Abstract
Background
Several somatic mutation hotspots were recently identified in the TERT promoter region in human cancers. Large scale studies of these mutations in multiple tumor types are limited, in particular in Asian populations. This study aimed to: analyze TERT promoter mutations in multiple tumor types in a large Chinese patient cohort, investigate novel tumor types and assess the functional significance of the mutations.
Methods
TERT promoter mutation status was assessed by Sanger sequencing for 13 different tumor types and 799 tumor tissues from Chinese cancer patients. Thymic epithelial tumors, gastrointestinal leiomyoma, and gastric schwannoma were included, for which the TERT promoter has not been previously sequenced. Functional studies included TERT expression by RT-qPCR, telomerase activity by the TRAP assay, and promoter activity by the luciferase reporter assay.
Results
TERT promoter mutations were highly frequent in glioblastoma (83.9%), urothelial carcinoma (64.5%), oligodendroglioma (70.0%), medulloblastoma (33.3%), and hepatocellular carcinoma (31.4%). C228T and C250T were the most common mutations. In urothelial carcinoma, several novel rare mutations were identified. TERT promoter mutations were absent in GIST, thymic epithelial tumors, gastrointestinal leiomyoma, gastric schwannoma, cholangiocarcinoma, gastric and pancreatic cancer. TERT promoter mutations highly correlated with upregulated TERT mRNA expression and telomerase activity in adult gliomas. These mutations differentially enhanced the transcriptional activity of the TERT core promoter.
Conclusions
TERT promoter mutations are frequent in multiple tumor types and have similar distributions in Chinese cancer patients. The functional significance of these mutations reflect the importance to telomere maintenance and hence tumorigenesis, making them potential therapeutic targets.
Keywords: Telomerase, promoter regions, Genetics, China, neoplasms, glioma
Introduction
Telomeres are essential structural elements that seal and protect the ends of chromosomes from recombination and end-to-end fusion. Maintenance of the telomeres requires the ribonucleoprotein complex known as telomerase. Telomerase consists of a multiple proteins, including telomerase reverse transcriptase (TERT) and its integral RNA subunit. In normal somatic cells, telomeres gradually shorten after successive rounds of cell division, resulting in senescence. Most tumor cells overcome this limitation by re-activating telomerase; however, the mechanisms of this reactivation process remain unknown [1].
Recently, two somatic mutations in the promoter region of TERT were reported in melanomas [2], [3]. The two most common mutations occurred at −124 and −146 base pairs upstream of the TERT ATG start site (hereafter referred to as C228T and C250T, respectively). These mutations occurred in nearly 70% of melanoma tumors and cell lines. Although the exact mechanism is unclear, each mutation independently generates a novel E-twenty-six (ETS) transcription factor binding site (GGAA/T) and has been shown to increase the transcriptional activity of the TERT promoter [3], [4]. Interestingly, TERT promoter mutations are not restricted to melanoma and occur frequently in several tumor types, including gliomas [4], [5], liposarcomas [4], urothelial carcinomas [4], [6], [7], [8], [9], and hepatocellular carcinomas [4], [10]. We sought to expand this work to search for additional TERT promoter mutations by investigating the TERT promoter mutation status of a large subset of cancers in a Chinese population, as most previous large-scale reports have been limited to Western populations. Furthermore, we assessed the TERT promoter mutation status of a number of tumor types that have not been investigated previously, including thymic epithelial tumors, gastrointestinal leiomyoma, and gastric schwannoma. Finally, we assessed the functional consequence and relevance of the most frequent mutations identified in this study.
Materials and methods
Tissue samples
A total of 799 tumor tissues from different tumor types were obtained from the archives of the Zhejiang Provincial People’s Hospital, between January 2007 and October 2013. All tissue samples were collected during surgical procedures with patients’ consent. FFPE tissue samples were fixed in formalin and later embedded in paraffin. The xenografts and snap-frozen primary tumor tissues used for RT-qPCR and TRAP assay were obtained from Duke University Preston Robert Tisch Brian Tumor Biorepository.
DNA extraction and PCR amplification
DNA was extracted and purified from 10 sections of 5 μm tumor FFPE samples using the TIANquick FFPE DNA Kit (TIANGEN BIOTECH, China) according to the manufacturer’s protocol. DNA was extracted from fresh tissue samples using the AxyPrep Genomic DNA Isolation Kit (Axygen Biosciences, China).
Sanger sequencing and mutation analysis
The amplification products were sent to BGI (Beijing Genomics Institute, China) for Sanger sequencing. Bi-directional sequence analysis of PCR products were conducted using the BigDye terminator v3.1 sequencing kit with the ABI PRISM 3730 automated next generation genetic analyzer (Applied Biosystems, USA). Mutation status was determined using Mutation Surveyor (SoftGenetics, USA) and FinchTV (Geospiza, USA).
Quantification of telomerase activity by TRAP assay
Telomerase activity was measured using the TRAPEZE Telomerase Detection Kit (Millipore, USA). All procedures were conducted as described in the manufacturer’s instructions. About 20 mg of tissue sections from snap-frozen xenografts or primary tumors were homogenized. In each TRAP experiment, 2 μl of extract containing 1 μg of protein was used. The TRAP product signals were visualized and captured by BioRad ChemiDoc MP Image system.
Reverse transcription quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from snap-frozen primary tumor tissue using E.Z.N.A. Total RNA Kit I (Omega Bio-Tek, USA), according to the manufacturer’s instructions. To measure TERT mRNA expression, 1 μg of total RNA from each sample was used as a template for cDNA synthesis using the RNA to cDNA EcoDry Premix, cDNA synthesis kit (TAKARA, Japan). TERT expression levels were then determined by quantitative real-time PCR using the SensiFAST SYBR No-Rox kit (Bioline, USA), normalized to GAPDH expression. TERT mRNA expression of a brain stem glioma cell line SF7761 (transduced with TERT) was used as a reference [11].
DNA constructs and site-directed mutagenesis
The promoter region of TERT (−424 to +65) was amplified from normal blood genomic DNA using forward (5′-CGGGGTACCGGCCGATTCGACCTCTCT-3′) and reverse (5′-CCGCTCGAGAGCACCTCGCGGTAGTGG-3′) primers. The PCR product was cloned into pGL3 basic plasmid using KpnI and XhoI digestion. The constructs with different TERT promoter mutations were generated by using the QuikChange site-directed mutagenesis kit (Stratagene, USA).
Cell culture and luciferase reporter assay
U87-MG cell line was obtained from the American Type Culture Collection (ATCC) and was authenticated using short tandem repeat analysis by the Duke University DNA Analysis Facility. The cells were cultured in MEM (10% FBS) in a 37°C incubator with 5% CO2. For the luciferase reporter assay, U87-MG cells were seeded in 24-well tissue culture plates and then transfected with wild-type or mutant TERT promoter reporter (Life Technologies, USA). After 24 h of transfection, the cells were lysed. Luciferase activity was determined by the Dual-Luciferase Reporter Assay System as described in the manufacturer’s instructions (Promega, USA). Renilla luciferase was used to normalize transfection efficiency.
Please see supplementary materials and methods for detailed protocols, primer sequences, and PCR programs.
Results
Sequencing analysis of the TERT promoter mutations in Chinese cancer patients
We evaluated TERT promoter mutations successfully in 799 tumor specimens and identified 268 samples (33.5%) with TERT promoter mutations. Overall, the hotspot mutations C228T (64.6%) and C250T (23.9%) were the most common and were mutually exclusive. These TERT promoter mutations occurred in many tumor types, including glioblastoma, medulloblastoma, urothelial carcinoma, hepatocellular carcinoma, and gallbladder carcinoma. TERT promoter mutations were absent in many types of tumors, including thymic epithelial tumor, thymic neuroendocrine carcinoma, GIST, gastrointestinal leiomyoma, gastric schwannoma, gastric cancer, pancreatic cancer, meningioma and cholangiocarcinoma (Table 1).
Table 1.
Frequency of TERT promoter mutations in various tumor types in a Chinese population
| Tumor type# | No. of tumors | mutated (%) No. of tumors |
|---|---|---|
| Glioma | 121 | 66 (54.5%) |
| Diffuse Astrocytoma (II) | 40 | 8 (20.0%) |
| Anaplastic Astrocytoma (III) | 12 | 4 (33.3%) |
| Glioblastoma (IV) | 56 | 47 (83.9%) |
| Oligodendroglioma (II-III) | 10 | 7 (70.0%) |
| Medulloblastoma | 6 | 2 (33.3%) |
| Urinary tract cancer | 292 | 188 (64.4%) |
| Urothelial carcinomas of bladder | 240 | 148 (61.7%) |
| Urothelial carcinomas of the upper urinary tract | 52 | 40 (76.9%) |
| Hepatocellular carcinoma | 35 | 11 (31.4%) |
| Gallbladder carcinoma | 2 | 1 (50%) |
No mutations were found in Pilocytic Astrocytoma (n=1), Subependymal giant cell astrocytoma (n=1), Pleomorphic Xanthoastrocytoma (n=1), Meningioma (n=77), Cholangiocarcinoma (n=6), GIST (n=76), Gastrointestinal leiomyoma (n=5), Gastric schwannoma (n=1), Gastric Cancer (n=74), Pancreatic cancer (n=46), Thymic cancer (n=5), Thymic neuroendocrine carcinoma (n=2), Thymoma (n=47).
CNS tumors
Gliomas are the most common type of primary brain tumor in China. Glioblastoma multiforme (GBM), a highly lethal high-grade astrocytoma, accounts for nearly half of all gliomas [12]. We investigated a total of 204 brain tumors, including 77 meningiomas, 56 glioblastomas, 55 other astrocytomas, 10 oligodendrogliomas and 6 medulloblastomas. All TERT promoter mutations were C228T or C250T, except for 1 case of C242T+C243T. TERT promoter mutations were highly prevalent in adult GBM (83.9%, 47/56), oligodendroglioma (70%, 7/10), diffuse astrocytoma (20%, 8/40), anaplastic astrocytoma (33.3%, 4/12) and medulloblastoma (33.3%, 2/6). In gliomas of grades II–IV and II–III, TERT promoter mutations were associated with an older age at diagnosis (p <0.0005 and p <0.05, respectively, Table S3). No TERT promoter mutations were identified in meningioma (n=77) (Tables 1, S2, and S4-A).
Urinary tract cancers
Urothelial carcinoma of the bladder is the most common urologic tumor in China [13]. We investigated a total of 293 urothelial carcinoma samples. Overall, 64.4% (188/292) contained TERT promoter mutations, with 61.7% (149/240) found in urothelial carcinoma of the bladder and 76.9% (40/52) found in those of the upper urinary tract (Table 1). Additionally, 69.5% (91/131) of infiltrating and 60.2% (97/161) non-invasive tumors harbored TERT promoter mutations. The majority of these mutations were C228T (39.7%, 116/292) and C250T (14.4%, 42/292). We also identified 10 additional mutations: A161C (n=12), C242T+C243T (n=6), C158A (n=3), C228A (n=2), G149T (n=2), C250T+C242T+C243T (n=1) and G245A (n=1), T198G (n=1), C193T (n=1), C190T+C184T (n=1) (Figure S1, Tables 1, S2, and S4-B). TERT promoter mutations were not significantly associated with age differences in these two groups (Table S3).
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide, with 55% of all cases being in Chinese patients [14]. In hepatocellular carcinoma samples, 31.4% (11/35) samples had TERT promoter mutations, among them 25.7% (9/35) were C228T and 5.7% were C250T (Tables 1, S2, and S4-C).
Cholangiocarcinoma and Gallbladder carcinoma
Cholangiocarcinoma is a rare tumor with a median survival of <24 months and with the highest rates in Asia [15]. No samples (0/9) contained TERT promoter mutations. We also identified one C228T mutation in a gallbladder carcinoma (GBC) sample (Tables 1, S2, S4-C). However due to its rarity, we were only able to collect a total of 2 GBC samples in this study.
Thymic epithelial tumors
While thymic epithelial tumors are rare, they are the most common neoplasms of the anterior mediastinum [16]. We show for the first time that TERT promoter mutations are absent in all (0/54) cases of thymic epithelial tumor studied, including 5 cases of thymic carcinoma, 2 cases of thymic neuroendocrine carcinoma and 47 cases of thymoma (Tables 1 and S4-F).
Gastrointestinal tumors
Gastric cancer, GIST, gastrointestinal leiomyoma, and gastric schwannoma samples were also included in this study. 10–30% of GISTs have malignant behavior, while gastric schwannomas and leiomyomas are largely benign [17]. We are the first to study the TERT promoter mutation status in gastrointestinal leiomyoma and schwannoma. No TERT promoter mutations were identified in gastric cancer of both intestinal and diffuse types (0/74), GIST (0/76), gastrointestinal leiomyoma (0/6), and gastric schwannoma (0/1) (Tables 1 and S4-D).
Pancreatic cancer
Pancreatic cancer is the sixth leading cause of death from malignancy in China and has a dismal overall cumulative 5-year survival rate of <3% primarily due to late diagnosis. The incidence and mortality of pancreatic cancer have also increased in China over the last decades [18]. Among samples analyzed with Lauren classifications ranging from low to high, TERT promoter mutations were notably absent (0/46) (Tables 1 and S4-E).
TERT promoter mutations upregulate TERT mRNA expression and telomerase activity in glioma
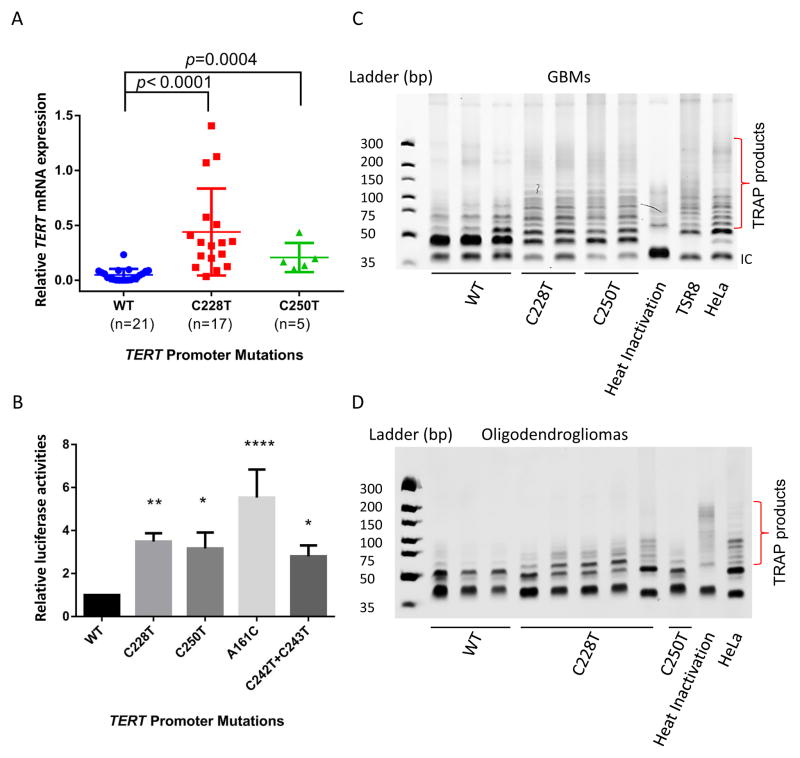
Consistent with previous reports, our study shows a high frequency of TERT promoter mutations in gliomas [4]. To assess whether TERT promoter mutations affect TERT mRNA expression, we assessed TERT mRNA expression in 43 primary glioma tumor tissues, including 18 GBMs, 17 oligodendrogliomas, and 8 astrocytomas (Table S4-H). As shown in Figure 2a, TERT mRNA expression levels in both C228T and C250T promoter mutant samples were significantly higher (P<0.001) than in TERT promoter wild-type samples, suggesting that TERT promoter mutations activate TERT expression.
Figure 2. Correlation of TERT promoter mutations and TERT mRNA expression as well as telomerase activation in gliomas and increase transcriptional activity of the TERT core promoter in a glioma cell line.
(A) TERT mRNA expression in both C228T and C250T TERT promoter-mutated gliomas was significantly higher than gliomas without mutation. TERT mRNA expression was measured by RT-qPCR and normalized to GAPDH expression first, and then the expression of TERT of all the samples were normalized to the SF7761 cell line. P values were determined by the Mann-Whitney test. (B) U87-MG cells were transfected with luciferase constructs containing the TERT core promoter (from −424 to +65) with the appropriate variants for 24 h. The luciferase activities were measured and normalized by Renilla luciferase activities. Results are mean±SD of three independent experiments. P values were determined by ANOVA with the Dunnett Test (*P <0.05, ** P<0.01, **** P<0.0001). (C) Both C228T and C250T TERT promoter mutations are associated with telomerase activation in xenografts; (D) Both C228T and C250T TERT promoter mutation upregulate telomerase activity in primary tumor tissues.
We also investigated whether TERT promoter mutations regulate telomerase activity. Using a repeat amplification protocol (TRAP) assay, we first evaluated telomerase activity in seven xenografts derived from adult GBMs (Table S4-G). As shown in Figure 2c, TERT promoter mutant xenografts (both C228T and C250T) exhibited high telomerase activity as indicated by the 6 bp incremental ladder of TRAP products on gel electrophoresis. However, in xenografts without TERT promoter mutations, the typical incremental TRAP ladder was almost undetectable. This result suggests that TERT promoter mutations are required for telomerase activation in the xenografts of adult gliomas. To further strengthen these findings, we examined the telomerase activity in 9 adult oligodendroglioma primary tumor samples (Table S4-G). Compared to xenografts, telomerase activity was slightly lower in primary tumor tissues. However, in agreement with the phenomena observed in xenografts, the typical TRAP products were only detected in the TERT promoter mutant samples but not in the TERT wide-type samples (Figure 2d).
TERT promoter mutations enhance the transcriptional activity of the TERT core promoter
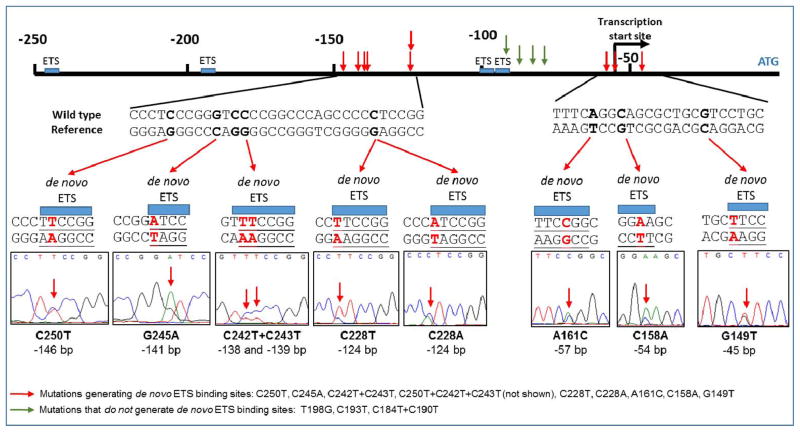
In addition to the two most common TERT promoter mutations, C228T and C250T, several other TERT promoter mutations were also identified in this study. Most of the mutations seen in multiple samples generate the ETS/TCF binding motif CCGGAA/T (Figure 1 and Table S1). We aimed to assess whether these common TERT promoter mutations play a similar role in increasing TERT promoter activity. For this purpose, the wild-type and several mutated TERT core promoters were determined using a luciferase reporter assay in U87-MG cells. We assayed the four most frequently mutated TERT core promoters identified in our study: C228T, C250T, A161C, and C242T+C243T. The transcriptional activities of all the mutated TERT core promoter constructs are significantly higher (from 2.8- to 5.3-fold) than the wild-type TERT core promoter. Among the single mutants, the A161C mutant has the strongest activity, whereas the C242T+C243T tandem mutated construct shows relatively weak up-regulation (2.8-fold, Figure 2b).
Figure 1. TERT promoter mutations identified in 799 tumor samples and potential ETS binding sites.
Arrows indicate locations of mutations identified (red: potential de novo ETS binding site generated, green: does not generate site).
Discussion
Since the first discovery of the TERT promoter mutations in melanoma [2], [3], many studies have been performed to establish their role as a novel genetic mechanism in human tumorigenesis. Subsequent research has revealed high frequencies of TERT promoter mutations in glioblastoma [4], [5], bladder cancer [6], hepatocellular carcinoma [10], and other human cancers [4]. However, most large-scale reports surveying multiple tumors are based on Western populations, with limited studies of Asian, in particular Chinese, populations.
Here, we report for the first time TERT promoter mutations in various types of cancer in a large cohort of 799 Chinese patients. We show that mutations in the promoter region of TERT occur frequently in glioblastoma (83.9%), oligodendroglioma (70%), urinary tract cancer (64.4%), hepatocellular carcinoma (31.4%), and medulloblastoma (33.3%). Previous studies have shown similar mutation percentages for these tumors GBM (70% [4], 83% [5]), diffuse astrocytomas (19% [5], 15% [9]) and anaplastic astrocytomas (25% [5]). Slightly higher frequencies of TERT promoter mutations have been reported in HCC (44.2% [4], 50.9% [10]) and urothelial carcinoma (83% [6], 74% [7]), although other reports reflect the opposite trend in urothelial carcinoma of the bladder (66% [4], 65.4% [8]) and upper urinary tract (47.3% [4]). This may be due to population differences or regional differences in carcinogen exposure. Previously, it has been speculated that cancers bearing frequent TERT promoter mutations largely originate in tissues that are not constantly self-renewing under normal conditions [4]. In our study, the cancer types that lacked TERT promoter mutations may have other mechanisms for telomere maintenance. To further understand these mechanisms, other factors must be considered, including TERT promoter methylation, genomic structural rearrangements in the TERT promoter, TERT copy number, and alternative lengthening of telomeres (ALT) [4], [19], [20], [21].
In addition to previously reported C228T, C250T, A161C, C158A, G149T, C242T+C243T, and G245A mutations, we identified five novel mutations: T198G, C193T, C190T+C184T, and C250T+C242T+C243T. At present we can only assume these mutations are somatic pending validation sequencing in matched normal tissue DNA. However, it is still interesting to note aside from C228T, C250T, and C242T+C243T, the other 8 mutations we identified were only found in bladder cancer. This pattern is also seen in melanoma, which likely reflects consequences of UV-exposure [2], [22]. Further study is warranted to investigate the etiology of this mutation bias in bladder cancer, which may be due to carcinogen exposure.
The TERT promoter has not been sequenced previously in thymic epithelial tumors, gastrointestinal leiomyoma, or gastric schwannoma. Our result suggests that TERT promoter mutation is absent in these tumor types.
In addition, we have found a notable absence of TERT promoter mutations in gastric cancer, pancreatic cancer, GIST and meningiomas in Chinese patients, consistent with reports in other populations [4], [9]. A recent report has found that TERT promoter mutations occurred in 28% of meningiomas undergoing malignant transformation [23]. Only 1 of 76 meningiomas we sequenced were invasive (grade III) which may explain this difference. We also identified a TERT C228T mutation in gallbladder carcinoma (1/2). However GBC samples were limited and additional samples are needed for further investigation.
In this study, we evaluated the functional consequences of TERT promoter mutations in gliomas. TERT mRNA expression levels were markedly higher in the samples that harbored TERT promoter mutations, which is consistent with previous findings in follicular thyroid adenoma [24] and adrenal tumors [25]. We found that TERT promoter mutations are highly correlated with telomerase activation using the TRAP assay in both xenografts and primary tumor tissues. The findings imply that TERT promoter mutations are highly correlated with telomerase activation in adult gliomas. However, the exact effect of this telomerase activation on changes in state and length of telomeres needs further investigation.
Among the mutations identified (Table S1), C228T, C250T, C242T+C243T, C250T+C242T+C243T, A161C, C158A and G149T generate a CCGGAA/T or GGAA/T motif, which is a possible transcription factor binding site for Ets transcription factors (Figure 1). We compared the transcriptional activity of the high frequency (n≥6) mutations using a luciferase reporter assay and found that they could all increase TERT promoter activity.
Our findings show similar distribution of TERT promoter mutations in tumor types from a Chinese population. Alongside our functional assays, this work reflects the importance of these mutations to telomere maintenance and human tumorigenesis. Development of sensitive assays for detection of these mutations may aid in earlier diagnosis of several tumor types.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81372598, No.81071991, No.81000016), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents, Major projects of Science and Technology Department of Zhejiang Province (No.2011C13036-1, 2013T301-13). the V Foundation, the Accelerate Brain Cancer Cure Foundation, the Slomo and Cindy Silvian Foundation, the Voices Against Brain Cancer Foundation, the Pediatric Brain Tumor Foundation Institute at Duke, the James S. McDonnell Foundation, American Cancer Society Research Scholar Award RSG-10-126-01-CCE, and National Cancer Institute Grants 5R01-CA140316, 5 P50 NS020023-30, and 5 P01 CA154291-01. The authors would like to thank Stephen Keir, Diane Satterfield, Lisa Ehinger, Merrie Thomas, and David Lister for assistance in collection of samples at Duke University.
Abbreviations Used
- TERT
telomerase reverse transcriptase
- TRAP
telomeric repeat amplification protocol
- GIST
gastrointestinal stromal tumor
- ETS
E-twenty-six
- FFPE
formalin-fixed, paraffin-embedded
- DNA
deoxyribonucleic acid
- mRNA
messenger ribonucleic acid
- RT-qPCR
reverse-transcriptase quantitative polymerase chain reaction
- PCR
polymerase chain reaction
- BGI
Beijing Genomics Institute
- GBC
gallbladder carcinoma
- HCC
hepatocellular carcinoma
- GBM
glioblastoma multiforme
- ALT
alternative lengthening of telomeres
- MEM
minimum essential media
- C228T
mutation at chr5: 1,295,228 C>T. All other TERT promoter mutations are stated in this fashion (i.e. C250T is mutation at chr5: 1,295,250 C>T)
Footnotes
Conflict of Interest
Under agreements between Duke University and Blueprint Medicines H.Y. is entitled to a share of the royalties received by the University on sales of products related to genes described in this manuscript. H.Y. receives royalties from Agios Pharmaceuticals, Sanofi-Aventis and Personal Genome Diagnostics. S.W. and H.Y. are co-founders and own stocks of Beijing Pangenomics Technology, Co. Ltd.
References
- 1.Gomez DE, Armando RG, Farina HG, Menna PL, Cerrudo CS, Ghiringhelli PD, et al. Telomere structure and telomerase in health and disease (review) Int J Oncol. 2012;41(5):1561–9. doi: 10.3892/ijo.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–61. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 3.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267–76. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
- 6.Hurst CD, Platt FM, Knowles MA. Comprehensive Mutation Analysis of the TERT Promoter in Bladder Cancer and Detection of Mutations in Voided Urine. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 7.Kinde I, Munari E, Faraj SF, Hruban RH, Schoenberg M, Bivalacqua T, et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013;73(24):7162–7. doi: 10.1158/0008-5472.CAN-13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachakonda PS, Hosen I, de Verdier PJ, Fallah M, Heidenreich B, Ryk C, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci U S A. 2013;110(43):17426–31. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 10.Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218. doi: 10.1038/ncomms3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashizume R, Smirnov I, Liu S, Phillips JJ, Hyer J, McKnight TR, et al. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J Neurooncol. 2012;110(3):305–13. doi: 10.1007/s11060-012-0973-6. [DOI] [PubMed] [Google Scholar]
- 12.Xue QC, Pu PY, Yang YS, Shen CH. A survey of 790 cases of astrocytoma. Clin Neurol Neurosurg. 1990;92(1):27–33. doi: 10.1016/0303-8467(90)90004-o. [DOI] [PubMed] [Google Scholar]
- 13.Cai DW, Liu XF, Bu RG, Chen XN, Ning L, Cheng Y, et al. Genetic polymorphisms of MTHFR and aberrant promoter hypermethylation of the RASSF1A gene in bladder cancer risk in a Chinese population. J Int Med Res. 2009;37(6):1882–9. doi: 10.1177/147323000903700625. [DOI] [PubMed] [Google Scholar]
- 14.Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, et al. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin Liver Dis. 2010;19(3):311–7. [PubMed] [Google Scholar]
- 15.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 16.Venuta F, Rendina EA, Anile M, de Giacomo T, Vitolo D, Coloni GF. Thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg. 2012;60(1):1–12. doi: 10.1007/s11748-011-0814-0. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 18.Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas. 2005;31(1):13–22. doi: 10.1097/01.mpa.0000168220.97967.d1. [DOI] [PubMed] [Google Scholar]
- 19.Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46(12):1267–73. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 20.Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26(3):319–30. doi: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castelo-Branco P, Choufani S, Mack S, Gallagher D, Zhang C, Lipman T, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–42. doi: 10.1016/S1470-2045(13)70110-4. [DOI] [PubMed] [Google Scholar]
- 22.Populo H, Boaventura P, Vinagre J, Batista R, Mendes A, Caldas R, et al. TERT Promoter Mutations in Skin Cancer: the Effects of Sun Exposure and X-Irradiation. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.163. [DOI] [PubMed] [Google Scholar]
- 23.Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High Incidence of Activating TERT Promoter Mutations in Meningiomas Undergoing Malignant Progression. Brain Pathol. 2013 doi: 10.1111/bpa.12110. [DOI] [PMC free article] [PubMed]
- 24.Wang N, Liu T, Sofiadis A, Juhlin CC, Zedenius J, Hoog A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014;120(19):2965–79. doi: 10.1002/cncr.28800. [DOI] [PubMed] [Google Scholar]
- 25.Liu T, Brown TC, Juhlin CC, Andreasson A, Wang N, Backdahl M, et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr Relat Cancer. 2014;21(3):427–34. doi: 10.1530/ERC-14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.