Abstract
In a sample of 50 verbally fluent adolescents and adults with autism spectrum disorders (ASD) (age: 16-31 years; verbal IQ: 72-140), we examined the pattern of response and associations between scores on common measures of depressive symptoms, participant characteristics, and clinical diagnosis of depressive disorders. Beck Depression Inventory, 2nd edition (BDI-II) item descriptives in this ASD sample were compared to previously published data from a large typically developing sample, with results suggesting that cognitive-attributional symptoms of depression may be particularly prevalent in ASD. Scores on a variety of self- and parent-report depression measures were not associated with chronological age or verbal IQ, and were relatively highly correlated with each other and with clinical diagnosis of a mood disorder. The BDI-II and the Adult Self-Report “Depressive” scale best identified both depressed and non-depressed participants in this sample, though neither was particularly strong. Validation studies of depression measures in the ASD population are necessary to advance research into this prevalent and impairing comorbidity.
Keywords: autism spectrum disorder, depression, self-report, Beck Depression Inventory, Adult Behavior Checklist, Adult Self Report
Findings from clinic-based and community studies suggest that depression is highly prevalent in individuals with autism spectrum disorder (ASD) (Lugnegard, Hallerback, & Gillberg, 2011; Mayes, Calhoun, Murray, & Zahid, 2011; Mazefsky, Conner, & Oswald, 2010; Leyfer et al., 2006; Howlin, 2000; Kim, Szatmari, Bryson, Streiner, & Wilson, 2000). Despite the pressing need for research into the causes, consequences, and treatment of co-occurring depression in ASD, progress is complicated by obstacles in adequately assessing depressive symptoms in individuals with ASD. Observation of depression in ASD may be impeded by differences in depression presentation and phenomenology between ASD and typically developing populations. Assessment of most depression criteria in the general population relies on self-report, which in turn relies on insight and communication skills often absent or impaired in ASD. We address both in turn.
Presentation of depression in ASD
Characteristics of autism can complicate observation of, and eventual diagnosis based on, depressive symptoms. Common symptoms of depression, such as those related to sleep, ability to concentrate, and communication of affect through facial expression or intonation may be easily masked by pre-existing symptoms of autism (Stewart, Barnard, Pearson, Hasan, & O'Brien, 2006). Nevertheless, a number of depressive symptoms common in the general population tend to be observed in cases with comorbid ASD, including sadness and tearfulness (Lainhart & Folstein, 1994; Perry et al., 2001; Stewart et al., 2006), apathy, anhedonia, and loss of interest in activities (Clarke, Littlehouse, Corbett, & Joseph, 1989; Gillberg, 1985), decreased self-care (Clarke, Baxter, Perry, & Prasher, 1999; Wing, 1981), and psychomotor retardation (Ghaziuddin & Tsai, 1991). A review of relatively small studies (Stewart et al., 2006) summarized that feelings of worthlessness or guilt are not endorsed frequently in ASD, perhaps due in part to difficulties with self-report. Other possible depressive symptoms more specific to or common in ASD might include irritability and agitation, increase in social withdrawal beyond an individual's baseline, a change in the character of obsessions (with fixations taking on a more morbid tone), and an increase in compulsive behavior (Ghaziuddin, 2005). Increased self-injury and regression of adaptive skills may be particularly significant symptoms of depression in less cognitively able individuals with ASD (Magnuson & Constantino, 2011). Overall, the presentation of depression in ASD depends on age, level of intelligence, and level of verbal skills. While depression or depressive symptoms can occur across the entire autism spectrum (Stewart et al., 2006), individuals who have more verbal skills or milder ASD symptoms seem to be either particularly affected or more easily identified (Cederlund, Hagberg, & Gillberg, 2010; Hurtig et al., 2009).
Self-report on depressive symptoms in ASD
Most standard depression diagnostic measures require self-report and rely on both the insight to recognize symptoms and the verbal aptitude to describe them. Effects of age, intellectual functioning, and level of verbal skills must be considered carefully in interpreting self-report data. Individuals with ASD who have intact or minimally-impaired cognitive and verbal abilities can better report depressed mood and loss of interest in previously enjoyed activities than can less able individuals within the population. However, even those with relatively well-developed language often have difficulty expressing feeling or mood states and may fail to use abstract concepts or metaphors (Rieffe, Terwogt, & Kotronopoulou, 2007; Perry, Marston, Hinder, Munden, & Roy, 2001; Hill, Berthoz, & Frith, 2004). It is possible that this could translate into a paradox (as yet untested) in which individuals with ASD more accurately identify emotions in others (e.g., through interpreting facial expressions) than in themselves (we thank an anonymous reviewer for this comment). Research suggests that individuals on the autism spectrum tend to perceive, remember, and interpret both social and nonsocial information differently, and often exhibit limited insight and perspective-taking skill (Johnson, Filliter, & Murphy, 2009; Hedley & Young, 2006; Stewart et al., 2006; Beebe & Risi, 2003; Blackshaw, Kinderman, Hare & Hatton, 2001; Hare, 1997). For example, in a 2004 paper by Hill and colleagues, 27 adults with high-functioning autism (HFA; i.e., ASD and IQ>70), had much more difficulty identifying and describing feelings, and had more externally oriented thinking, than did 35 adult controls and 47 ASD family members. Almost 85% of the ASD group fell in the slightly or severely impaired ranges on the Toronto Alexithymia Scale, a questionnaire that operationalizes deficiency in understanding, processing, and/or describing emotions, whereas 79-83% of the control groups fell in the unimpaired range on this measure.
Validity of depression diagnosis is potentially compromised when individuals with ASD do not have sufficient ability to communicate about abstractions in order to describe their internal states (Costello, Egger, & Angold, 2005). Despite the implied difficulty of reporting on their feelings, however, the ASD group in the Hill et al. sample endorsed high levels of depressive symptoms on the Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996), with 75% meeting clinical cut-offs for depressive concern versus 27% of the relatives and 17% of the typical controls. In a 2010 study by Cederlund and colleagues, scores on the Beck Depression Inventory (BDI) were consistent with clinical diagnoses of depression in a sample of 76 young men with Asperger syndrome. This is a promising start, though validation studies of depression inventories and interviews are needed in ASD samples.
Informant report on depressive symptoms in ASD
Because of the inherent difficulties with self-report in ASD, most measures of the autism phenotype are based on parent report, even for adults. However, in the general depression literature, corroborating reports of an adult's depressive symptoms from other informants (e.g., spouse or parent) have received negligible attention. This has been an obstacle to validation studies of general depression symptom measures in adults with ASD – what should such instruments be validated against, as gold standard indicators of depression in ASD? This remains unclear. Even if parent report of an adult's depressive symptoms were accessible, self- and other-ratings often exhibit minimal association in various contexts and populations. For example, White & Roberson-Nay (2009) found little agreement between parent- and self-reports of anxiety in their school-aged ASD sample (n=20). In such cases, we do not know which raters produce data more reflective of the true clinical phenomenon. Therefore, descriptive analyses continue to be informative, though not conclusive (see also White, Schry, & Maddox, 2012).
Study aims
To comment on the phenomenology of depression in ASD, we highlight patterns of depressive symptom endorsement in a verbal adolescent and adult ASD sample, and compare our BDI-II item data to previously published means from a large typically developing sample of approximately the same age (Dozois, Dobson, & Ahnberg, 1998). We next describe the associations between several common measures of depressive symptoms, chronological age, verbal IQ, and clinical diagnosis of depressive disorders in our ASD sample. As we used adapted parent report measures for assessment of adult children's depressive symptoms, we will comment on the relationship between self- and parent-report of depressive symptoms in ASD. We hope that this work will illuminate the profile of depressive symptomatology in ASD and help inform choices of depression assessment or screening measures in future ASD research.
Methods
Participants
This sample represents data from 50 adolescents and adults with best estimate clinical diagnoses of an autism spectrum disorder. Inclusion was limited to those with a verbal IQ (VIQ) of 70 or greater and reading comprehension at the fifth-grade level or beyond, due to hypotheses tested in a parent study (Gotham, Bishop, Brunwasser, & Lord, 2014). Participants ultimately ranged in age from 16 to 31 years old (M=20.7 years, SD=3.9). Mean VIQ was 105 (SD=17.7; Range=72-140) and nonverbal IQ was 101 (SD=15.8; Range=73-138). Data were available from 5 females (10% of the sample). Race and ethnicity of the sample was 82% Caucasian (n=41), 12% African American (n=6), and one person (2%) each from the Asian/Pacific Islander, American Indian, and ‘two or more racial affiliations’ categories. See Table 1 for a detailed description of the sample. See Gotham et al., 2014 for more eligibility and demographic information about this sample.
Table 1.
Sample Description
| N | Range | Mean(SD) | |
|---|---|---|---|
| Age in Years | 50 | 16-31 | 20.7 (3.9) |
| VIQ | 50 | 72-140 | 105.0 (17.7) |
| NVIQ | 49 | 73-138 | 101.0 (15.8) |
| ADI-R Social | 35 | 2-30 | 16.0 (8.3) |
| ADI-R CommV | 35 | 0-24 | 13.4 (6.2) |
| ADI-R CommNV | 35 | 0-14 | 7.3 (4.4) |
| ADI-R RRB | 44 | 0-12 | 5.4 (2.7) |
| ADOS Comm | 35 | 0-5 | 2.8 (1.3) |
| ADOS Soc | 35 | 2-14 | 6.7 (2.7) |
| ADOS Comm-Soc | 35 | 3-18 | 9.5 (3.2) |
| ADOS Stereo | 35 | 0-6 | 0.9 (1.5) |
| VCST | 49 | 33-113 | 76.8 (17.4) |
| VDLST | 50 | 36-107 | 74.4 (14.1) |
| VSST | 50 | 25-107 | 72.4 (15.5) |
| VABCST | 49 | 28-104 | 72.0 (13.8) |
| BDI-II | 50 | 0-28 | 9.9 (8.2) |
| SRDQ | 48 | 32-72 | 51.8 (9.9) |
| ASR Withdrawn T | 18 | 50-87 | 66.1(11.6) |
| ASR Depressive T | 18 | 50-82 | 64.6(10.9) |
| ASR Internalizing T | 18 | 51-82 | 66.7(10.3) |
| CDRS | 46 | 14-62 | 26.6 (10.5) |
| CDI-Parent, Func | 21 | 1-14 | 6.9 (3.5) |
| CDI-Parent, Emot | 21 | 0-14 | 8.1 (3.3) |
| ABCL Withdrawn T | 29 | 50-97 | 67.6(12.4) |
| ABCL Depressive T | 29 | 50-85 | 65.0(8.8) |
| ABCL Internalizing T | 29 | 50-90 | 67.2(10.2) |
| AMAS | 18 | 10-29 | 20.2(6.3) |
| SCAS-P | 21 | 6-55 | 25.1(13.1) |
Note. VIQ=Verbal IQ; NVIQ=Nonverbal IQ; ADI-R Social=ADI-R Social Total; ADI-R CommV=ADI-R Communication Total for Verbal Subjects; ADI-R CommNV=ADI-R Communication Total for Nonverbal Subjects; ADI-R RRB=ADI-R Restricted, Repetitive Behaviors Total; ADOS Comm=ADOS Communication Total (Module 4); ADOS Soc=ADOS Reciprocal Social Interaction Total (Module 4); ADOS Comm-Soc=ADOS Communication+Reciprocal Social Combined Total (Module 4); ADOS Stereo=ADOS Stereotyped Behavior and Restricted Interests Total (Module 4); VCST=Vineland II Communication standard score; VDLST=Vineland II Daily Living Skills standard score; VSST=Vineland II Socialization standard score; VABCST=Vineland II Overall Adaptive Behavior Composite standard score; BDI-II=Beck Depression Inventory-II total; SRDQ=Self-Report Depression Questionnaire total; ASR Withdrawn T=Adult Self-Report ‘Withdrawn’ subscale T-score; CDRS=Children's Depression Rating Scale total score (adapted for adults); CDI-Parent, Func=Children's Depression Inventory, Parent Version (adapted for adults), Functional Scale; CDI-Parent, Emot=Children's Depression Inventory, Parent Version (adapted for adults), Emotional Scale; ABCL Withdrawn T=Adult Behavior Checklist ‘Withdrawn’ subscale T-score; AMAS=Adult Manifest Anxiety Scale; SCAS-P= Spence Children's Anxiety Scale-Parent.
Data were collected from two subsets of participants: 29 individuals (58% of the sample) were seen as part of an ongoing longitudinal research protocol to assess then 16-22 year old participants who had been consecutive ASD referrals at age 2 (Lord et al., 2006). An additional 21 families were recruited specifically for this study, via current or previous participation with the University of Michigan Autism and Communication Disorders Center (UMACC) or public recruitment. Because of the practical difficulties of combining lengthy study protocols, a number of the longitudinal study participants received some but not all of the current measures of interest. For example, the Children's Depression Inventory, Adult Behavior Checklist, and Adult Self-Report (see below) were available only from the participants collected at UMACC (n=21; Age M=23.3 years, SD=4.9 years; VIQ M=106, SD=18.1; 86% Caucasian; 14% Female). See Table 1 for sample size per measure.
Ten participants received a secondary diagnosis of a current active mood disorder (20% of sample; n=7, Major Depressive Disorder; n=2, Dysthymic Disorder; n=1 Mood Disorder-Not Otherwise Specified). Twelve individuals received a diagnosis of a current anxiety disorder (24% of the sample; n=4, Generalized Anxiety Disorder; n=3, Obsessive-Compulsive Disorder; n=5, Anxiety Disorder-Not Otherwise Specified).
Procedures
The data collection protocol included a packet of questionnaires and a face-to-face assessment for both the adolescent or adult participant with ASD (i.e., proband) and his/her parent. Data were collected and clinical diagnoses assigned by advanced graduate students and research assistants, all of whom had undergone extensive training to achieve research reliability on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, & Lord, 2003); this took place under the supervision of licensed clinical psychologists. All relevant clinicians/examiners discussed the case and came to a consensus agreement about all clinical diagnoses based on all available information. Questionable cases were reviewed by authors [K.G., C.L.]. The University of Michigan Institutional Review Board in Health and Behavioral Sciences approved all procedures related to this study.
Proband measures
Probands completed the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), the Neale Analysis of Reading Ability (Neale, 1997) or the Wide Range Achievement Test (WRAT; Wilkinson & Robertson, 2006) reading comprehension subtests in order to verify reading comprehension necessary to complete questionnaires, the ADOS (Lord et al., 2000) to confirm ASD diagnosis, the Adult Manifest Anxiety Scale--Adult Self-Report (AMAS-A; Reynolds, Richmond, & Lowe, 2003), as well as the following measures of depressive symptoms:
The Beck Depressive Inventory, 2nd edition (BDI-II; Beck, Steer, & Brown, 1996), a 21-item self-report questionnaire designed for adolescents and adults that measures emotions related to depression, somatic/physical symptoms, and lifestyle changes on a 0-3 scale. It has been found to have high internal consistency, as well as strong convergent validity (Dozois et al., 1998).
The 32-item, closed-ended Self-Report Depression Questionnaire (SRDQ; Reynolds & Baker, 1988), designed to measure the physical, cognitive, and behavioral aspects of depression in adults with mild to moderate intellectual disability (Esbensen, Seltzer, Greenberg, & Bensen, 2005), and found to have high internal consistency, criterion and predictive validity compared to established clinical interview scales in this focal population (Esbensen et al., 2005).
The Adult Self Report (ASR; Achenbach & Rescorla, 2003), a 123-item self-report measure for adults aged 18-59 that assesses a wide variety of symptom areas, including anxiety/depression, withdrawal/depression, somatic complaints, social problems, thought problems, attention-deficit/hyperactivity problems, rule-breaking behavior, aggressive behavior, and affective problems. These domains stand alone as well as contribute to Internalizing and Externalizing scales. Note that the Youth Self Report (YSR; Achenbach, 1991), a similar measure intended for children aged 11 to 18, was collected in the UMACC subsample but is not reported on here due to very small sample size.
Parent measures
Parent participants completed a face-to-face assessment consisting of the Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, & Lord, 2003), the second edition of the Vineland Adaptive Behavior Scales (Sparrow, Cicchetti, & Balla, 2005) to assess proband adaptive functioning, the Spence Children's Anxiety Scale-Parent (SCAS-P; Nauta, et al., 2004), as well as the following depression measures:
The Children's Depression Rating Scale (CDRS; Poznanski & Mokros, 1996), a semi-structured interview for use with children, young adolescents, or their parents that has been shown to have good internal consistency and convergent validity with established global functioning scales (Mayes, Bernstein, Haley, Kennard, & Emslie, 2010).
The Children's Depression Inventory, parent-rated version (CDI-P; Kovacs, 1992) is intended for children aged 7-17 and assesses depressive symptoms within the domains of Emotional and Functional problems as discrete scales.
The Adult Behavior Checklist (ABCL; Achenbach & Rescorla, 2003), an 118-item scale designed to be rated by parents of adult children aged 18 to 59. The ABCL is based on a multi-factor model, for which domains of withdrawn, somatic problems, and anxious-depressed are indicators of a more general “Internalizing” factor (Tenneij & Koot, 2007). Note that the Child Behavior Checklist (CBCL/6-18; Achenbach, 1991), a version of this measure intended for children aged 6 to 18, was collected in the BLINDED subsample but is not reported on here due to very small sample size.
We adapted the CDRS, CDI-P, and SCAS-P for parents of adult children by providing developmentally appropriate options (e.g., “school/work,” “classmates/co-workers”) and minimally updated wording (“hang out/ socialize” rather than “play with”); no changes were made to symptom content.
Design and analyses
We first described BDI-II item endorsement by magnitude in this sample, using t-tests to compare published means from a large undergraduate sample. We examined bivariate correlations between the several measures of depressive symptoms, including (1) self-report questionnaires: raw scores on the BDI-II, SRDQ, and the withdrawn, depressive, and internalizing scales of the ASR; (2) parent-report instruments: raw scores on the CDRS interview, the CDI, and the withdrawn, depressive, and internalizing scales of the ABCL; and (3) between all measures and participant chronological age and verbal IQ. Intraclass correlations indicated internal reliability for the four depression measures with available item data.
Clinical diagnosis of any mood disorder (with a binary yes/no coding) was cross-tabulated against ranges of clinical concern in those depression measures (BDI-II raw, CDRS, ABCL, and ASR T-scores) with clinical cut-offs that could be calculated for the age range of our sample. Chi-square analysis was used to assess gender distribution by depressive disorder status. Logistic regression modeling was used to evaluate the relation between continuous depression raw scores and clinical diagnosis of mood disorder, as well as to assess significant independent contributions of the depression measures (i.e., over each other) as predictors of clinical diagnosis. Across all analyses, we applied a criterion-standard p-value of .01 to balance the number of analyses performed with the increased Type II error associated with a relatively small sample size.
Results
Depressive symptom endorsement in ASD
Ten individuals in our sample met criteria for a clinical diagnosis of a current mood disorder (20% of the overall sample). Of note, 3 out of 5 women had depressive diagnoses, versus 7 out of 45 men (χ2(df)=5.56(1); p=.02). In order to comment on specific depressive symptom endorsement in ASD, we present BDI-II item means in order of magnitude within depressed and non-depressed participants separately (see Table 2). Given the small sample size with comorbid depression, these results are considered exploratory.
Table 2.
Patterns in endorsement of Beck Depression Inventory, 2nd edition, items
| Highest to Lowest Item Means in Non-Depressed ASD | Highest to Lowest Item Means in Depressed ASD | By Magnitude of Mean Difference (From ASD > TYP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDI-II Item | n=40 | BDI-II Item | n=10 | BDI-II Item | Current Study N=50 (ASD) | Dozois et. al N=1022 (TYP) | Group difference | t* | |||
| Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | ||||||||
| 5 | guilty feelings | 0.68(0.66) | 2 | pessimism | 1.60(.97) | 5 | guilty feelings | 0.77(0.69) | 0.39(0.56) | 0.38 | 21.34 |
| 16 | changes sleeping | 0.68(0.92) | 6 | punishment feelings | 1.50(1.10) | 3 | past failure | 0.7(0.88) | 0.35(0.60) | 0.35 | 18.04 |
| 3 | past failure | 0.59(0.87) | 15 | loss of energy | 1.30(1.10) | 6 | punishment feelings | 0.52(0.85) | 0.24(0.60) | 0.28 | 14.48 |
| 8 | self-criticism | 0.59(0.68) | 3 | past failure | 1.10(.87) | 14 | worthlessness | 0.48(0.80) | 0.21(0.54) | 0.27 | 15.47 |
| 13 | indecisiveness | 0.55(0.72) | 5 | guilty feelings | 1.10(.74) | 2 | pessimism | 0.67(0.86) | 0.41(0.56) | 0.26 | 14.30 |
| 2 | pessimism | 0.42(0.64) | 7 | self-dislike | 1.10(1.28) | 13 | indecisiveness | 0.63(0.79) | 0.4(0.69) | 0.23 | 10.52 |
| 18 | changes in appetite | 0.42(0.79) | 8 | self-criticism | 1.10(.99) | 1 | sadness | 0.46(0.50) | 0.27(0.50) | 0.19 | 12.12 |
| 14 | worthlessness | 0.37(0.75) | 11 | agitation | 1.10(.99) | 8 | self-criticism | 0.7(0.78) | 0.55(0.68) | 0.15 | 6.96 |
| 19 | diff to concentrate | 0.37(0.63) | 19 | diff to concentrate | 1.10(1.10) | 9 | suicidal thoughts | 0.27(0.49) | 0.18(0.41) | 0.09 | 6.93 |
| 1 | sadness | 0.34(0.48) | 20 | tiredness/fatigue | 1.00(.94) | 12 | loss of interest | 0.46(0.74) | 0.37(0.61) | 0.09 | 4.64 |
| 11 | agitation | 0.34(0.67) | 16 | changes sleeping | 1.00(.82) | 7 | self-dislike | 0.48(0.90) | 0.41(0.72) | 0.07 | 3.04 |
| 12 | loss of interest | 0.34(0.67) | 1 | sadness | .90(.32) | 11 | agitation | 0.5(0.80) | 0.55(0.62) | −0.05 | 2.52 |
| 7 | self-dislike | 0.32(0.70) | 12 | loss of interest | .90(.88) | 19 | diff to concentrate | 0.52(0.80) | 0.59(0.72) | −0.07 | 3.07 |
| 20 | tiredness/fatigue | 0.32(0.57) | 13 | indecisiveness | .90(.99) | 4 | loss of pleasure | 0.29(0.58) | 0.38(0.56) | −0.09 | 5.11 |
| 15 | loss of energy | 0.29(0.57) | 14 | worthlessness | .90(.88) | 17 | irritability | 0.38(0.49) | 0.48(0.61) | −0.10 | 5.27 |
| 6 | punishment feelings | 0.26(0.55) | 21 | loss interest sex | .90(1.44) | 15 | loss of energy | 0.5(0.80) | 0.64(0.64) | −0.14 | 6.86 |
| 17 | irritability | 0.26(0.45) | 17 | irritability | .80(.42) | 16 | changes sleeping | 0.74(0.90) | 0.88(0.71) | −0.14 | 6.17 |
| 9 | suicidal thoughts | 0.21(0.47) | 4 | loss of pleasure | .70(.95) | 18 | changes in appetite | 0.46(0.74) | 0.63(0.77) | −0.17 | 7.03 |
| 21 | loss interest sex | 0.19(0.57) | 18 | changes in appetite | .60(.52) | 20 | tiredness/fatigue | 0.46(0.71) | 0.65(0.62) | −0.19 | 9.68 |
| 4 | loss of pleasure | 0.18(0.39) | 9 | suicidal thoughts | .50(.53) | 10 | angry | 0.06(0.25) | 0.31(0.64) | −0.25 | 12.73 |
| 10 | angry | 0.03(0.16) | 10 | angry | .20(.42) | 21 | loss interest sex | 0.34(0.87) | Not Reported | NA | NA |
Note. TYP=General population sample
All comparisons significant at p<.001.
We also compared BDI-II item means and standard deviations from our entire ASD sample to those published in a large sample of undergraduates from the general population (N=1022; Dozois et al., 1998; referred to as TYP for typically developing). The two samples were similar in age [M(SD) in years=21(3.9) ASD; 21(4.5) TYP]. Though the TYP sample was 67% female (compared to 10% in our sample, consistent with recent data on gender ratios in ASD; Whitely, Todd, Carr, & Shattuck, 2010), Dozois et al. found no significant differences in BDI-II scores across women and men. They did not report the proportion of participants qualifying for a clinical diagnosis of depression in their sample, however, like our ASD sample, their participants were neither selected nor excluded on this attribute. Because of the vast difference in sample sizes, significance testing was not informative, however in Table 2 we also present BDI-II items ordered from more strongly endorsed in this ASD sample to those more strongly endorsed in the TYP sample. In our ASD sample, participants tended to report cognitive symptoms of depression (e.g., guilty feelings, sense of failure, pessimism) more so than most somatic and affect-related symptoms, and with greater relative prevalence than did the general college sample. In depressed participants with ASD, loss of energy also tended to be strongly endorsed.
Associations between depression measures and participant characteristics
See Table 3 for the association between the different measures of depressive symptoms, anxiety symptoms, chronological age, and verbal IQ. Overall, scores on the various depression instruments were not associated with participant age or verbal IQ, though there was a trend toward significance between the BDI-II and chronological age (r=.27, p=.06).
Table 3.
Association between depression and anxiety instruments, age, and verbal IQ
|
Pearson r
p-value n |
BDI-II | SRDQ | CDI-Total | CDI-F | CDI-E | CDRS-R | ASR-W | ASR-D | ASR-I | ABCL-W | ABCL-D | ABCL-I | AMAS-A | SCAS-P | Verbal IQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDI-II | -- | ||||||||||||||
| SRDQ | 0.7 | ||||||||||||||
| < 0.01 | -- | ||||||||||||||
| 48 | |||||||||||||||
| CDI-Total | 0.63 | 0.52 | |||||||||||||
| 0.002 | 0.02 | -- | |||||||||||||
| 21 | 21 | ||||||||||||||
| Functional | 0.67 | 0.4 | 0.84 | ||||||||||||
| 0.001 | 0.07 | <0.001 | -- | ||||||||||||
| 21 | 21 | 21 | |||||||||||||
| Emotional | 0.28 | 0.36 | 0.82 | 0.39 | |||||||||||
| 0.23 | 0.11 | <0.001 | 0.08 | -- | |||||||||||
| 21 | 21 | 21 | 21 | ||||||||||||
| CDRS-raw | 0.52 | 0.47 | 0.81 | 0.73 | 0.62 | ||||||||||
| <0.001 | 0.001 | <0.001 | <0.001 | 0.003 | -- | ||||||||||
| 46 | 44 | 21 | 21 | 21 | |||||||||||
| ASR Withdrawn | 0.43 | 0.38 | 0.43 | 0.76 | −0.06 | 0.4 | |||||||||
| 0.07 | 0.12 | 0.07 | <0.001 | −0.82 | 0.1 | -- | |||||||||
| 18 | 18 | 18 | 18 | 18 | 18 | ||||||||||
| Depressive | 0.617 | 0.81 | 0.45 | 0.43 | 0.22 | 0.28 | 0.56 | ||||||||
| 0.01 | < 0.001 | 0.06 | 0.08 | 0.38 | 0.26 | 0.02 | -- | ||||||||
| 18 | 18 | 18 | 18 | 18 | 18 | 18 | |||||||||
| Internalizing | 0.73 | 0.82 | 0.52 | 0.55 | 0.22 | 0.31 | 0.69 | 0.88 | |||||||
| 0.001 | <0.001 | 0.03 | 0.02 | 0.39 | 0.22 | 0.001 | <0.001 | -- | |||||||
| 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | ||||||||
| ABCL Withdrawn | 0.31 | 0.03 | 0.73 | 0.86 | 0.29 | 0.42 | 0.57 | 0.3 | 0.36 | ||||||
| 0.11 | 0.88 | 0.001 | <0.001 | 0.24 | 0.02 | 0.01 | 0.23 | 0.14 | -- | ||||||
| 29 | 29 | 18 | 18 | 18 | 29 | 18 | 18 | 18 | |||||||
| Depressive | 0.307 | 0.27 | 0.65 | 0.64 | 0.4 | 0.61 | 0.67 | 0.58 | 0.54 | 0.47 | |||||
| 0.11 | 0.16 | 0.003 | 0.01 | 0.1 | <0.001 | 0.002 | 0.01 | 0.02 | 0.01 | -- | |||||
| 29 | 29 | 18 | 18 | 18 | 29 | 18 | 18 | 18 | 29 | ||||||
| Internalizing | 0.45 | 0.3 | 0.75 | 0.67 | 0.51 | 0.6 | 0.46 | 0.46 | 0.47 | 0.66 | 0.82 | ||||
| 0.013 | 0.11 | <0.001 | 0.003 | 0.03 | 0.001 | 0.06 | 0.05 | 0.04 | <0.001 | <0.001 | -- | ||||
| 29 | 18 | 18 | 18 | 18 | 29 | 18 | 18 | 18 | 29 | 29 | |||||
| AMAS-Adult | 0.55 | 0.63 | 0.43 | 0.3 | 0.3 | 0.21 | 0.3 | 0.75 | 0.76 | 0.13 | 0.34 | 0.28 | |||
| 0.02 | 0.01 | 0.07 | 0.22 | 0.23 | 0.41 | 0.22 | <0.001 | <0.001 | 0.61 | 0.16 | 0.26 | -- | |||
| 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | ||||
| SCAS-Parent | 0.49 | 0.58 | 0.62 | 0.43 | 0.54 | 0.37 | 0.15 | 0.46 | 0.45 | 0.51 | 0.47 | 0.73 | 0.41 | ||
| 0.02 | 0.01 | 0.003 | 0.51 | 0.01 | 0.1 | 0.55 | 0.06 | 0.06 | 0.03 | 0.04 | 0.001 | 0.09 | -- | ||
| 21 | 21 | 21 | 21 | 21 | 21 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | |||
| Verbal IQ | −0.12 | −0.06 | 0.34 | 0.28 | 0.26 | 0.08 | 0.16 | 0.19 | 0.09 | 0.23 | 0.27 | 0.11 | 0.39 | 0.27 | |
| 0.41 | 0.68 | 0.14 | 0.22 | 0.25 | 0.6 | 0.54 | 0.45 | 0.71 | 0.24 | 0.15 | 0.58 | 0.11 | 0.24 | -- | |
| 50 | 48 | 21 | 21 | 21 | 46 | 18 | 18 | 18 | 29 | 29 | 29 | 18 | 21 | ||
| Current Age | 0.27 | 0.19 | 0.08 | −0.05 | 0.21 | 0.16 | −0.2 | −0.16 | −0.06 | −0.26 | 0.17 | −0.01 | −0.01 | −0.01 | 0.12 |
| 0.06 | 0.19 | 0.73 | 0.82 | 0.37 | 0.28 | 0.42 | 0.54 | 0.81 | 0.17 | 0.39 | 0.99 | 0.96 | 0.99 | 0.42 | |
| 50 | 48 | 21 | 21 | 21 | 46 | 18 | 18 | 18 | 29 | 29 | 29 | 18 | 21 | 50 | |
Note: Bold-type font (for correlation coefficients) and italic-type font (for p-values) indicate significance at the p<.01 criterion standard. BDI-II= Beck Depression Inventory-II total; SRDQ=Self-Report Depression Questionnaire total; CDI-F=Children's Depression Inventory, Functional Scale; CDI-E=Children's Depression Inventory, Emotional Scale; CDRS-R=Children's Depression Rating Scale total (raw scores); ASR-W=Adult Self Report, Withdrawn Scale; ASR-D=Adult Self Report, Depressive Scale; ASR-I=Adult Self Report, Internalizing Scale; ABCL-W=Adult Behavior Checklist, Withdrawn Scale; ABCL-D=Adult Behavior Checklist, Depressive Scale; ABCL-I=Adult Behavior Checklist, Internalizing Scale; AMAS-Adult=Adult Manifest Anxiety Scale total; SCAS-P=Spence Children's Anxiety Scale-Parent total.
Associations between depression measures within and across raters
As expected, association was highest within raters: the BDI-II and SRDQ were correlated at r=.70 (p<.001), and the correlation coefficients of the Adult Self-Report Internalizing and Depressive scales ranged from .62 to .82 in relation to these measures (the ASR Withdrawn scale was less highly correlated). Parent measures, both interview (CDRS) and questionnaires, generally were significantly correlated [for example, r=.81 (p<.001) for the CDRS-CDI-P; r=.75 (p<.001) for the CDI-P-ABCL Internalizing], with the exception of the CDI-P Emotional scale with the ABCL Withdrawn (r=.29, p=.24) and Depressive scales (r=.40, p=.10). Of note, all associations within parent-report were stronger for the CDI-P Functional Scale than the Emotional Scale, and these two subscales were not strongly associated with each other (r=.39, p=.08) in the small subsample for which CDI-P data were available.
Reliability across self and parent informants was mixed. Within the same format (questionnaire), they tended to be moderately associated, with most correlation coefficients ranging from 0.3 (ABCL-Internalizing and SRDQ) to 0.67 (BDI-II and CDI-P Functional). Weaker associations were observed when they included the CDI-P Emotional subscale, the Withdrawn scales from both the ASR and ABCL, or the ABCL Depressive subscale. In particular, the parent-rated CDI Emotional scale did not show any significant associations with any self-report measure. Surprisingly, associations across both raters and formats were significant: the parent interview (CDRS) was significantly correlated with all self-report questionnaires from r=.47 to .76.
Internal reliability of depression measures
Intraclass correlation coefficients (Cronbach's alphas) were acceptable to strong for the BDI-II (.87), SRDQ (.90), CDI-P (.73), and the CDRS (.85).
Association between clinical diagnosis and instrument ranges of concern
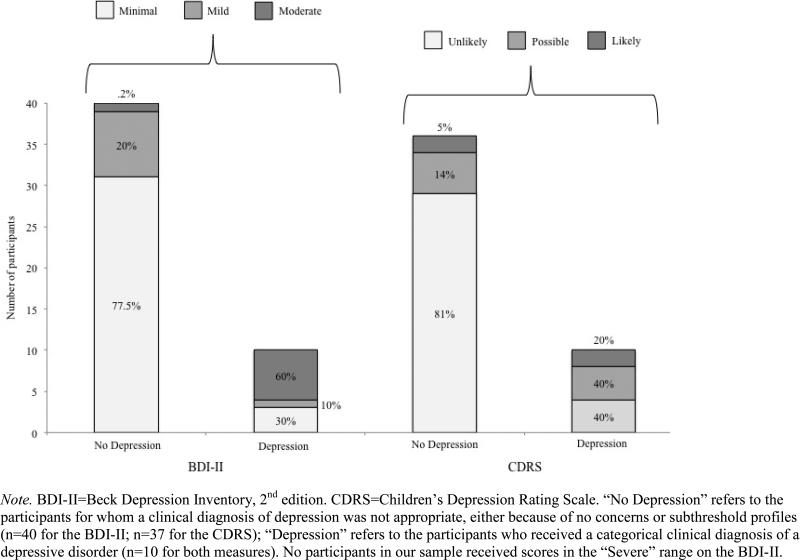
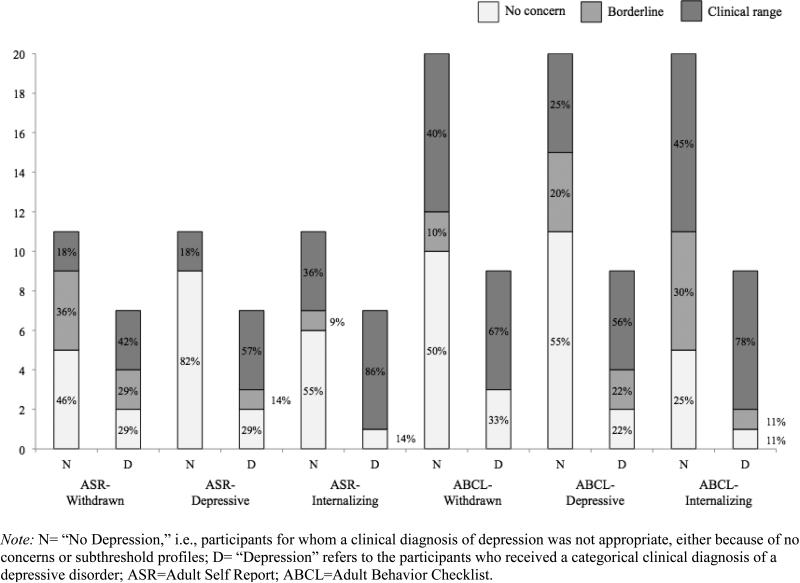
Of the ten participants with depressive disorders, 70% scored in clinical ranges of concern on the BDI-II (1 in Mild, 6 in Moderate), and 60% fell into the “possible” or “likely” depression ranges based on age-independent CDRS T-scores. Approximately 20% of non-depressed participants’ scores also fell in the clinical ranges on these measures. See Figure 1 for details on the number and percentages within each range of concern on the BDI-II and CDRS, based on depressive disorder status, and Figure 2 for the same in the ASR and ABCL.
Figure 1.
Percentages of participants in various ranges of concern on the Beck Depression Inventory-II and the Children's Depression Rating Scale interview.
Figure 2.
Percentages of participants in various ranges of concern on the Adult Self-Report and Adult Behavior Checklist
Of the depressed participants with available Adult Behavior Checklist data (n=9) or Adult Self-Report data (n=7), 71-89% fell into the Borderline or Clinical concern ranges on the Internalizing and Depressive indices -- though 45-75% of non-depressed participants also fell in these ranges. The ASR Depressive subscale appeared to best categorize participants by depressive status (71% of depressed participants in concern ranges and 82% of non-depressed participants in the No Concern range).
Association between clinical diagnosis and dimensional scores
Most self- and parent-report depression measures were associated with clinical diagnosis of depression, at or approaching criterion-standard significance. This included the BDI-II [B(SE)=0.27(0.08), OR=1.31, p=0.002]; SRDQ [B(SE)=0.12 (0.05), OR=1.13, p=0.007]; CDI-P total [B(SE)=0.28 (0.13), OR=1.33, p=0.03]; CDRS [B(SE)=0.12 (0.04), OR=1.17, p=0.005]; and the Internalizing scales on the ASR [B(SE)=0.09(0.05), OR=1.10, p=0.06] and ABCL [B(SE)=0.08 (0.04), OR=1.10, p=0.05]. Further, we used logistic regression modeling to assess for significant independent contributions of each measure to the prediction of depression diagnosis. Only the BDI-II approached significance as making an independent contribution over the SRDQ and CDRS in predicting clinical diagnosis of depression [B(SE)=0.24 (0.10), OR=1.27 p=0.02]. In the smaller subsample with available CDI-P, ASR, and ABCL data, only BDI-II scores accounted for a trend-level proportion of the variance [B(SE)=0.21 (0.11), OR=1.23, p=0.06 against CDI-P and ABCL; and B(SE)=0.30 (0.16), OR=1.35, p=0.06 against ASR]. No parent-report measure contributed to clinical diagnosis of depression above another.
Discussion
Summary of primary aims and results
The aim of this paper was to describe rates of depressive symptom endorsement in ASD and comment on the relationships between scores on common measures of depressive symptoms, participant characteristics (age and verbal IQ), and clinical diagnosis of depressive disorders in this population. It is encouraging to note that, in this sample of individuals with ASD spanning ages 16 to 31 and verbal IQs of 72-140, depression symptom measures were independent of age and verbal IQ. We were surprised to find that cognitive symptoms of depression (i.e., those associated with negative attributions about self and situation, including pessimism, sense of failure or punishment, guilt) were among the most frequently endorsed BDI-II items in our ASD sample (see also Gotham et al., 2014), and in fact were endorsed at higher rates in this sample than in previously published data from a similarly-aged general population sample (Dozois et al., 1998). This stands in contrast to the idea (derived from early reports) that these symptoms might be particularly difficult to express in ASD and therefore less useful markers of this comorbidity (Stewart et al., 2006; Magnuson & Constantino, 2011). Taken together, these findings support the value of self-report on depressive symptoms in the verbally fluent ASD population.
When viewed in light of the rich history of research on cognitive theories of depression (e.g., Beck, Rush, Shaw, & Emery, 1979), our novel findings for cognitive-emotional symptoms of depression in ASD might imply that the phenomenon of depression in ASD is consistent with the typical presentation in the general population, versus an atypical or ASD-specific presentation. Of note, some of these cognitive-emotional symptoms (such as guilt and a sense of failure) were also reported by the non-depressed ASD subsample with greater frequency/severity than was noted in a similar-age general population sample. Perhaps some aspect of ASD confers additional risk for depression (for example, cognitive rigidity that leads to depressive-type rumination; c.f. Gotham et al., 2014). Future research is needed to identify basic mechanisms of risk for depression that may be associated with ASD, as well as processes associated with “tipping” into development of true comorbid depression in this population.
As intended, most depression instrument scores were significantly associated with clinical diagnosis of a depressive disorder. Of those measures with clinical cut-offs applicable to this sample, some under-identified participants with mood disorders (e.g., 60% sensitivity on the CDRS) and many others over-identified non-depressed participants (e.g., 25-55% specificity on the ABCL subscales). Depressive status was best identified by clinical cut-points on the Adult Self-Report Depressive subscale (71% sensitivity and 82% specificity within the small subsample with available data) and the Beck Depression Inventory, 2nd edition (70% sensitivity; 78% specificity). When interpreting these results, it is important to note that the sensitivity statistics in particular are based on a very small sample of 10 individuals with co-occurring ASD and depressive disorders.
Though the depression instruments differ in their administration methods and raters, they were relatively highly correlated with each other in this sample, with the exception of minimal association between parent ratings of emotional symptoms of depression (CDI-P Emotional scale) and the self-report questionnaires. As expected, association between instruments was highest within raters, but many cross-informant associations approached significance as well, suggesting acceptable agreement between parent- and self-report of depression symptoms on these measures in this sample. It is particularly encouraging that our self-report measures were highly correlated, given that the BDI-II and ASR were developed for use in the general population and the SRDQ was created specifically to rate depression in individuals with intellectual disability. Though our sample included only participants with verbal IQs of 70 and above, it was possible that the simplified language on the SRDQ would lead to a stronger performance in individuals with a developmental disorder characterized by deficits in social-communication and insight. This does not seem to be the case within this verbally fluent ASD sample.
Issues in choosing instruments and raters of depression in ASD
This study supports existing evidence of the high prevalence of depressive features in ASD, with 20% of the sample meeting criteria for a clinical diagnosis of a mood disorder, and a large percentage of the non-depressed participants falling into clinical ranges of concern on a variety of measures (see Figures 1 and 2), particularly the Adult Behavior Checklist. In analyses of continuous scores (rather than clinical cut-offs) on these instruments, most of the scales tested were significantly associated with clinical diagnosis of a mood disorder. The BDI-II was the only measure that made a significant independent contribution to the prediction of clinical depression diagnosis (i.e., accounted for more variance than other measures when entered into the same model). There was a trend toward a significant positive relationship between BDI-II scores and chronological age; this could reflect a “real” developmental phenomenon of depressive symptomatology increasing with age (Kessler et al., 2003; Mayes et al., 2011).
We observed weak relation in the CDI-P Emotional subscale to both self-report questionnaires and depression diagnostic status, suggesting that the ability to rate internal emotional symptoms is important in making a diagnosis of depressive disorders within ASD as they are currently conceptualized. Parents are less likely to know and accurately rate the internal experiences of their adolescent and adult children, versus observing and interpreting their physical symptoms and lifestyle changes associated with depression. It may be even more difficult for non-self raters to report on emotional symptoms of depression in the ASD population than it is in the typically developing population (see Hurtig et al., 2009; Lopata et al., 2010). Again we conclude that parent report of depressive symptoms in adolescents and adults with ASD should not necessarily be weighted more heavily than self-report, despite potential limitations to self-report in this population. More work is needed to identify a possible depressive phenotype in individuals with limited emotional insight and/or minimal communication strategies. Development of dedicated parent measures of depressive symptoms in adult children would be valuable across the ability range within ASD.
Limitations
We lacked a ‘gold-standard’ standardized measure (e.g., the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID-I; First & Gibbon, 2004]) to aid in making the clinical diagnoses of depressive disorders, although the CDRS is a well-respected interview that assesses similar information. Our analyses are somewhat circular in that we examined depression measures for association with clinical diagnoses of depression, while the clinical diagnoses were based in part on information from these measures. On the other hand, as we see from the BDI-II and CDRS analyses (Figure 1), as many as 40% of participants (4 out of 10) who were given a clinical diagnosis of a depressive disorder did not have elevated scores that fell into the ranges of concern on one or both of those measures. This indicates broad variability in reported symptoms within the depressed and non-depressed groups, as well as the fact that clinical diagnoses of depressive disorders were not assigned solely on the basis of instrument scores.
We were unable to find parent measures of depressive symptoms in adult children, and therefore we adapted common parent-report measures intended for children through adolescents. The wording and example changes were minimal and likely had little impact on the assessments (e.g., replacing “play with” with “hang out,” or “at school” with “at school/work”). However, it is possible that the symptoms of depression assessed on a childhood measure might not be as applicable to depression in adults, inherently limiting the sensitivity of the measures. Similarly, we do not know if the performance of the CDRS would be improved if T-scores were applied only within the intended age range of the measure. Unfortunately, for those measures that had distinct age-appropriate versions (the CBCL and ABCL, YSR and ASR), administering the correct version based on age later forced us to exclude a portion of our overall sample from specific analyses (e.g., all YSR data) due to small sample sizes. In terms of sample size limitations, we conducted planned testing on a priori hypotheses, and therefore we rely on future replication in larger independent samples rather than setting a higher criterion standard to correct for number of analyses.
Conclusions
Depression instrument scores were minimally associated with age and verbal IQ in this sample that ranged from mid-adolescence through mid-adulthood and borderline cognitive functioning through above average intelligence. The ability of these instruments to quantify depressive symptoms within a “more able” ASD population does not appear to be confounded by these participant characteristics. We observed, however, that clinical cut-offs on several depression measures tended to over-identify participants who did not have clinical diagnoses of depressive disorders, perhaps reflective of the high level of depressive symptomatology in adolescents and adults with ASD who are “subthreshold” for depressive disorders, or potentially due to overlap between depressive and ASD symptoms. In addition, approximately 30-40% of depressed participants were misidentified by measures that had reasonable specificity (the ASR Depressive subscale, BDI-II, and CDRS). We observed that cognitive symptoms of depression (i.e., guilt, feelings of worthlessness) were proportionally more evident in this ASD sample than in a large, typically developing undergraduate sample (Dozois et al., 1998), which indicates the need to revisit our understanding of depression phenomenology in the ASD population. Other future directions include evaluating self-report interviews of depressive symptoms, covarying continuous measures of reading ability in the evaluation of questionnaires, and developing the means to assess mood disorders in individuals with ASD who have limited language and intellectual disabilities.
Acknowledgments
We thank the families that participated in this research, the previous staff of University of Michigan Autism and Communication Disorders Center (UMACC), and Susan White, Somer Bishop, and Christina Jacobsen. This work was supported by the National Institutes of Health [grant numbers MH57167, MH066469, T32-MH18921, R01MH081873-01A1, RC1MH089721, R01HD065277], BLINDED Graduate School, and the Blue Cross Blue Shield of BLINDED Foundation.
Footnotes
Declaration of Conflicting Interests
Dr. Lord receives royalties from the publisher of the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS), mentioned in this paper. Dr. Gotham receives royalties from the ADOS-2, the second edition of the measure described here. Both of these authors donate to charity all royalties from clinics and projects in which they are involved.
References
- Achenbach T. Manual for the Youth Self-Report and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2003. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Author; Washington, DC: 2000. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guilford; New York: 1979. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. The Psychological Corporation; San Antonio, TX.: 1996. [Google Scholar]
- Beebe D, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke M, Dattilio F, Freeman A, editors. Cognitive therapy with children and adolescents: A casebook for clinical practice. 2nd ed. Guilford Press; New York, NY: 2003. pp. 369–401. [Google Scholar]
- Blackshaw A, Kinderman P, Hare D, Hatton C. Theory of mind, causal attribution and paranoia in Asperger syndrome. Autism. 2001;5(2):147–163. doi: 10.1177/1362361301005002005. [DOI] [PubMed] [Google Scholar]
- Cederlund M, Hagberg B, Gillberg C. Asperger syndrome in adolescent and young adult males. Interview, self - and parent assessment of social, emotional, and cognitive problems. Research in Developmental Disabilities. 2010;31(2):287–298. doi: 10.1016/j.ridd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Clarke D, Baxter M, Perry D, Prasher V. The diagnosis of affective and psychotic disorders in adults with autism: Seven case reports. Autism. 1999;3:149–164. [Google Scholar]
- Clarke DJ, LittleJohns CS, Corbett JA, Joseph S. Pervasive developmental disorders and psychoses in adult life. British Journal of Psychiatry. 1989;155:692–699. [PubMed] [Google Scholar]
- Costello EJ, Egger H, Angold A. 10-Year research update review: The epidemiology of child and adolescent psychiatric disorders: I. Methods and publish health burden. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(10):972–986. doi: 10.1097/01.chi.0000172552.41596.6f. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Kazdin AE. Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin. 2005;131:483–509. doi: 10.1037/0033-2909.131.4.483. [DOI] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory—II. Psychological Assessment. 1998;10(2):83–89. [Google Scholar]
- Esbensen AJ, Seltzer MM, Greenberg JS, Benson BA. Psychometric evaluation of a self-report measure of depression for individuals with mental retardation. Journal Information. 2005;110(6) doi: 10.1352/0895-8017(2005)110[469:PEOASM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M. Mental health aspects of autism and aspergers syndrome. Jessica Kingsley Publishers; London, England: 2005. [Google Scholar]
- Ghaziuddin M, Tsai L. Depression in autistic disorder. British Journal of Psychiatry. 1991;159:721–723. doi: 10.1192/bjp.159.5.721. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Asperger's syndrome and recurrent psychosis--a case study. Journal of Autism & Developmental Disorders. 1985;15(4):389–397. doi: 10.1007/BF01531783. [DOI] [PubMed] [Google Scholar]
- Gotham K, Bishop S, Brunwasser S, Lord C. Rumination and perceived impairment associated with depressive symptoms in a verbal adolescent-adult ASD sample. Autism Research. 2014;7(3):381–391. doi: 10.1002/aur.1377. PMCID: PMC4429601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DJ. The use of cognitive-behavioral therapy with people with Asperger syndrome: A case study. Autism. 1997;1(2):215–225. [Google Scholar]
- Harrell FE. Regression modeling strategies. Springer; New York: 2001. [Google Scholar]
- Hedley D, Young R. Social comparison processes and depressive symptoms in children and adolescents with Asperger syndrome. Autism. 2006;10(2):139–153. doi: 10.1177/1362361306062020. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. Journal of Autism and Developmental Disorders. 2004;34:229–235. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Howlin P. Outcome in adult life for more able individuals with autism or Asperger syndrome. Autism. 2000;4(1):63–83. [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Hurtig T, Kuusikko S, Mattila M, Haapsamo H, Ebeling H, Jussila K, Joskitt L, Pauls D, Moilanen I. Multi-informant reports of psychiatric symptoms among high-functioning adolescents with Asperger syndrome or autism. Autism. 2009;13(6):583–598. doi: 10.1177/1362361309335719. [DOI] [PubMed] [Google Scholar]
- Johnson S, Filliter J, Murphy R. Discrepancies between self- and parent-perceptions of autistic traits and empathy in high functioning children and adolescents on the autism spectrum. Journal of Autism and Developmental Disorders. 2009;39:1706–1714. doi: 10.1007/s10803-009-0809-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder. Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kim J, Szatmari P, Bryson S, Streiner D, Wilson F. The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism. 2000;4(2):117–132. [Google Scholar]
- Kovacs M. Children's Depression Inventory. Multi-Health Systems; North Tonawanda, NY: 1992. [Google Scholar]
- Lainhart JE, Folstein SE. Affective disorders in people with Autism: a review of published cases. Journal of Autism and Developmental Disorders. 1994;24(5):587–601. doi: 10.1007/BF02172140. [DOI] [PubMed] [Google Scholar]
- Laugeson E, Frankel F, Mogil C, Dillon A. Parent-assisted social skills training to improve friendships in teens with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(4):596. doi: 10.1007/s10803-008-0664-5. [DOI] [PubMed] [Google Scholar]
- Leyfer O, Folstein S, Bacalman S, Davis N, Dinh E, Morgan J, Tager-Flusberg H, Lainhart J. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36 doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lopata C, Toomey JA, Fox JD, Volker MA, Chow SY, Thomeer ML, et al. Anxiety and depression in children with HFASDs: Symptom levels and source differences. Journal of Abnormal Psychology. 2010;38:765–776. doi: 10.1007/s10802-010-9406-1. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore P, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Research in Developmental Disabilities. 2011;32(5):1910–1917. doi: 10.1016/j.ridd.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Magnuson KM, Constantino JN. Characterization of depression in children with autism spectrum disorders. Journal of developmental and behavioral pediatrics: JDBP. 2011 doi: 10.1097/DBP.0b013e318213f56c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Ahuja M, Smith LA. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Research in Autism Spectrum Disorders. 2011;5(1):474–485. [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Scale—Revised in adolescents. Journal of Child and Adolescent Psychopharmacology. 2010;20(6):513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta M, Scholing A, Rapee R, Abbott M, Spence S, Waters A. A parent report measure of children's anxiety. Behaviour Research and Therapy. 2004;42(7):813–839. doi: 10.1016/S0005-7967(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Neale MD. Neale Analysis of Reading Ability – Revised. NFER-Nelson; Windsor, UK: 1997. [Google Scholar]
- Perry DW, Marston GM, Hinder SAJ, Munden AC, Roy A. The phenomenology of depressive illness in people with a learning disability and autism. Autism. 2001;5:265–275. doi: 10.1177/1362361301005003004. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children's Depression Rating Scale Revised (CDRS-R) Western Psychological Services; Los Angeles, CA: 1996. [Google Scholar]
- Reynolds WM, Baker J. Assessment of depression in persons with mental retardation. American Journal on Mental Retardation. 1988;93(1):93–105. [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO, Lowe PA. The Adult Manifest Anxiety Scale. Western Psychological Services; 2003. [Google Scholar]
- Rieffe C, Terwogt MM, Kotronopoulou K. Awareness of single and multiple emotions in high-functioning children with autism. Journal of autism and developmental disorders. 2007;37(3):455–465. doi: 10.1007/s10803-006-0171-5. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised–WPS. WPS ed. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Sletta O, Valas H, Skaalvik E. Peer-relations, loneliness and self-perceptions in school aged children. The British Journal of Educational Psychology. 1996;66(4):431. doi: 10.1111/j.2044-8279.1996.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd ed. American Guidance Service Inc.; Circle Pines, MN: 2005. [Google Scholar]
- Spence SH, Barrett PM, Turner CM. Psychometric properties of the Spence Children's Anxiety Scale with young adolescents. Journal of Anxiety Disorders. 2003;17(6):605–625. doi: 10.1016/s0887-6185(02)00236-0. [DOI] [PubMed] [Google Scholar]
- Stewart M, Barnard L, Pearson J, Hasan R, O'Brien G. Presentation of depression in autism and Asperger syndrome: A review. Autism. 2006;10:103–113. doi: 10.1177/1362361306062013. [DOI] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory—Second Edition in a sample of college students. Depression and Anxiety. 2004;19(3):187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Tenneij NH, Koot HM. A preliminary investigation into the utility of the Adult Behavior Checklist in the assessment of psychopathology in people with low IQ. Journal of Applied Research in Intellectual Disabilities. 2007;20(5):391–400. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; San Antonio, TX.: 1999. [Google Scholar]
- White SW, Robertson-Nay R. Anxiety, social deficits, and loneliness in youth with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(7):1006–1013. doi: 10.1007/s10803-009-0713-8. [DOI] [PubMed] [Google Scholar]
- White SW, Schry AR, Maddox BB. Brief report: the assessment of anxiety in high- functioning adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012;42(6):1138–1145. doi: 10.1007/s10803-011-1353-3. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Todd L, Carr K, Shattuck P. Gender ratios in autism, Asperger syndrome and autism spectrum disorder. Autism Insights. 2010;2010(2):17–24. [Google Scholar]
- Wierzbicki M. A parent form of the Children's Depression Inventory: Reliability and validity in nonclinical populations. Journal of Clinical Psychology. 1987;43(4):390–397. doi: 10.1002/1097-4679(198707)43:4<390::aid-jclp2270430409>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4, professional manual. Psychological Assessment Resources; Lutz, FL: 2006. [Google Scholar]
- Wing L. Language, social, and cognitive impairments in autism and severe mental retardation. Journal of Autism and Developmental Disorders. 1981;11(1):31–44. doi: 10.1007/BF01531339. [DOI] [PubMed] [Google Scholar]