Abstract
Bariatric surgery is an effective and increasingly common treatment for severe obesity and its many co-morbidities. Among the side effects of these procedures are detrimental effects on bone and mineral metabolism. This review explores the skeletal response to bariatric surgery, potential mechanisms for these changes and strategies for management. Bone disease among bariatric surgery patients is influenced by pre-operative abnormalities in bone and mineral metabolism related to morbid obesity. Changes that occur after surgery are specific to procedure type, with the most pronounced abnormalities in calciotropic hormones and bone loss seen after procedures that result in the most malabsorption. The most consistent site for bone loss after all bariatric procedures is at the hip, although available BMD data are limited by issues associated with DXA technology, including artifact introduced by adipose tissue itself. The bone loss that occurs after bariatric surgery is likely multifactorial. Proposed mechanisms include skeletal unloading, abnormalities in calciotropic hormones, as well as changes in gut hormones. There are very limited data on fracture risk in the bariatric population, and this is a critical area for additional research. Current treatment should be geared toward correcting nutritional deficiencies and following BMD in high-risk patients.
Introduction
Bariatric surgery has become an increasingly common treatment for severe obesity1, 2, as it results in significant, sustained weight loss 3, reverses many complications of obesity 4, 5 and decreases mortality 6, 7. However, there are several detrimental effects of these procedures, among them deleterious effects on bone and mineral metabolism, including vitamin D deficiency, hyperparathyroidism and bone loss. The bone loss that occurs after bariatric surgery is likely multifactorial. Proposed mechanisms include skeletal unloading, abnormalities in calciotropic hormones, as well as changes in gut hormones. Increased bone turnover may be associated with physiological adaptation of the skeleton to unloading, or it may be associated with pathophysiological changes like increased parathyroid hormone (PTH). Whether the skeletal changes that occur after bariatric surgery are pathological and are associated with skeletal fragility remains to be seen. This review will explore the skeletal response to bariatric surgery, potential mechanisms for these changes and strategies for management. As other recent reviews have specifically explored the relationship between gut hormones and adipokines with bone, we will not address that topic in detail so as not to be duplicative of the recent contributions to the literature.
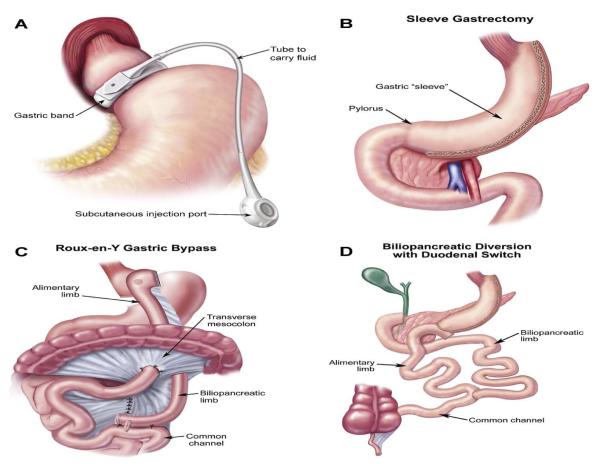
The rate of weight loss and resolution of co-morbidities after bariatric surgery varies by procedure 8. The skeletal effects are similarly procedure specific(see Figure 1). Current surgeries involve reduction in stomach size (restriction), delayed mixing of food with bile salts and pancreatic juices (malabsorption), or a combination of both. Gastric banding (GB) is a purely restrictive procedure, in which a silicone band placed around the proximal stomach creates a pouch that holds only a small amount of food. In sleeve gastrectomy (SG), more than 80% of the stomach is transected. Nutrients rapidly pass through the gastric conduit, resulting in altered gut hormones and metabolism.1 Roux-en-Y gastric bypass (RYGB) has both restrictive and malabsorptive features. Restriction occurs through the creation of a small gastric pouch from the proximal stomach, which is then anastamosed to the proximal jejunum to form an alimentary tract. Food content mixes with bile and pancreatic secretions in the distal jejunum. In biliopancreatic diversion with duodenal switch (BPD/DS), food bypasses most of the small intestine; agastric sleeve is anastamosed to the distal ileum where food mixes with digestive enzymes8. In RYGB and BPD/DS, the intestinal surface area available for caloric absorption is reduced, leading to malabsorption of minerals and fat-soluble vitamins. Hormonal changes that occur as a result of SG, RYGB and BPD/DS are important mediators of weight loss and may affect bone loss as well.
Figure 1.
Common Bariatric Surgery Procedures (from Atlas of Metabolic and Weight Loss Surgery, Jones et al. Cine-Med, 2010.8
Search Strategy
We searched PubMed and Ovid MEDLINE using the search terms “bariatric surgery” and “bone”. We primarily selected publications from the past 5 years, but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. This review focused on prospective data when available, and on studies that reported bone mineral density (BMD) changes at sites used by the World Health Organization in their diagnostic criteria for osteoporosis, lumbar spine (LS), total hip (TH), femoral neck (FN) and 1/3 radius (1/3R). Other recent review articles are cited to provide readers with more details and references than can be accommodated in this manuscript.
Causes of Abnormal Bone Metabolism In Obese Individuals
Before discussing the effects of bariatric surgery on bone, it is important to review the skeletal consequences of obesity. Vitamin D deficiency is widespread9, 10, with potential causes including inadequate dietary intake of foods and supplements containing vitamin D despite high overall caloric intakes 11, limited sunlight exposure 12, and decreased bioavailability of vitamin D secondary to sequestration of the fat soluble vitamin in excess adipose tissue13. Individuals who are the most severely obese, African Americans and decreased sun exposure appear to be at greatest risk9. Hyperparathyroidism is common, and while this may be secondary to vitamin D deficiency, an independent relationship between PTH and obesity has been reported14, 15. Emerging data also support a direct relationship between bone and fat16, 17. One study found an inverse correlation between total fat mass and BMD.18 Osteoblasts and adipocytes differentiate from the same mesenchymal precursor, and increased marrow fat is one proposed mechanism for low BMD among obese individuals19. Visceral fat in particular may have negative effects on bone formation, structure and strength, while subcutaneous fat may be protective20, 21. In a recent study of obese women had higher levels of PTH, bone specific alkaline phosphatase (BSAP), leptin, fibroblast growth factor-23 (FGF-23) and lower 1,25-dihydroxyvitamin D than controls14. Leptin levels predicted both PTH and FGF-23.
The relationship between body mass index (BMI) and fracture risk is complex, differs across skeletal sites22. Recent data question the long-held notion that obesity is protective of bone. Relative to body weight, microarchitecture and strength are lower in obese women compared to controls 23. While data are limited, obese patients may be at increased fracture risk, particularly at peripheral sites24-27. This may relate in part to an increased risk of falls24. Increased intramuscular adipose tissue may result in impaired mobility and muscle strength28. In addition, these constraints on mobility in obese individuals may result in altered patterns of falling, with increased propensity toward backward and sideways falls. Men and women may be at risk for fractures at different anatomical sites29.
Limitations of Bone Studies in the Bariatric Population
There are several limitations common to many studies of bone disease in the bariatric population. The majority of studies performed are small, and have substantial drop–out rates, particularly the few that follow subjects beyond one year post-operatively. There is a great deal of heterogeneity among studies relating to the age, sex and race of patients, surgical techniques, sites evaluated by DXA and DXA techniques. Supplementation of calcium and vitamin D is usually ancillary to the study protocol as part of clinical care and compliance is not assessed. In many studies, information on supplementation is not reported at all.
Limitations due to DXA technology include the fact that obesity itself and changes in fat mass introduce artifacts that may compromise accuracy and precision.30-33 Measurements by QCT34 and high resolution peripheral QCT (HR-pQCT)35 may be less affected by changes in body fat. Although newer machines can accommodate patients up to approximately 200 kg or 450 pounds, the 136 kg or 300 pound weight limit of many DXA machines means that many studies were only able to perform axial DXA measurements (of the spine and hip) on a sub-set of patients, giving them less power and making the reported results less reliable.
Skeletal Consequences of Bariatric Surgery
Gastric Banding
After GB, patients typically lose 20-30% of initial body weight and 41-54% of excess body weight36, 37. However, weight regain is common 38, 39. Although vitamin D deficiency has been documented prior to GB, post-operatively vitamin D remains stable or increases and PTH remains stable40, 41. Increased bone resorption, measured by C-Telopeptide (CTX), is evident by 6 months, and persists for at least 24 months after surgery40. Although it is beyond the scope of this review to discuss in detail, circulating estrogen and leptin levels decline, both related to decreased fat tissue.42
Bone density studies after GB are complicated by artifact in spine DXA measurements that may be introduced if the band overlies the spine. Spuriously high follow-up values occur if affected vertebrae are not properly identified and excluded. Studies have reported that LS BMD does not change, or increases slightly 40, 41, 43, 44. Estimates of hip bone loss have been variable, from no change at 1 year in study of only premenopausal women, to 6% at 24 months40, 41. In vertical banded gastroplasty (VBG), another restrictive procedure, a decline of 9.9% at the FN was reported after one year 43, while in an older study there was no change.45
Sleeve Gastrectomy
Sleeve gastrectomy is becoming increasingly common, with weight loss falling between the purely restrictive and malabsorptive procedures (1 to 2 year weight loss: 20-30%; excess BMI loss: 45-80%).36, 46-50. Data on bone metabolism after SG are limited. In one study, where 95% of subjects had D deficiency, and 43% elevated PTH at baseline, 25OHD increased and PTH decreased post-operatively, although no information is available regarding supplement use or adherence46. A prospective study, with higher baseline levels found 25OHD increased but PTH did not change48.
Data on skeletal outcomes are limited. Increased bone turnover markers have been reported at one year 48. Bone loss has been reported as early as 6 months after SG (TH :-5.2% and FN :-7.0%, with had a small decrease at the LS)51. In a small prospective study, eight women who underwent SG had significant bone loss at the spine (4.6%) and hip(total hip 8.3% and FN 7.1%) at one year.48 In contrast, a retrospective study in which most patients were vitamin D deficient pre-operatively, LS BMD increased over 2 years.46 The contradictory findings in the latter study may be explained by its retrospective study design, selection bias in the subjects referred for DXA, and treatment of vitamin D deficiency. Larger, prospective studies are needed to elucidate the changes after SG.
Roux-en-Y gastric Bypass
The majority of available data on changes in bone after bariatric surgery comes from studies of RYGB. After RYGB, patients typically lose 35% of initial weight, or 62-75% of excess body weight47, 52-54, significantly greater than with GB.55, 56. Calcium malabsorption has been documented as early as 3 months after RYGB53, 54 with reduced true fractional absorption of calcium57, and secondary hyperparathyroidism52, 53, 58, 59. Early studies showed high rates of vitamin D deficiency60, 61. Although aggressive vitamin D repletion has led to less post-operative vitamin D deficiency, improvements have not been commensurate with supplementation, suggesting significant impairment in vitamin D absorption after RYGB. We found stable 25 OHD levels despite more than 200% increases in vitamin D intake (mean of 658 IU/d at baseline, 1698 IU/d at 12 months)53, and no increase in 25OHD levels on doses of approximately 5000 IU daily52. Further reductions in 25OHD and increases in PTH have been reported at year 3 compared to year 1 after RYGB62, but without information on compliance with supplements.
Bone turnover increases as early as 3 months after RYGB 53, 54. Resorption markers increase steadily by up to 200% over the first 12-18 months 52, 63, 64. Increases in bone formation markers are less exuberant52, 53, and have not been uniformly reported54. There are no long-term longitudinal data elucidating the duration of the increased bone turnover. In one cross-sectional study, osteocalcin and BSAP were still elevated compared to obese controls 10 years after RYGB.65
While cross-sectional comparisons did not demonstrate consistent differences in BMD in RYGB patients compared to controls 65, 66, prospective studies have demonstrated clear declines in BMD in the first year following RYGB. Declines in hip BMD range between 8-11%17, 52-54, 62, 67-69. Few studies have evaluated changes beyond one year; in one study, hip BMD decreased by 3% between years 1 and 3 62. Changes at the spine are more variable, some studies report small decreases 24, 54, 63, 67-69, while others have not 52, 53, 65, 66. One study reported that a further small decline between one and 3 years 62. Some66 but not all52 studies have reported that menopausal status affects change in BMD after RYGB, with postmenopausal women losing the greatest amounts of bone.
BMD at the radius has only been measured in a few longitudinal studies, and most have found no change34, 52-54 although one study did report a decline.69 Other studies were limited by inclusion of patients who underwent other malabsorptive procedures.70,71.
A recent study that followed subjects after RYGB with DXA and central QCT reported declines at the LS by both imaging modalities. However, the decline at the hip by DXA was not seen by QCT, raising the possibility that artifactual changes may significantly affect hip measurements.63. In other work, when bone mineral apparent density (BMaD) was calculated to compensate for potential artifact related to changes in bone size, BMaD did not decrease significantly at the spine.53 As previously discussed, there is substantial artifact using any imaging modality in this population. Additional work is needed to clarify which modality is the most accurate.
Few studies have compared bone loss after RYGB with other procedures, and those that have are limited by small sample sizes. Typically, RYGB subjects lose more weight and more bone at the hip and FN at one year than those who undergo GB44, 52 or VBG.72. The one study that found similar bone loss at the spine and hip after SG and RYGB also had similar excess weight loss and calciotropic hormone levels eliminating the differences in those factors that might be expected to affect bone density73.
Biliopancreatic Diversion with Duodenal switch
BPD/DS is typically reserved for the super obese patients (BMI>50 kg/m2). Mean excess weight loss is 70-80% 74-76. After BPD/DS more than 50% of patients have vitamin D deficiency and estimates of secondary hyperparathyroidism range from 60-100% even with aggressive supplementation75, 77-79. Markers of bone turnover increase significantly74, 80. A randomized trial found more postoperative 25 OHD deficiency in BPD/DS versus RYGB, and while PTH levels did not differ, supplement use was far greater among BPD/DS patients81. Decreased bone formation and increased resorption by histomorphometry was reported after an early form of BPD 82. The majority (73%) of subjects had defective mineralization. A small decrease in LS BMD and stable hip density was seen 4 and 10 years after BPD/DS80, but results were very heterogeneous, with BMD increasing and decreasing in similar numbers of subjects. Another study that only measured only LS BMD found significant declines at one year, unattenuated by high dose calcium supplementation.74
Changes in Bone Quality and Microarchitecture After Bariatric Surgery
In addition to bone density and bone turnover, microarchitecture is an important property contributing to bone strength and fracture risk83. Evaluating changes in skeletal microarchitecture can better elucidate the nature of bone loss after bariatric surgery, but current data are limited. Bone biopsies at baseline and 4 years post-operatively in a group of patients who underwent BPD/DS demonstrated decreased cortical thickness, while trabecular bone volume increased 80. Mineralization declined over the four year follow-up, with an increase in osteoid volume, and bone formation rate increased. These data raise the possibility that a decline in mineralization could explain some of the post-operative reduction in BMD. While this mechanism may play a role in the BPD/DS population, it is unlikely that defective mineralization plays a significant role in decreased BMD seen in recent studies of other procedures (GB, SG or RYGB), given that in response to robust supplementation the majority of subjects had 25OHD levels close to 20 ng/ml or 50 nmol/L. In our recent prospective study using HR-pQCT in 22 women (RYGB, SG, and GB), cortical area, density, thickness, and total density all decreased at the tibia one year after surgery. Declines in cortical bone were predicted by the increase in PTH. The sub-group of RYGB patients lost more weight, had more cortical bone loss than those with GB or SG and had declines in cortical load share estimated by finite element analysis84. Again, it is important to note that HR-pQCT may also be affected by changes in sub-cutaneous fat.
Mechanisms of Bone Loss After Bariatric Surgery
Unloading
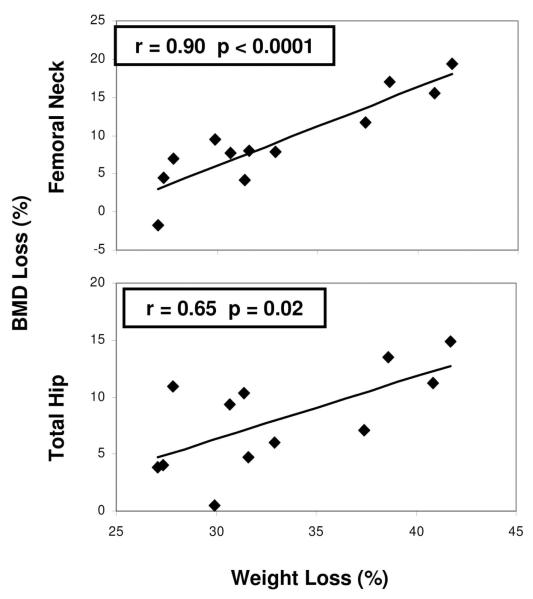
Mechanical loading of bone is an important determinant of bone size, mass and biomechanical properties. Changes in loading can induce compensatory increases in localized bone remodeling85, likely mediated through osteocytes and the sclerostin pathway. Bone loss has been observed in the setting of skeletal unloading in other populations, including patients with spinal cord injury86, and bed rest 87. Hip bone loss has been documented in individuals who lose even small amounts of weight from caloric restriction 88-92. The hip typically carries a load approximately two to three times body weight 93, therefore drastic weight loss after bariatric surgery may particularly affect this site. A strong association between extent of weight loss after bariatric surgery and amount of bone loss has been documented bymost40, 45, 51-53, 62, 67 but not all studies.63 We found that after RYGB, declines at the TH (r=0.65, p=0.02) and FN (r=0.90, p<0.0001) were associated with the extent of weight loss (Figure 2)53. Our HR-pQCT study demonstrating pronounced changes at the tibia but not the radius, suggests that there may be an interaction between PTH and weight bearing52. This hypothesis could explain the lack of change at the spine and radius observed in many studies.
Figure 2.
Relationship between decline in BMD at the hip (FN and TH) and extent of weight loss at 1 year after RYGB. From Fleisher et al. JCEM 2008.53
Changes in calcium, vitamin D and PTH
Vitamin D deficiency in obese individuals may result in metabolic and skeletal abnormalities that antedate but are only detected after surgery. The variable rates of vitamin D deficiency at baseline, and marked disparities in repletion regimens also complicate our understanding of the impact of bariatric surgery on calciotropic hormones. In RYGB and BPD/DS, active absorption of calcium, 80% of which occurs in the duodenum and jejunum, is impaired. Additionally absorption of vitamin D which occurs in the jejunum and ileum is affected by delayed mixing of ingested nutrients with bile acids and pancreatic enzymes 57, 94. Secondary hyperparathyroidism and bone loss may develop as a consequence 53, 66. Also after SG and RYGB, gastric acid production is reduced, which may affect calcium absorption.95 Calcium absorption may be further compromised by use of proton pump inhibitors, common in bariatric patients.96
Vitamin D levels are now routinely checked prior to surgery, and patients are supplemented with calcium and vitamin D post-operatively. Both observational 97 and randomized trials98 have shown that despite extremely high supplementation doses, 25OHD levels are often in the lower end of the normal range, suggesting decreased absorption of vitamin D or increased distribution to the adipose tissue.
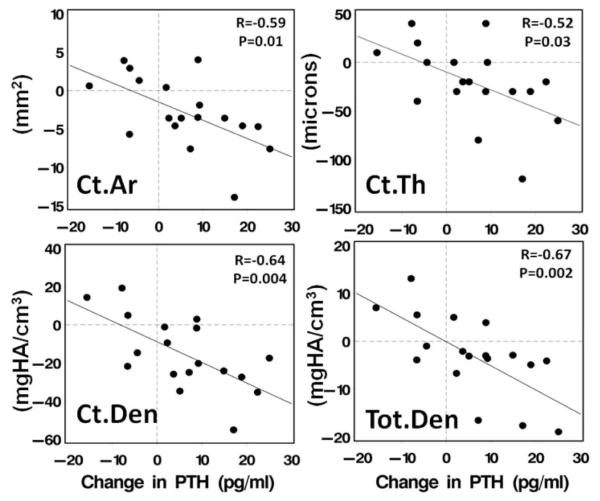
Many 52, 53, 60, 65, 66, but not all 54, 64 studies, have documented increased PTH following bariatric surgery. The decline in urinary calcium and rise in PTH that has been reported is consistent with calcium malabsorption, also despite aggressive supplementation. A significant rise in PTH levels, even within the normal range, can still have consequences. The differential effects of PTH on cancellous bone (anabolic) and cortical bone (catabolic), well described in primary hyperparathyroidism and osteoporosis therapy99, may be evident after bariatric surgery46, 52, 53. In studies that found postoperative increases in PTH levels, lumbar spine BMD did not decline52, 53, 65, 66. Conversely, spine BMD declined in some54 but not all46 studies in which PTH was stable or decreased. These findings suggest that increased PTH may be protective of the predominantly cancellous bone at the LS. PTH may affect hip bone loss as well; with increased PTH associated with greater bone loss at the FN53 and with cortical bone loss at the tibia 52 (Figure 3).
Figure 3.
Association between change in PTH and cortical area, cortical thickness, cortical density and total density by HR-pQCT at the tibia. From Stein et al. JCEM 2013.52
Changes in 25OHD have also been proposed to affect bone density. Subjects with increased or stable 25OHD had less bone loss at the FN than those whose 25OHD declined, suggesting that postoperative maintenance of 25OHD stores is important to hip bone preservation52. In another study, subjects randomized to high dose vitamin D had less hip bone loss than those who received only 800 IU daily98.
Changes in other hormones
A detailed discussion of the complex and procedure specific hormonal changes that occur after bariatric surgery is summarized in other recent reviews.47, 95, 100 Very little is known about the impact of these changes on bone metabolism following bariatric surgery. Declines in leptin after bariatric surgery101, may be associated with increased osteoclast activity 102. One study found the decrease in leptin after RYGB correlated with an increase in bone resorption measured by NTX 64. A differential effect of leptin on cortical and trabecular bone has been proposed, and merits further exploration 47. Adiponectin, an adipokine that is inversely associated with extent of fat mass, may also have an impact on post-operative changes in bone, although in vitro and animal studies have produced conflicting results103. In a prospective study, adiponectin levels almost doubled 12 months after RYGB and adiponectin levels were associated with a decrease in total body BMD.67
Gut hormones change substantially after SG, RYGB and BPD/DS and may also influence bone metabolism following bariatric surgery. Peptide YY (PYY), secreted by L-cells in the intestinal mucosa, inhibits food intake and helps regulate energy homeostasis. Levels of PYY increase following SG and RYGB but not GB 101 In animal studies, PYY was inversely related to osteoblast activity.104 Ghrelin and GLP-1 may impact bone metabolism but there are no data relating changes in these hormones to altered skeletal metabolism following bariatric surgery. Lower levels of insulin and amylin after surgery could also result in increased osteoclast recruitment and inhibition of osteoblast activity 102.
Changes in gonadal hormones may also play a role in bone loss after bariatric surgery. Hypogonadism is common in obese individuals. As adipose tissue is the primary source of estrogen in postmenopausal women and men, decreased production after loss of adipose may lead to a relative increase in bone resorption. To date, some studies have shown reported increased bone loss in postmenopausal women 62, others have not52. Conversely, there is data to support normalization of gonadal hormones after bariatric surgery 105. This topic merits further exploration. Sarcopenia could also play a role in bone loss, as lean body mass also declines after bariatric surgery.52, 62 An association between decreased lean mass and femoral neck bone loss has been reported.62. The loss of muscle mass could potentially increase falls and fracture risk. Although there is one retrospective study that suggests an increase in falls after bariatric surgery,106 additional data are needed.
Fracture Risk After Bariatric Surgery
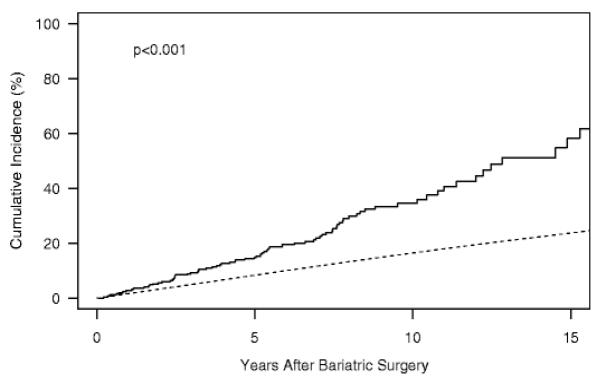
There is very little data regarding the risk of fracture following bariatric surgery. A retrospective cohort study from the United Kingdom found no significantly increased risk of fracture in 2079 bariatric surgery patients compared to 10442 matched controls 84. However, mean follow-up was only 2.2 years, and a trend toward increased fracture risk was noted in all patients after 3-5 years and in those with the greatest decline in BMI. Furthermore, the majority of subjects in this study underwent gastric banding, thus the results may not be representative of the risk for patients who undergo those types of surgery associated with greater bone loss. A historical cohort study (median follow-up: 7.7 years)compared fracture incidence in 258 subjects who underwent bariatric surgery, primarily RYGB, with expected incidence in a community based population107. Bariatric subjects had an increased incidence of all fracture, fractures due to minimal or moderate trauma and specifically of fractures at the hip spine, wrist of humerus. Risk was greatest for appendicular fractures; half of all fractures occurred in the foot, leg or hand. Vitamin D deficiency and lower preoperative activity were predictors of fracture. Associations with PTH changes or physical activity after surgery were not examined. Both of these studies are limited by their retrospective design as well as potential difficulties in selecting appropriate controls. There are no prospective fracture data.
Management of Post-Operative Nutritional Deficiencies And Bone Loss
While there is evidence that vitamin D deficiency in obese patients prior to bariatric surgery can be effectively treated with cholecalciferol and ergocalciferol based regimens,9 there are no interventional studies of vitamin D treatment that begin prior to surgery and continue post-operatively. After surgery, the separate effects of calcium and vitamin D on PTH levels cannot be discerned, making it difficult to determine whether calcium intake is adequate. The effects of vitamin D can be more readily assessed using 25OHD levels as an endpoint. Parent vitamin D in doses up to 800 IU daily have been shown to be inadequate in restoring vitamin D sufficiency after RYGB 66, 98, 108. Patients randomized to 50,000 IU weekly of ergocalciferol after RYGB had higher 25OHD levels at one year than those who received 800 IU daily.98 In another randomized trial 45 patients undergoing RYGB received vitamin D in doses of 800, 2,000, or 5,000 IU daily in addition to 2 grams of calcium. Levels of 25OHD increased in all subjects, and to a greater extent in the subjects receiving 2000 and 5000 IU compared to 800 IU daily. However, groups had significantly different baseline 25OHD and PTH levels, medication adherence was variable and almost half of all patients dropped out by the 24-month visit. Two subjects receiving 5000 IU daily developed hypercalciuria, measured by spot urine calcium collections109. Data from larger studies that include 24 hour urinary calcium measurements are needed to determine safe and effective regimen(s) for vitamin D repletion.
Earlier this year, AACE, TOS and ASBMS updated clinical practice guidelines for the peri-operative nutritional, metabolic and nonsurgical support of bariatric surgery patients 1. These guidelines now address several issues pertaining to bone health (Table 1). While the guidelines are based on the best available data, many of the recommendations regarding bone health are not evidence-based. The Endocrine Society 2010 guidelines are similar; pre-operative and annual DXA are recommended until BMD stabilizes after RYGB, BPD and BPD/DS.110
Table 1.
2013 AACE/TOS/ ASMBS Guidelines for Management of Bone Health in Patients after Bariatric Surgery1
| Prior to Surgery | After Surgery | |
|---|---|---|
| All Patients | Measure 25OHD |
|
|
RYGB and BPD/DS (in addition to measures outlined above) |
Measure areal BMD by DXA at spine and hip |
|
It is important to note that despite significant declines, BMD remains in the normal range in the vast majority of patients. For patients found to have osteoporosis on the basis of a T-score below -2.5 or the presence of a low trauma fracture, a metabolic work-up including serum PTH, calcium, phosphorus, 25OHD and 24 hour urine calcium is advised.1 The 2013 guidelines emphasize that adequate calcium and vitamin D intake are critical. It is important to recognize that compliance with supplementation decreases over time and subsequently the risk of inadequate calcium, vitamin D deficiency and secondary hyperparathyroidism increase. Pharmacologic therapy should only be administered after adequate replacement of calcium and vitamin D. The management guidelines recommend considering bisphosphonate treatment for bariatric surgery patients with osteoporosis. Intravenous therapy is recommended given the associated risks and concerns about absorption with oral bisphosphonates.
There is no evidence supporting the use of antiresorptive therapy to treat the bone loss that occurs following bariatric surgery. In fact, use of these agents is associated with a high risk of adverse events in this population. Oral bisphosphonates are associated with risk of reflux and anastomotic ulceration. Hypocalcemia and tetany may complicate intravenous bisphosphonate use in patients with low calcium or vitamin D. Although not addressed in the guidelines, other parenteral therapies may have particular risks in this population, teriparatide because of the risk of secondary hyperparathyroidism and denosumab because of concerns about severe hypocalcemia.
Summary and Future Directions
Bariatric surgery offers an effective treatment of morbid obesity, and also its many comorbidities. As the prevalence of bariatric procedures increases, so does the need for more information about the potentially deleterious effects on bone. Bone disease among bariatric surgery patients is influenced by pre-operative abnormalities in bone and mineral metabolism related to morbid obesity. Changes that occur after surgery are specific to procedure type, with the most pronounced abnormalities in calciotropic hormones and bone loss seen after procedures that result in the most malabsorption. The most consistent site for bone loss after all bariatric procedures is at the hip, although available BMD data are limited by issues associated with DXA technology, including artifact introduced by adipose tissue itself. Potential mechanisms for bone loss after bariatric surgery include unloading, calcium and vitamin D deficiency and secondary hyperparathyroidism, and other hormonal changes. There are very limited data on fracture risk in the bariatric population. Current treatment should be geared toward correcting nutritional deficiencies and following BMD in high-risk patients.
The studies outlined in this review provide an important foundation for understanding metabolic bone disease in the bariatric population, and also highlight the many remaining gaps in our knowledge. Larger prospective studies are needed to validate existing data. Further, as SG becomes an increasingly prevalent procedure more studies are needed to elucidate the associated skeletal changes. The long-term skeletal changes after bariatric surgery are unknown: studies must address the question of whether bone loss continues after the first year or levels off as the velocity of weight loss slows. The effect on skeletal indices of weight regain, common after GB, is unknown.
As postmenopausal women may be at particular risk of bone loss following bariatric surgery, studies with larger groups of postmenopausal women are needed to quantify bone loss and fracture risk. Adolescent patients, who are now undergoing bariatric surgery in increasing numbers, are another population who may be at particular risk, since their skeletons are still achieving peak bone mass. Additional studies focusing on the role of lean mass, and changes in physical activity and falls will help elucidate the effects of sarcopenia on bone loss and potentially fracture risk in this population. Whether exercise regimens may mitigate postoperative bone loss as has been shown in other populations undergoing weight loss remains to be seen. Finally, the most important question of whether the reported hormonal abnormalities and bone loss after bariatric surgery translate into increased fracture risk remains unanswered. Additional studies that address this question will be critical in determining the type of follow-up and treatment necessary for skeletal health in this burgeoning population.
Figure 4.
Cumulative incidence of fracture among Olmsted County, Minnesota, residents following bariatric surgery in 1985–2004 (solid line) vs. expected incidence among community men and women (dashed line). From Nakamura et al. Osteoporosis Int 2013.107
Acknowledgements
This work was supported by K23 DK084337 (Stein) and K24 DK074457 (Silverberg)
Footnotes
Disclosures
The authors have no conflicts of interest
Author Contributions
Literature review and manuscript drafting: EMS Manuscript editing and review: SJS
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013;9(2):159–91. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19(12):1605–11. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 3.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric Surgery versus Intensive Medical Therapy in Obese Patients with Diabetes. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric Surgery versus Conventional Medical Therapy for Type 2 Diabetes. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 7.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 8.Jones DB, Schneider BE, Olbers T. Atlas of Metabolic and Weight Loss Surgery. Cine_Med; Woodbury, Connecticut: 2010. [Google Scholar]
- 9.Stein EM, Strain G, Sinha N, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf) 2009;71(2):176–83. doi: 10.1111/j.1365-2265.2008.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censani M, Stein EM, Shane E, et al. Vitamin D Deficiency Is Prevalent in Morbidly Obese Adolescents Prior to Bariatric Surgery. ISRN obesity. 2013;2013 doi: 10.1155/2013/284516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 12.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34(11):2359–63. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 13.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 14.Grethen E, Hill KM, Jones R, et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. The Journal of clinical endocrinology and metabolism. 2012;97(5):1655–62. doi: 10.1210/jc.2011-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores L, Osaba MJ, Andreu A, Moize V, Rodriguez L, Vidal J. Calcium and vitamin D supplementation after gastric bypass should be individualized to improve or avoid hyperparathyroidism. Obes Surg. 2010;20(6):738–43. doi: 10.1007/s11695-010-0138-7. [DOI] [PubMed] [Google Scholar]
- 16.Reid IR. Relationships between fat and bone. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(5):595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 17.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27(1):1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- 18.Hsu YH, Venners SA, Terwedow HA, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83(1):146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 19.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR American journal of roentgenology. 2004;183(6):1761–5. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 20.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. The Journal of clinical endocrinology and metabolism. 2009;94(9):3387–93. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. The Journal of clinical endocrinology and metabolism. 2013;98(6):2562–72. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson H, Kanis JA, Oden A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2017. [DOI] [PubMed] [Google Scholar]
- 23.Sornay-Rendu E, Boutroy S, Vilayphiou N, Claustrat B, Chapurlat RD. In Obese Postmenopausal Women, Bone Microarchitecture and Strength Are Not Commensurate to Greater Body Weight: The Os des Femmes de Lyon (OFELY) Study. J Bone Miner Res. 2013;28(7):1679–87. doi: 10.1002/jbmr.1880. [DOI] [PubMed] [Google Scholar]
- 24.Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. The American journal of medicine. 2011;124(11):1043–50. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20(12):2090–6. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 26.Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25(2):292–7. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- 27.Compston JE, Flahive J, Hosmer DW, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: The global longitudinal study of osteoporosis in women (GLOW) J Bone Miner Res. 2013 doi: 10.1002/jbmr.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. Journal of aging research. 2012;2012:629637. doi: 10.1155/2012/629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Premaor MO, Compston JE, Fina Aviles F, et al. The association between fracture site and obesity in men: A population-based cohort study. J Bone Miner Res. 2013;28(8):1771–7. doi: 10.1002/jbmr.1878. [DOI] [PubMed] [Google Scholar]
- 30.Binkley N, Krueger D, Vallarta-Ast N. An overlying fat panniculus affects femur bone mass measurement. J Clin Densitom. 2003;6(3):199–204. doi: 10.1385/jcd:6:3:199. [DOI] [PubMed] [Google Scholar]
- 31.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knapp KM, Welsman JR, Hopkins SJ, Fogelman I, Blake GM. Obesity increases precision errors in dual-energy X-ray absorptiometry measurements. J Clin Densitom. 2012;15(3):315–9. doi: 10.1016/j.jocd.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Madsen OR, Jensen JE, Sorensen OH. Validation of a dual energy X-ray absorptiometer: measurement of bone mass and soft tissue composition. European journal of applied physiology and occupational physiology. 1997;75(6):554–8. doi: 10.1007/s004210050204. [DOI] [PubMed] [Google Scholar]
- 34.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27(1):119–24. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colt E, Akram M, Javed F, Shane E, Boutroy S. [Accessed August 16, 2012];Comparison of the Effect of Surrounding Fat on Measurements of BMD by DXA and High Resolution Quantitative Computerized Tomography. J Bone Miner Res. 2011 26(Suppl 1) Available at http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007757-89. [Google Scholar]
- 36.Dixon JB, Straznicky NE, Lambert EA, Schlaich MP, Lambert GW. Surgical approaches to the treatment of obesity. Nature reviews Gastroenterology & hepatology. 2011;8(8):429–37. doi: 10.1038/nrgastro.2011.112. [DOI] [PubMed] [Google Scholar]
- 37.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 38.Romy S, Donadini A, Giusti V, Suter M. Roux-en-Y gastric bypass vs gastric banding for morbid obesity: a case-matched study of 442 patients. Archives of surgery. 2012;147(5):460–6. doi: 10.1001/archsurg.2011.1708. [DOI] [PubMed] [Google Scholar]
- 39.Suter M, Calmes JM, Paroz A, Giusti V. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg. 2006;16(7):829–35. doi: 10.1381/096089206777822359. [DOI] [PubMed] [Google Scholar]
- 40.Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burckhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: potential role of serum C telopeptides for follow-up. International journal of obesity. 2005;29(12):1429–35. doi: 10.1038/sj.ijo.0803040. [DOI] [PubMed] [Google Scholar]
- 41.Pugnale N, Giusti V, Suter M, et al. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric banding in obese pre-menopausal women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(1):110–6. doi: 10.1038/sj.ijo.0802177. [DOI] [PubMed] [Google Scholar]
- 42.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? The Journal of clinical endocrinology and metabolism. 2002;87(3):1184–8. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 43.Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13(3):383–8. doi: 10.1381/096089203765887705. [DOI] [PubMed] [Google Scholar]
- 44.von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism: clinical and experimental. 2004;53(7):918–21. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Cundy T, Evans MC, Kay RG, Dowman M, Wattie D, Reid IR. Effects of vertical-banded gastroplasty on bone and mineral metabolism in obese patients. The British journal of surgery. 1996;83(10):1468–72. doi: 10.1002/bjs.1800831046. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Tovar J, Oller I, Priego P, et al. Short- and Mid-term Changes in Bone Mineral Density After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2013;23(7):861–6. doi: 10.1007/s11695-013-0866-6. [DOI] [PubMed] [Google Scholar]
- 47.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(1):52–67. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 48.Nogues X, Goday A, Pena MJ, et al. [Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass] Cirugia espanola. 2010;88(2):103–9. doi: 10.1016/j.ciresp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Annals of surgery. 2011;254(3):410–20. doi: 10.1097/SLA.0b013e31822c9dac. discussion 20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demaria EJ, Winegar DA, Pate VW, Hutcher NE, Ponce J, Pories WJ. Early postoperative outcomes of metabolic surgery to treat diabetes from sites participating in the ASMBS bariatric surgery center of excellence program as reported in the Bariatric Outcomes Longitudinal Database. Annals of surgery. 2010;252(3):559–66. doi: 10.1097/SLA.0b013e3181f2aed0. discussion 66-7. [DOI] [PubMed] [Google Scholar]
- 51.Pluskiewicz W, Buzga M, Holeczy P, Bortlik L, Smajstrla V, Adamczyk P. Bone mineral changes in spine and proximal femur in individual obese women after laparoscopic sleeve gastrectomy: a short- term study. Obes Surg. 2012;22(7):1068–76. doi: 10.1007/s11695-012-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. The Journal of clinical endocrinology and metabolism. 2013;98(2):541–9. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. The Journal of clinical endocrinology and metabolism. 2008;93(10):3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. The Journal of clinical endocrinology and metabolism. 2004;89(3):1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Annals of surgery. 2009;250(4):631–41. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 56.Spivak H, Abdelmelek MF, Beltran OR, Ng AW, Kitahama S. Long-term outcomes of laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass in the United States. Surgical endoscopy. 2012;26(7):1909–19. doi: 10.1007/s00464-011-2125-z. [DOI] [PubMed] [Google Scholar]
- 57.Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity. 2006;14(11):1940–8. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casagrande DS, Repetto G, Mottin CC, et al. Changes in bone mineral density in women following 1-year gastric bypass surgery. Obes Surg. 2012;22(8):1287–92. doi: 10.1007/s11695-012-0687-z. [DOI] [PubMed] [Google Scholar]
- 59.Youssef Y, Richards WO, Sekhar N, et al. Risk of secondary hyperparathyroidism after laparoscopic gastric bypass surgery in obese women. Surgical endoscopy. 2007;21(8):1393–6. doi: 10.1007/s00464-007-9228-6. [DOI] [PubMed] [Google Scholar]
- 60.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. The American journal of the medical sciences. 2005;329(2):57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 61.El-Kadre LJ, Rocha PR, de Almeida Tinoco AC, Tinoco RC. Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2004;14(8):1062–6. doi: 10.1381/0960892041975505. [DOI] [PubMed] [Google Scholar]
- 62.Vilarrasa N, San Jose P, Garcia I, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21(4):465–72. doi: 10.1007/s11695-010-0338-1. [DOI] [PubMed] [Google Scholar]
- 63.Yu EW, Bouxsein M, Roy AE, et al. Bone loss after bariatric surgery: Discordant results between DXA and QCT bone density. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruno C, Fulford AD, Potts JR, et al. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. The Journal of clinical endocrinology and metabolism. 2010;95(1):159–66. doi: 10.1210/jc.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ott MT, Fanti P, Malluche HH, et al. Biochemical Evidence of Metabolic Bone Disease in Women Following Roux-Y Gastric Bypass for Morbid Obesity. Obes Surg. 1992;2(4):341–8. doi: 10.1381/096089292765559936. [DOI] [PubMed] [Google Scholar]
- 66.Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40–7. doi: 10.1038/oby.2004.7. [DOI] [PubMed] [Google Scholar]
- 67.Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1):41–6. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 68.Vilarrasa N, Gomez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860–6. doi: 10.1007/s11695-009-9843-5. [DOI] [PubMed] [Google Scholar]
- 69.Pereira FA, de Castro JA, dos Santos JE, Foss MC, Paula FJ. Impact of marked weight loss induced by bariatric surgery on bone mineral density and remodeling. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2007;40(4):509–17. doi: 10.1590/s0100-879x2007000400009. [DOI] [PubMed] [Google Scholar]
- 70.Sinha N, Shieh A, Stein EM, et al. Increased PTH and 1.25(OH)(2)D levels associated with increased markers of bone turnover following bariatric surgery. Obesity. 2011;19(12):2388–93. doi: 10.1038/oby.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson JM, Maher JW, Samuel I, Heitshusen D, Doherty C, Downs RW. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2005;9(8):1106–10. doi: 10.1016/j.gassur.2005.07.012. discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 72.Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Annals of surgery. 2006;244(5):715–22. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vilarrasa N, de Gordejuela AG, Gomez-Vaquero C, et al. Effect of Bariatric Surgery on Bone Mineral Density: Comparison of Gastric Bypass and Sleeve Gastrectomy. Obes Surg. 2013 doi: 10.1007/s11695-013-1016-x. [DOI] [PubMed] [Google Scholar]
- 74.Tsiftsis DD, Mylonas P, Mead N, Kalfarentzos F, Alexandrides TK. Bone mass decreases in morbidly obese women after long limb-biliopancreatic diversion and marked weight loss without secondary hyperparathyroidism. A physiological adaptation to weight loss? Obes Surg. 2009;19(11):1497–503. doi: 10.1007/s11695-009-9938-z. [DOI] [PubMed] [Google Scholar]
- 75.Hewitt S, Sovik TT, Aasheim ET, et al. Secondary hyperparathyroidism, vitamin d sufficiency, and serum calcium 5 years after gastric bypass and duodenal switch. Obes Surg. 2013;23(3):384–90. doi: 10.1007/s11695-012-0772-3. [DOI] [PubMed] [Google Scholar]
- 76.Feng JJ, Gagner M. Laparoscopic biliopancreatic diversion with duodenal switch. Seminars in laparoscopic surgery. 2002;9(2):125–9. [PubMed] [Google Scholar]
- 77.Newbury L, Dolan K, Hatzifotis M, Low N, Fielding G. Calcium and vitamin D depletion and elevated parathyroid hormone following biliopancreatic diversion. Obes Surg. 2003;13(6):893–5. doi: 10.1381/096089203322618722. [DOI] [PubMed] [Google Scholar]
- 78.Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2004;8(1):48–55. doi: 10.1016/j.gassur.2003.09.020. discussion 4-5. [DOI] [PubMed] [Google Scholar]
- 79.Moreiro J, Ruiz O, Perez G, et al. Parathyroid hormone and bone marker levels in patients with morbid obesity before and after biliopancreatic diversion. Obes Surg. 2007;17(3):348–54. doi: 10.1007/s11695-007-9063-9. [DOI] [PubMed] [Google Scholar]
- 80.Marceau P, Biron S, Lebel S, et al. Does bone change after biliopancreatic diversion? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2002;6(5):690–8. doi: 10.1016/s1091-255x(01)00086-5. [DOI] [PubMed] [Google Scholar]
- 81.Aasheim ET, Hofso D, Sovik TT. Vitamin supplements after bariatric surgery. Clin Endocrinol (Oxf) 2010;72(1):134–5. doi: 10.1111/j.1365-2265.2009.03611.x. [DOI] [PubMed] [Google Scholar]
- 82.Compston JE, Vedi S, Gianetta E, Watson G, Civalleri D, Scopinaro N. Bone histomorphometry and vitamin D status after biliopancreatic bypass for obesity. Gastroenterology. 1984;87(2):350–6. [PubMed] [Google Scholar]
- 83.Cheung AM, Detsky AS. Osteoporosis and fractures: missing the bridge? JAMA. 2008;299(12):1468–70. doi: 10.1001/jama.299.12.1468. [DOI] [PubMed] [Google Scholar]
- 84.Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. Bmj. 2012;345:e5085. doi: 10.1136/bmj.e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frost HM. Bone “mass” and the “mechanostat”: a proposal. The Anatomical record. 1987;219(1):1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 86.Maimoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal cord. 2006;44(4):203–10. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- 87.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13(10):1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 88.Salamone LM, Cauley JA, Black DM, et al. Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr. 1999;70(1):97–103. doi: 10.1093/ajcn/70.1.97. [DOI] [PubMed] [Google Scholar]
- 89.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20(3):455–63. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012;27(3):619–27. doi: 10.1002/jbmr.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16(1):141–7. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 92.Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN, Jr., Shapses SA. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13(6):1045–50. doi: 10.1359/jbmr.1998.13.6.1045. [DOI] [PubMed] [Google Scholar]
- 93.Bergmann G, Deuretzbacher G, Heller M, et al. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34(7):859–71. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 94.Shaker JL, Norton AJ, Woods MF, Fallon MD, Findling JW. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1991;1(3):177–81. doi: 10.1007/BF01625450. [DOI] [PubMed] [Google Scholar]
- 95.Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. International journal of obesity. 2012;36(11):1373–9. doi: 10.1038/ijo.2012.115. [DOI] [PubMed] [Google Scholar]
- 96.Deitel M. Bariatric surgery, proton pump inhibitors, and possibility of osteoporosis. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2010;6(4):461–2. doi: 10.1016/j.soard.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 97.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25- hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 98.Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux en-Y gastric bypass: a randomized prospective clinical trial. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2009;5(4):444–9. doi: 10.1016/j.soard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4(3):283–91. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 100.Michalakis K, le Roux C. Gut hormones and leptin: impact on energy control and changes after bariatric surgery--what the future holds. Obes Surg. 2012;22(10):1648–57. doi: 10.1007/s11695-012-0698-9. [DOI] [PubMed] [Google Scholar]
- 101.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. International journal of obesity. 2009;33(7):786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31(5):547–55. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 103.Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochemical and biophysical research communications. 2005;331(2):520–6. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 104.Wong IP, Driessler F, Khor EC, et al. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PloS one. 2012;7(7):e40038. doi: 10.1371/journal.pone.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. European journal of endocrinology / European Federation of Endocrine Societies. 2013;168(6):829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 106.Berarducci A, Haines K, Murr MM. Incidence of bone loss, falls, and fractures after Roux-en-Y gastric bypass for morbid obesity. Applied nursing research : ANR. 2009;22(1):35–41. doi: 10.1016/j.apnr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 doi: 10.1007/s00198-013-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.da Rosa CL, Dames Olivieri Saubermann AP, Jacqueline J, Pereira SE, Saboya C, Ramalho A. Routine Supplementation Does Not Warrant the Nutritional Status of Vitamin D Adequate after Gastric Bypass Roux-En-Y. Nutricion hospitalaria : organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2013;28(1):169–72. doi: 10.3305/nh.2013.28.1.6166. [DOI] [PubMed] [Google Scholar]
- 109.Goldner WS, Stoner JA, Lyden E, et al. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: a prospective, randomized pilot clinical trial. Obes Surg. 2009;19(2):173–9. doi: 10.1007/s11695-008-9680-y. [DOI] [PubMed] [Google Scholar]
- 110.Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2010;95(11):4823–43. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]