1.
Incorporation profiles of insulin into PMAA or P(MAA-g-EG) microparticles containing different ratios of MAA:EG. PMAA (○), Molar ratio of MAA:EG; 4:1 (■) and 1:1 (●). Each value represents mean±S.D., n=3.
Previously, Moriyama et al. showed that insulin was preferentially partitioned into the PEG moieties and became entangled with PEG chains in the system of hyaluronic acids grafted with PEG [13]. It was considered that the negatively charged insulin in a physiological pH range was mainly partitioned into the PEG phase because hyaluronic acid is a polyelectrolyte which is negatively charged. Similarly in these complexation gels, high partitioning of insulin into P(MAA-g-EG)(1:1) was most likely observed due to high PEG contents in the polymer. Protein is highly attracted to the polymeric surfaces and in the case of insulin, it has higher affinity to the hydrophobic materials than to the hydrophilic ones [14]. Accordingly we observed near complete insulin uptake in both the P(MAA-g-EG)(4:1) and PMAA microparticles.
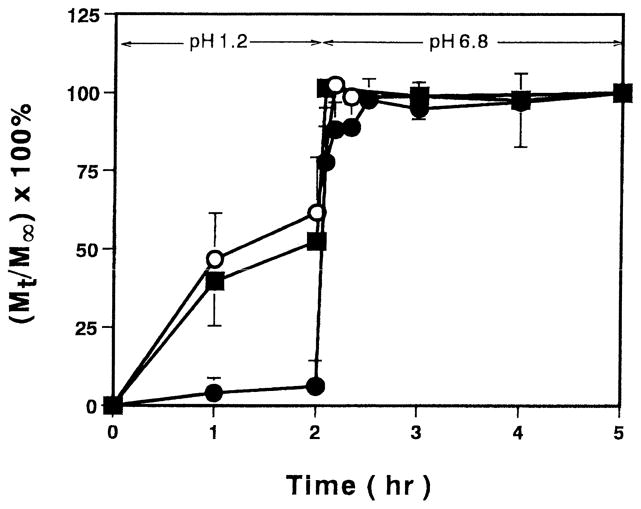
The pH-dependent, pulsatile release of insulin from the microparticles was performed by swelling the initially dry, insulin-incorporated particles in pH 1.2 solution for 2 h and pH 6.8 solution for the next 3 h. The fractional release of insulin, defined here as the ratio of the amount released at any time (Mt) to the total amount released after 5 h (M∞) is shown in Fig. 2. For the initial period during which the PEG-containing microparticles were complexed, only 6% of the insulin was released from the P(MAA-g-EG)(1:1). This implies that this polymer has the desired protective effect for oral delivery of the drug as a significant fraction of insulin remains in the polymer as the microparticles pass through the low pH environment of the stomach. When the microparticles were transferred to the high pH solution, the particles swelled rapidly and a rapid burst-type release of insulin occurred. In contrast, for the carriers containing increased amounts of MAA, more than 50% of the insulin was released from the microparticles during the first 2 h in acidic media. The remaining insulin was released rapidly upon changing the pH to 6.8. The results indicate that these carriers exhibit less than ideal properties for oral delivery of insulin as more than half of the drug will be released into the stomach where it will be readily digested by proteolytic enzymes.
Fig. 2.
Oscillatory release profiles of insulin from PMAA or P(MAA-g-EG) microparticles containing different ratios of MAA:EG. PMAA (○), Molar ratio of MAA:EG; 4:1 (■) and 1:1 (●). Each value represents mean±S.D., n=3.
Previously, we reported that the mesh size in these hydrogels varied significantly with small changes in pH due to the complexation / decomplexation behavior [5]. In low pH solutions in which complexation occurs, the network mesh sizes for microparticles were as low as 70 Å, but as the pH was increased, the physical cross-links dissociated and the polymer chains elongated resulting in a significant increase in the network mesh size to almost 210 Å [5]. Additionally, complexation was found to be greater in gels containing a 1:1 molar ratio than materials containing the 4:1 ratio of MAA / EG, and accordingly the gels with the 1:1 MAA / EG ratio have a smaller network mesh in the acidic media [5]. As such, the P(MAA-g-EG)(1:1) was better able to retard the release rate of insulin in the low pH solution. On the contrary, in the higher pH solutions, decomplexation occurred and the gels swelled due to the ionization of the PMAA side groups resulting in a mesh size almost three times greater than that of the complexed gels. This corresponds to a nearly 10-fold increase in the effective area for diffusion, which results in the rapid release rate from both gels. For the PMAA gels in low pH solutions, the decrease of the network mesh size was only due to the hydrophobic nature of PMAA chains under these conditions and the chains were in a coiled conformation. However, as no complexation occurred in these gels, the mesh size was larger than that of the complexation gels and the release rate in the low pH solution was faster. In PMAA hydrogels, in high pH solutions, the acid groups in PMAA ionized and the mechanism for swelling was the same as in the P(MAA-g-EG) microparticles. Thus, the network mesh size of three microparticles in pH 6.8 solution was almost the same [5]. Although there was no significant difference in insulin release in high pH solution, insulin release from P(MAA-g-EG)(1:1) was slightly retarded. It was reported that insulin appeared to partition into the PEG phase, and intermolecular interactions could retard insulin leakage [13]. Therefore, it may be reasonable for retardation of insulin release from the microparticles having higher PEG contents. As the P(MAA-g-EG)(1:1) showed the greatest change in insulin release rate from the low pH to high pH solutions, this polymer was selected for further evaluation as a drug delivery system.
3.2. Drug incorporation and release experiments
The incorporation profiles of theophylline, a hydrophilic solute with a molecular weight of 180, and vancomycin, a glycopeptide antibiotic with a molecular weight of 1486, into P(MAA-g-EG)(1:1) microparticles are shown in Fig. 3. In contrast to the profiles of insulin, complete partitioning to the microparticles was not seen in both drugs. For the case of theophylline, ~40% of theophylline partitioned into the polymer slowly over 96 h. However, the uptake of vancomycin into the microparticles was initially rapid and the maximum partition reached ~65% at 72 h.
Fig. 3.
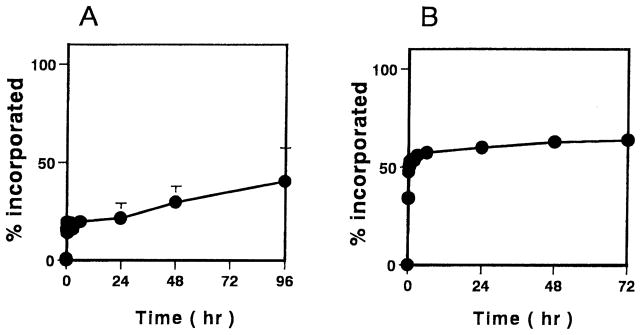
Incorporation profiles of theophylline (A) and vancomycin (B) using P(MAA-g-EG) microparticles. Molar ratio of MAA:EG; 1:1. Each values represents mean±S.D., n=3.
The pulsatile release behavior of theophylline and vancomycin is shown in Fig. 4. In the release study, both theophylline and vancomycin were gradually released from the microparticles of P(MAA-g-EG)(1:1) in the acidic solution. As compared to insulin, both of these drugs were released from the gels more rapidly in the low pH-environment. In the neutral solution, both drugs showed oscillatory release behavior, however, the differences in the release rates between the low pH and high pH are not as dramatic as those observed for insulin. This behavior was due to the fact that as the drug size decreases, the ability for the drug to move through the complexed network increases. However, as the complexes dissociate and the gels swell to a large degree, the network mesh size is sufficiently large so as to have little effect on the rate of diffusion of the drug.
Fig. 4.
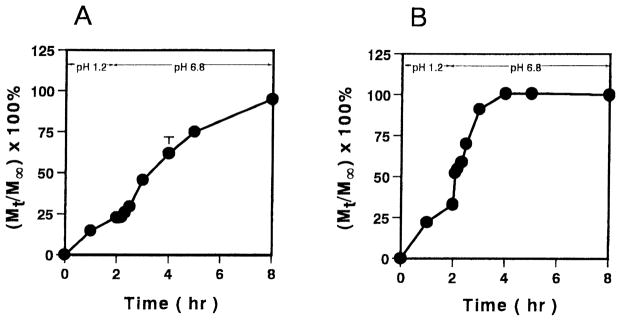
Oscillatory release profiles of theophylline (A) and vancomycin (B) using P(MAA-g-EG) microparticles. Molar ratio of MAA:EG; 1:1. Each values represents mean±S.D., n=3.
3.3. Drug incorporation ratio
The various drug incorporation ratios are shown in Table 2. The results clearly indicate that a significant fraction of insulin can be loaded into the microparticles. Vancomycin also showed ~15% of incorporation efficiency. On the contrary, the other hydrophilic drugs could not be incorporated extensively. For the case of insulin, the incorporation efficiency was similar to the amount that partitioned into the gel in the partitioning experiment. However, for the other drugs the incorporation ratio was much less than the partitioning amount. This is attributed to the fact that the smaller drugs were ‘squeezed’ out of the gel when they were washed with the acidic solution, while insulin was sufficiently large to remain entrapped in the gel.
Table 2.
The drug incorporation ratio
| Drug | Incorporation ratio |
|---|---|
| Theophylline | 1.1±0.5 |
| Vancomycin | 14.5±0.3 |
| Insulin | 87.4±6.8 |
| FITC-D-4 | 7.0±0.3 |
| FITC-D-10 | 5.2±0.2 |
| FITC-D-20 | 5.2±0.7 |
Molar ratio of MAA:EG; 1:1. Each value represents mean±S.D., n=3–5.
The ratio of the solute radius to the network mesh size was a significant factor in the overall behavior of P(MAA-g-EG) hydrogels [15]. However, the differences in the loading efficiency could also be attributed to the complex nature of the drugs. In our experiments, we studied molecules of varying molecular size and structure. For example, insulin is a large peptide drug and composed of two polypeptide chains [16]. In the neutral solution, insulin self-associates into dimers and higher oligomers occur. Vancomycin is also a relatively large molecule and possesses a complicated structure. The molecule is polycyclic and contains various components that are interconnected in a seven-member peptide chain held in the form of three rings [17]. Theophylline is a relatively small molecular weight, aromatic heterocyclic compound. The incorporation ratio for FITC-Ds was also significantly less than for insulin. Assuming a spherical shape of the drugs, FITC-D-4 has similar solute radius to insulin [18], however, the uptake was much lower than that of insulin. Additionally, considering insulin more likely may exist in the hexameric form in the incorporation medium [19], the apparent molecular weight of insulin would be much larger than FITC-D-10 and FITC-D-20. However, both FITC-D-10 and FITC-D-20 were not incorporated into the microparticles efficiently; rather, their uptake was much lower than that of insulin. Unlike peptide drugs, dextrans are rod-shaped and closely resemble an ellipsoid which in turn could result in the difficulty of loading [20]. Thus, in addition to the molecular weight, the complex nature and shape of the molecule is a significant factor for the incorporation efficiency of the microparticles.
4. Conclusions
In this work, we have prepared P(MAA-g-EG) microparticles and studied the drug loading and release profiles. We have found that we can load almost 90% of insulin from solution into our microparticles in pH 7.4 solutions. Correspondingly, the polymer was capable of providing for retarding release from gels in low pH environments and releasing the drug rapidly in high pH media in vitro. The difference in the release rates from the particles of P(MAA-g-EG) was maximized when the polymer contained equimolar ratios of MAA / EG. In this case, extremely low insulin release occurred from the polymer in solutions of pH 1.2. However, greater than 95% of the insulin loaded into the gels was released rapidly over a period of 1 h from the P(MAA-g-EG)(1:1) microparticles. In comparison, with other drugs of different nature and molecular size, we could not obtain such efficient loading or the dramatic differences in the drug release rates as a function of pH. Thus, our results indicate that P(MAA-g-EG)(1:1) microparticles are thought to be highly suitable as an oral carrier for peptide drugs, specifically insulin.
Acknowledgments
This work was supported by the Ministry and Education, Science, Sports, and Culture of Japan and a grant from the National Institutes of Health. The gift of insulin from Shimizu Pharmaceutical Company, Ltd., Shizuoka, Japan, is gratefully acknowledged.
References
- 1.Sen M, Uzun C, Guven O. Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide / maleic acid) hydrogels. Int J Pharm. 2000;203:149–157. doi: 10.1016/s0378-5173(00)00449-x. [DOI] [PubMed] [Google Scholar]
- 2.Traitel T, Cohen Y, Kost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials. 2000;21:1679–1687. doi: 10.1016/s0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 3.Ranjha NM, Doelker E. pH-sensitive hydrogels for site-specific drug delivery. I. Swelling behaviour of crosslinked copolymers of acrylic and methacrylic acid. STP PHARMA. 1999;9:335–340. [PubMed] [Google Scholar]
- 4.Lowman AM, Peppas NA. Design of oral delivery systems for peptides and proteins using complexation graft copolymer networks. In: Peppas NA, Mooney DJ, Mikos AG, Brannon-Peppas L, editors. Biomaterials, Carriers for Drug Delivery and Scaffolds for Tissue Engineering. AiChE; New York: 1997. pp. 21–23. [Google Scholar]
- 5.Lowman AM, Peppas NA. Analysis of the complexation / decomplexation phenomena in polyelectrolyte networks. Macromolecules. 1997;30:4659–4965. [Google Scholar]
- 6.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20:1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 7.Bai JPF, Chang LL, Guo JH. Effect of polyacrylic polymers on the lumenal proteolysis of peptide drugs in the colon. J Pharm Sci. 1995;84:1291–1294. doi: 10.1002/jps.2600841107. [DOI] [PubMed] [Google Scholar]
- 8.Lueßen HL, Bochard G, Verhoef JC, Lehr CM, de Boer AG, Junginger HE. Mucoadhesive polymers in peroral peptide drug delivery. II. Carbomer and polycarbophil are potent inhibitors of the intestinal proteolytic enzyme trypsin. Pharm Res. 1995;12:1293–1298. doi: 10.1023/a:1016213405081. [DOI] [PubMed] [Google Scholar]
- 9.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa H, Nagase M. Reversed-phase high-performance liquid chromatography of peptides. Yakugaku Zasshi. 1986;106:398–405. [Google Scholar]
- 11.Luksa J, Marusic A. Rapid high-performance liquid chromatographic determination of vancomycin in human plasma. J Chromatogr B Biomed Appl. 1995;667:277–281. doi: 10.1016/0378-4347(95)00033-f. [DOI] [PubMed] [Google Scholar]
- 12.Ishizaki T, Watanabe M, Morishita N. The effect of assay methods on plasma levels and pharmacokinetics of theophylline. Br J Clin Pharmacol. 1979;7:333–341. doi: 10.1111/j.1365-2125.1979.tb00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriyama K, Ooya T, Yui N. Hyaluronic acid grafted with poly(ethylene glycol) as a novel peptide formulation. J Control Release. 1999;59:77–86. doi: 10.1016/s0168-3659(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 14.Sefton MV, Antonacci GM. Adsorption isotherms of insulin onto various materials. Diabetes. 1984;33:674–680. doi: 10.2337/diab.33.7.674. [DOI] [PubMed] [Google Scholar]
- 15.Lowman AM, Peppas NA. Solute transport analysis in pH-responsive, complexing hydrogels of poly(methacrylic acid-g-ethylene glycol) J Biomater Sci Polym Ed. 1999;10:999–1009. doi: 10.1163/156856299x00586. [DOI] [PubMed] [Google Scholar]
- 16.Klostermeyer H, Humbel RE. The chemistry and biochemistry of insulin. Angew Chem Int Ed. 1966;5:807–822. doi: 10.1002/anie.196608071. [DOI] [PubMed] [Google Scholar]
- 17.Williams DH, Kalman JR. Structural and mode of action studies on the antibiotic vancomycin. Evidence from 270-MHz proton magnetic resonance. J Am Chem Soc. 1977;99:2768–2774. doi: 10.1021/ja00450a058. [DOI] [PubMed] [Google Scholar]
- 18.Matsukawa Y, Lee VHL, Crandall ED, Kim KJ. Size-dependent dextran transport across rat alveolar epithelial cell monolayers. J Pharm Sci. 1997;86:305–309. doi: 10.1021/js960352x. [DOI] [PubMed] [Google Scholar]
- 19.Mitra R, Pezron I, Chu WA, Mitra AK. Lipid emulsions as vehicles for enhanced nasal delivery of insulin. Int J Pharm. 2000;205:127–134. doi: 10.1016/s0378-5173(00)00506-8. [DOI] [PubMed] [Google Scholar]
- 20.van Os CH, de Jong MD, Slegers JF. Dimensions of polar pathways through rabbit gallbladder epithelium. The effect of phloretin on nonelectrolyte permeability. J Membr Biol. 1974;15:363–382. doi: 10.1007/BF01870095. [DOI] [PubMed] [Google Scholar]