Abstract
Ricin is a potent ribotoxin considered to be a potentially dangerous bioterrorist agent due to its wide availability and the possibility of aerosol delivery to human populations. Studies in rodents and nonhuman primates have demonstrated that ricin delivered to the pulmonary system leads to acute lung injury and symptoms resembling acute respiratory distress syndrome. Increasing evidence suggests that the inflammatory effects triggered by ricin are responsible for its lethality. We demonstrated previously that ricin administered to the lungs of mice causes death of pulmonary macrophages and the release of proinflammatory cytokines, suggesting macrophages may be a primary target of ricin. Here we examined the requirement for macrophages in the development of ricinmediated pulmonary inflammation by employing transgenic (MAFIA) mice that express an inducible gene driven by the c-fms promoter for Fas-mediated apoptosis of macrophages upon injection of a synthetic dimerizer, AP20187. Administration of aerosolized ricin to macrophage-depleted mice led to reduced inflammatory responses, including recruitment of neutrophils, expression of proinflammatory transcripts, and microvascular permeability. When compared with control mice treated with ricin, macrophage-depleted mice treated with ricin displayed a reduction in pulmonary IL-1/3. Employing mice deficient in IL-1, we found that ricin-induced inflammatory responses were suppressed, including neutrophilia. Neutrophilia could be restored by co-administering ricin and exogenous IL-1β to IL-1α/β−/− mice. Furthermore, IL1Ra/anakinra cotreatment inhibited ricin-mediated inflammatory responses, including recruitment of neutrophils, expression of proinflammatory genes, and histopathology. These data suggest a central role for macrophages and IL-1 signaling in the inflammatory process triggered by ricin.
The U.S. Chemical Warfare Service began studying ricin as a weapon of war near the end of World War II. In recent years, ricin has become a tool of extremist groups in the United States and abroad as a result of ricin’s ease of production and high toxicity (1–4). The toxicity of ricin is ∼ 1000-fold greater by aerosol delivery to the respiratory system than by oral ingestion (5), suggesting that delivery of ricin in aerosol form would constitute an effective means of delivery to human populations by terrorist groups. Studies in rodents and nonhuman primates have demonstrated that ricin delivered into the pulmonary system leads to acute lung injury and symptoms resembling acute respiratory distress syndrome (ARDS)3 (6). ARDS is characterized by inflammation and increased permeability of the lung epithelial barrier (7), and it remains a leading cause of morbidity and mortality in clinical settings (8, 9). When administered to the lungs of animals, ricin induces a rapid massive migration of inflammatory cells, predominantly neutrophils, and causes apoptosis of alveolar macrophages (10). Subsequent effects of ricin exposure include pulmonary edema as well as apoptosis and necrosis of the endothelium and epithelium that constitute the lung surface barrier (11). In addition to direct effects of ricin on the pulmonary system, we have reported that intratracheal delivery of ricin to the lungs produces systemic consequences that produce inflammatory changes in multiple organs of the body, including the kidney, liver, and spleen (12).
Ricin is a 62-kDa protein consisting of two glycoprotein chains linked by a disulfide bridge. The 34-kDa B chain, a lectin that binds primarily to galactose-containing surface proteins, facilitates the internalization of the 32-kDa A chain, the toxic moiety. Following endocytosis and retrograde transfer through the Golgi apparatus, the A subunit of ricin enters the cytosol where it depurinates a single adenine (A4256 in mouse) in the 28S rRNA ribosomes. The depurination of the A4256 is directly responsible both for the inhibition of protein translation (13–15) and the initiation of upstream events that lead to inflammatory responses (16). We reported the mechanism connecting the inhibition of protein synthesis and the activation of proinflammatory phenomena by demonstrating that ricin mediates activation of stress-activated protein kinases (SAPKs) by producing lesions in the peptidyl transferase center of 28S rRNA (17). The two classes of SAPKs in mammalian cells are the JNKs and the p38 MAPKs, both of which are activated by upstream kinases called SAPKKs and SAPKKKs (18–21). SAPKs belong to the family of MAPKs, which also include ERKs, and are important mediators through which stress signals are transduced to modulate expression of proinflammatory genes (22–24). Recent studies have identified ZAK/MLK7 as the MAP3K whose activation by ricin and related toxins leads ultimately to the phosphorylation and activation of the SAPKs (25). We demonstrated that delivery of ricin to both pulmonary and extrapulmonary tissues mediated the activation of JNK and p38. Another early consequence of ricin exposure is the activation of NF-κB, a rapid-acting primary transcription factor that induces expression of genes encoding several proinflammatory cytokines and chemokines (12, 16, 26). Primary human airway epithelial cells and primary murine macrophages respond to ricin in vitro through activation of both MAPK and NF-κB (26, 27), but the specific cell types responsible for ricin’s lethal inflammatory effects in vivo remain unclear.
The pulmonary innate immune response is activated not only by pathogens but also by a wide variety of xenobiotic agents (28–30), and alveolar macrophages represent the first line of defense. Evidence from our laboratory suggests that macrophages play a role in mediating the proinflammatory effects of ricin in the lungs. Intra-tracheal administration of ricin leads to almost complete elimination of macrophages in the lungs (10) and intravascular administration of ricin leads to the death of macrophages in multiple organs (31, 32), suggesting that macrophages may serve as primary targets of ricin. Exposure of primary bone marrow-derived and alveolar murine macrophages to ricin in vitro leads to activation of SAPKs, increased mRNA transcripts encoding proinflammatory genes, and the increased production and release of TNF-α, suggesting the possibility that macrophages may mediate the early proinflammatory effects of ricin in vivo (26).
In the present study we investigated the role of pulmonary macrophages in ricin-mediated lung injury by employing a transgenic mouse line that harbors an inducible suicide gene in macrophages and their precursors (33). Using this model, we were able to achieve depletion of macrophages in the lungs of mice treated with the inducer. Compared with nondepleted mice, macrophage-depleted mice displayed markedly decreased inflammatory signs in response to ricin, including decreased recruitment of neutrophils, decreased expression of proinflammatory mediators, and reduced microvascular permeability. Employing mice deficient in IL-1α and IL-1β or wild-type mice co-treated with IL-1Ra, a recombinant human form of the IL-1 receptor antagonist, we determined that ricinmediated inflammation was dependent on IL-1 signaling. Taken together, our data suggest that the proinflammatory effects of aerosolized ricin are dependent on IL-1 signaling and the presence of pulmonary macrophages, either as the source of IL-1 or as the target cells upon which ricininduced IL-1 acts to amplify the inflammatory cascade.
Materials and Methods
Animals
C57BL/6J, MAFIA, and TNF-α−/− mice were purchased from The Jackson Laboratory. MAFIA mice are cataloged with The Jackson Laboratory as strain C57BL/6J–Tg(Csf1r–GFP, NGFR/FKBP12)2Bck/J. IL-1α/β−/− were developed as described (34). Male mice 8–10 wk of age and weighing 18–24 g were used throughout the experiments. Mice were housed under 12-h light-dark cycle and fed with standard diet ad libitum. All studies were conducted with approval by the Institutional Animal Care and Use Committee at Oregon Health and Science University. For both treatment and euthanasia, mice were anesthetized with an i.p. injection of a cocktail containing 0.75 mg of ketamine, 0.15 mg of xylazine, and 0.03 mg of acepromazine. For treatment, a PennCentury 23-gauge microsprayer (model IA-1C) was inserted into the opening of the trachea with the aid of a laryngoscope. A volume of 50 µl of saline or 0.3 µg/100 g of ricin diluted in saline was delivered by aerosol to the animal. The dose of ricin used was determined to be the lethal dose at which wild-type animals succumbed within 48–96 h. Exogenous recombinant IL-1β (EMD Chemicals) and IL-1Ra/anakinra (Kineret; Amgen) were delivered in the same manner at indicated concentrations. For survival experiments, animals were euthanized when they became moribund according to the criteria of lack of response to stimuli or lack of righting reflex.
Reagents and Abs
Ricin was purchased from Vector Laboratories. AP20187 was provided by ARIAD Pharmaceuticals. BD OptEIA mouse IL-β ELISA set was purchased from BD Biosciences. Anti-Gr-1/Ly6G (no. 550291) was purchased from BD Biosciences, and anti-cleaved caspase-3 (no. 9664) was purchased from Cell Signaling Technology.
In vivo depletion of macrophages
AP20187 was a gift from Ariad Pharmaceuticals. Lyophilized AP20187 was dissolved in 100% ethanol at a concentration of 62.5 mg/ml stock solution and was stored at −20°C As recommended by Ariad Pharmaceuticals, injection solutions consisted of 4% ethanol, 10% PEG-400, and 2% Tween 80 in water. All injections were administered i.p. within 30 min after preparation. The volume of injection solution was adjusted according to the average mouse body weight to deliver a dose of 10 mg/kg AP20187 per mouse in an average volume of 100 µl. Mice were injected daily for 5 days before ricin treatment.
Bronchoalveolar lavage (BAL) and neutrophil counts
At 48 h after treatment with ricin, lungs were lavaged four times with 1 ml of cold 0.7 × PBS. After lavage, the population of cells from the BAL fluid was examined using Cytospin (Thermo Fisher Scientific) slide preparations and staining by Hema 3 stain set (Fisher Healthcare). Polymorphonuclear neutrophils were identified morphologically and were counted. BAL slides from at least five animals from each treatment group were analyzed to ensure reproducibility.
RNA isolation
Lung tissues were dissected and were immediately frozen and ground in liquid nitrogen. RNA was extracted using TRIzol reagent in accordance with the manufacturer’s instructions and was further digested with DNase. Both reagents were purchased from Invitrogen.
Real-time PCR analysis
Two micrograms of RNA were reverse-transcribed in the presence of Super-Script II and oligo(dT) primer (both reagents were purchased from Invitrogen). The amplification of the cDNA was accomplished using the ABI Prism 7900HT sequence detection system (Applied Biosystems) in the presence of the commercially available SYBR Green PCR Master Mix (Applied Biosystems) and 20 µmol/L of the corresponding sense and antisense RT-PCR primers for 120-bp amplicons in a 40-cycle PCR. Fold induction in gene expression was measured using absolute quantitation of a standard curve in arbitrary units and using levels of GAPDH for normalization. The nucleotide sequences of the primers used in this study have been previously published (16). The denaturing, annealing, and extension conditions of each PCR cycle were 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s, respectively. RNA from five animals per group was analyzed by real-time PCR.
Histology and immunohistochemical analysis
Animals were sacrificed at 48 h unless otherwise indicated. After dissection, lungs were fixed in 4% paraformaldehyde solution for 24 h, at which time tissues were dehydrated and embedded in paraffin. For histology, 5-µm sections were mounted on glass slides, deparaffinized, and stained with H&E following standard procedures. For immunohistochemical analysis of activated caspase-3, Ag retrieval was performed by placing the deparaffinized slides in 10 mmol/L sodium citrate (pH 6) in a microwave oven for 10 min. Immunohistochemical detection of Gr-1/Ly6G did not require pretreatment for Ag retrieval. After blocking in serum, the slides were incubated with primary Abs overnight at 4°C at appropriate dilutions. Slides were further processed using the VectaStain Elite ABC kit (Vector Laboratories) according to the manufacturer’s recommendations using 3,3′-diaminobenzidine as substrate. Immunohistochemical analysis was performed on specimens from at least five animals in each group to verify reproducibility. For examination of embedded lungs, one lung from each animal was sliced transversely into 2-mm slices. To ensure thorough microscopic examination of the entire lung, all slices from each lung were processed, embedded, sectioned by microtomy, and mounted on a single glass slide for examination either by H&E or by immunohistochemistry.
Evans blue dye (EBD) assay
Forty-eight hours after ricin treatment, animals were anesthetized and injected with 50 mg/kg EBD diluted in saline to a total volume of 200 µl through the retro-orbital vein. The dye was allowed to circulate for 30 min, at which time the vasculature was perfused with 5 ml of saline through the right ventricle to remove residual EBD from the systemic circulation before dissection of the lungs. Lungs were removed, weighed, and homogenized; the dye was extracted in 1.5 ml of formamide. The OD of each sample was determined spectrophotometrically (absorbance 620 nm), and EBD concentration was calculated using a standard curve of known EBD dilutions. Values are expressed in fold induction over saline-control after being normalized for the dry weight of each tissue, and they represent the average of three animals per group.
ELISA
Lung tissue was homogenized in lysis buffer in PBS containing 2% Non-idet P-40 and Complete protease inhibitor cocktail (Roche) and centrifuged at 10,000 × g for 20 min at 4°C. The resulting supernatant was analyzed for IL-1β levels using the BD OptEIA mouse IL-1β ELISA set from BD Biosciences per the manufacturer’s instructions. The detection limit for IL-1β was 4 pg/ml. Absorbance of standards and samples were determined spectrophotometrically at 450 nm using a microplate reader (Bio-Rad). Results were plotted against the linear portion of the standard curve. Results are expressed as pg/ml IL-1β and represent the average of three animals per group.
Lung wet/dry weight ratio
Left lungs were excised and rapidly weighed for wet weight. Samples were oven dried at 65°C for 72 h to a stable dry lung weight. Data are presented as the ratio of lung wet to dry weight 48 h after either saline or ricin treatment. Five animals from each treatment group were analyzed for wet/dry weight ratios.
Statistical analysis
Individual groups were compared using unpaired t test analysis. To estimate p values, all statistical analyses were interpreted in a two-tailed manner. Values of p < 0.05 were considered to be statistically significant. Kaplan-Meier analysis was performed for survival curves. One-way ANOVA analysis was performed for EBD and wet/dry weight ratio experiments. All data are presented as means ± SEM.
Results
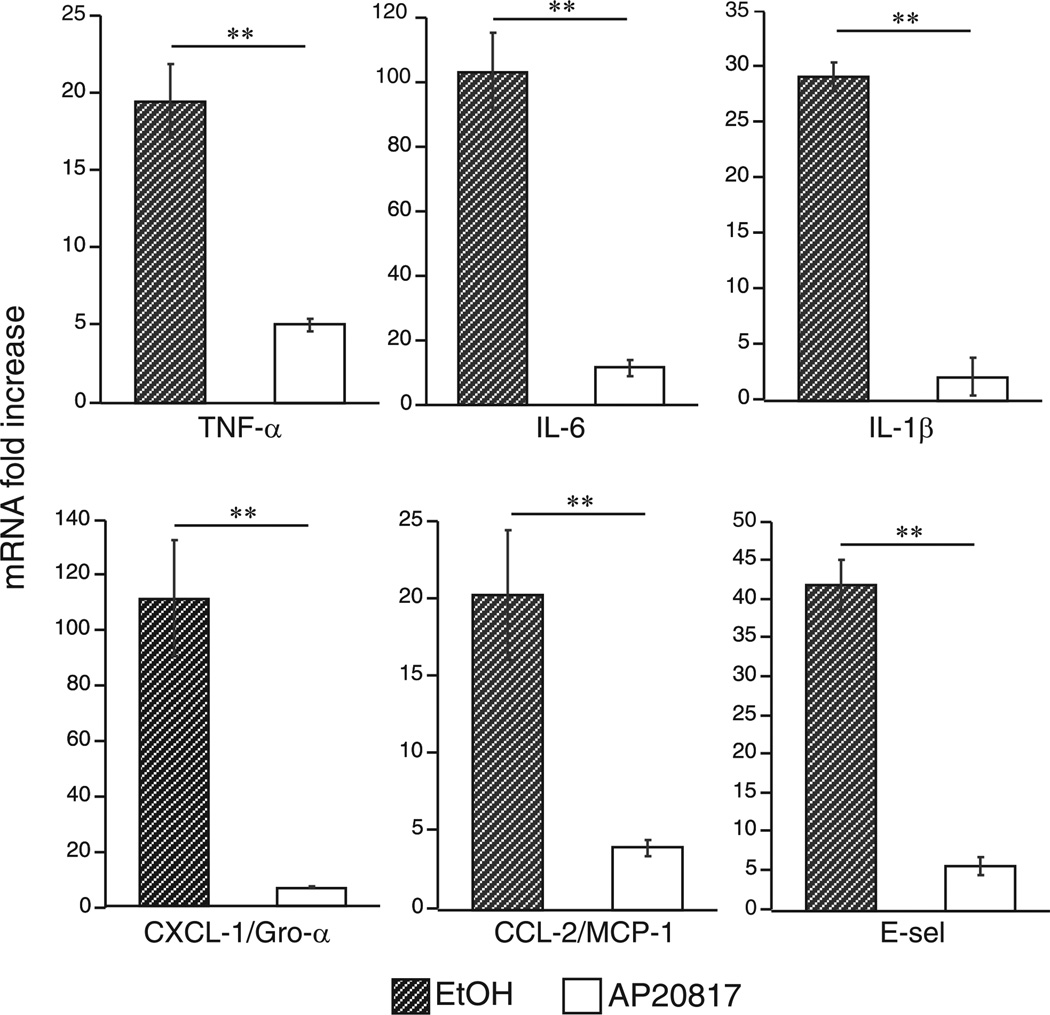
Exposure of primary murine macrophages to ricin in vitro causes activation of SAPKs in a dose-dependent manner and triggers the expression of key cytokines, chemokines, and cell-surface recognition molecules involved in proinflammatory signaling (26). To determine whether alveolar macrophages are required for ricinmediated expression of proinflammatory transcripts in vivo, we compared the responses of wild-type mice with mice depleted of macrophages just before ricin treatment. Transgenic MAFIA mice were treated with AP20187 for 5 days, at which time apoptotic macrophages were detected in the lung tissue and in Cytospin preparations of the cells from BAL fluid (data not shown). Apoptosis was confirmed both by morphologic features and immuno-cytochemical detection of activated caspase-3, and, consistent with results of previous studies characterizing the MAFIA mouse model, we achieved 90% ablation of macrophages using this method (33). Control and macrophage-depleted MAFIA mice were exposed to either aerosolized saline or 0.3 µg/100 g of aerosolized ricin, a lethal dose that consistently caused death in 100% of wild-type mice within 48–96 h. Forty-eight hours later, lysates of lung tissue were examined by quantitative real-time RT-PCR (qRT-PCR) for expression of mRNA transcripts that encode a variety of proinflammatory genes. When compared with MAFIA mice receiving ethanol vehicle, MAFIA mice receiving AP20187 to deplete macrophages exhibited significantly reduced expression (p < 0.01) of several proinflammatory cytokines (IL-1β, TNF-α, IL-6), chemokines (CXCL-1/Gro-α and CCL-2/MCP-1), and the cell adhesion molecule E-selectin (Fig. 1). These data indicate that the presence of macrophages was indeed required for the ricininduced expression of several proinflammatory mediators in vivo.
FIGURE 1.
Macrophage depletion reduces ricin-mediated expression of proinflammatory RNA transcripts. Mice pretreated for 5 days with ethanol vehicle (hatched bars) or AP20187 (white bars) were administered either saline or 0.3 µg/100 g ricin. Total RNA was purified from lung tissue harvested 48 h later and processed for real-time PCR. Values displayed represent fold induction over respective saline control, normalized to GAPDH (n = 5; **p < 0.01). Error bars represent SEM.
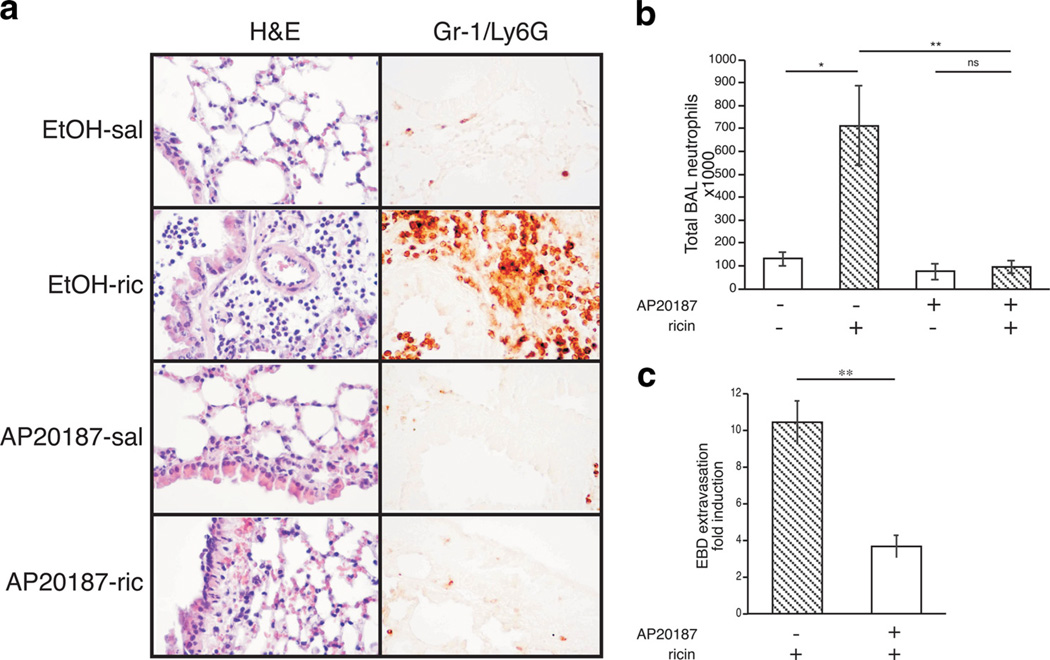
Recruitment of neutrophils to the lung parenchyma and alveolar spaces is a hallmark of acute pulmonary inflammation (35). In response to ricin, neutrophils accumulate in the BAL fluid and lung tissue of mice 48 h after administration of ricin (12). To determine whether macrophages were required for ricin to induce the recruitment of neutrophils to the lungs, lung sections and Cytospin preparations of BAL fluid were examined 48 h after administration of ricin. Compared with nondepleted mice, animals depleted of macrophages exhibited dramatically reduced numbers of neutrophils in both the lung parenchyma (Fig. 2a) and the BAL fluid (Fig. 2b), suggesting that the presence of macrophages was required for ricin-mediated neutrophil recruitment to the lungs.
FIGURE 2.
Macrophage depletion results in reduced ricin-mediated neu-trophil accumulation and barrier permeability. Mice pretreated for 5 days with ethanol vehicle or AP20187 were administered either saline or 0.3 µg/100 g ricin and euthanized 48 h later. a, Lung tissue sections (40×) stained with H&E and processed for immunohistochemical detection of Gr-1/Ly6G show ricin-mediated damage to alveolar epithelium and accumulation of Gr-1/Ly6G–positive cells in alveoli and airways (n= 5). b, Total neutrophils counted in the BAL fluid (n=5; p< 0.05, **p < 0.01). c, Microvascular permeability as measured by Evans blue dye (EBD) permeability assay and expressed as fold induction over respective saline controls (n= 5; **p < 0.01). Error bars represent SEM.
Another hallmark of acute lung injury is enhancement of pulmonary capillary permeability to proteins and fluid across the endothelial cell barrier (36). EBD has been used as a marker of extravascular protein leakage due to its high affinity for binding albumin when injected into the bloodstream (37). To determine the role of alveolar macrophages in ricin-mediated leakage of extravascular proteins, MAFIA mice treated with ricin were injected i.v. with 50 mg/kg EBD 1 h before sacrifice. In lungs of MAFIA mice depleted of macrophages, the concentration of extravasated EBD after ricin exposure was reduced by 60% compared with MAFIA mice that had only received ethanol vehicle before ricin (Fig. 2c). This result suggests that ricin-induced vascular permeability to proteins across the lung endothelial barrier is at least partially dependent on the presence of macrophages.
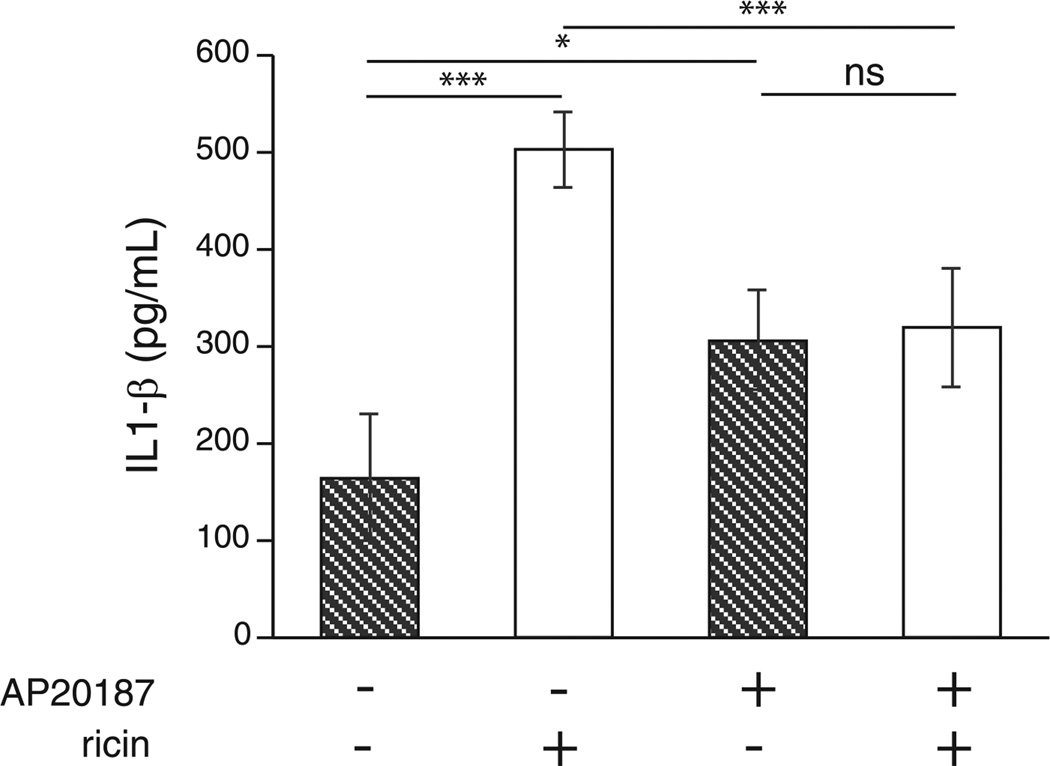
We showed previously that ricin exposure triggers the production and release of early response cytokines TNF-α and IL-1β (26), which are linked to the generation of inflammatory cascades in multiple lung pathologies (38, 39). TNF-α and IL-1 are considered to be initiator cytokines, inasmuch as their release from macrophages has been shown to orchestrate inflammatory responses. In view of the increased expression of mRNA transcripts encoding TNF-α and IL-1β in response to aerosolized ricin in MAFIA mice (Fig. 1), we sought to determine whether these cytokines were required for ricin to elicit inflammatory responses. To address this question, we first examined TNF-α and IL-1β protein levels in lung homogenates of MAFIA mice treated with ricin. Both groups of MAFIA mice treated with AP20187 exhibited slightly higher basal levels of IL-1β in the lungs compared with nondepleted mice. However, MAFIA mice whose macrophages had not been depleted displayed a 3- to 4-fold increase in IL-1β after exposure to ricin, while no ricin-mediated induction was observed in IL-1β levels of macrophage-depleted animals (Fig. 3). Meanwhile, TNF-α measured from lung homogenates did not change significantly between groups (data not shown), suggesting that IL-1β may play a larger role in the response to ricin in MAFIA mice.
FIGURE 3.
IL-1β protein is increased in lung tissue after ricin treatment in control but not macrophage-depleted mice. Protein levels were measured by ELISA from lung homogenates collected 48 hours after ricin treatment (n= 5; *, p< 0.05; ***p < 0.001; ns, not significant). Error bars represent SEM.
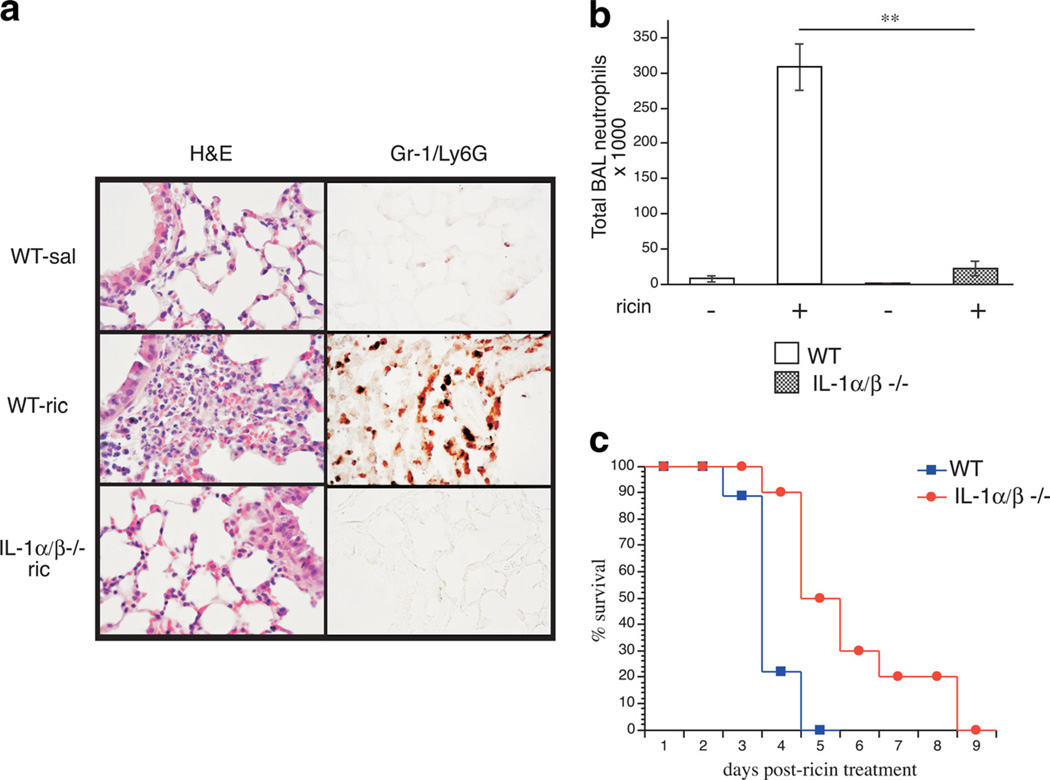
To further probe the roles of IL-1 and TNF-α in ricin-mediated inflammation, we compared responses of mice harboring null mutations in IL-1α and IL-1β (IL-1α/β−/−) and TNF-α (TNF-α−/−). Lung tissue sections prepared from IL-1α/β−/− at 48 h after ricin treatment showed markedly reduced vascular congestion, destruction of alveoli, and accumulation of neutrophils in the airways compared with wild-type tissue sections (Fig. 4a). Furthermore, IL-1-deficient animals had reduced neutrophil counts in the BAL fluid (Fig. 4b), improved survival compared with wild-type (Fig. 4c) and diminished pulmonary edema as measured by lung tissue wet/dry ratios (Table I). TNF-α−/− mice, however, failed to show improvement over wild-type animals in inflammatory responses after ricin treatment (data not shown). Taken together, the data in Fig. 3 and Table I indicate that IL-1 was required for ricin to mediate its proinflammatory effects in the pulmonary system and, furthermore, that TNF-α was dispensable for this response.
FIGURE 4.
IL-1α/β−/− mice have markedly reduced neutrophilia and pulmonary edema, and improved survival compared with wild-type mice. IL-1α/β−/− and wild-type animals were administered ricin and euthanized 48 h later. a, Representative photomicrographs of lung sections stained with H&E and labeled with anti-Gr-1/Ly6G. b, Total neutrophils counted in the BAL fluid (n= 5; **p < 0.01). c, Survival curves comparing IL-1α/β−/− (circles) and wild-type (squares) survival percentages over 9 days following ricin treatment (n= 10; p= 0.002). Error bars represent SEM.
Table I.
Lung wet/dry ratios
| Wild Type | IL 1 α/β−/− | |||
|---|---|---|---|---|
| Treatment | Mean | SEM | Mean | SEM |
| Saline | 4.58 | 0.17 | 4.80 | 0.09 |
| Ricin | 5.72 | 0.08a | 5.05 | 0.29b |
p < 0.05 vs saline.
p = 0.63 (NS) vs saline; p < 0.05 vs wild type.
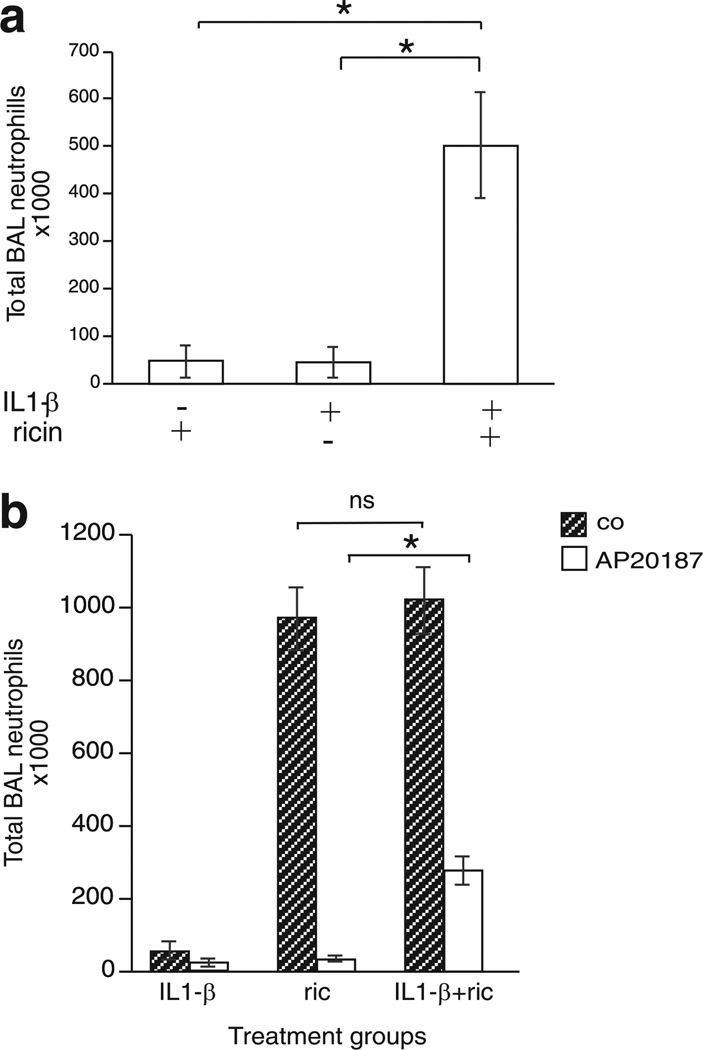
If the provoked release of IL-1 from alveolar macrophages were responsible for the proinflammatory effects of ricin, then the co-administration of exogenous IL-1 and ricin to IL-1α/β−/− mice should restore the inflammatory responses in these animals. To test this possibility, we administered 15 ng of IL-1β in the presence or absence of ricin and measured the accumulation of neutrophils in the BAL fluid. Whereas IL-1β alone failed to induce recruitment of neutrophils to the BAL fluid of IL-1α/β−/− mice, the co-administration of ricin and IL-1β to these mice resulted in the accumulation of BAL neutrophils to levels observed in wild-type mice treated with ricin alone (compare Figs. 5a and 2b). Taken together with the data shown in Figs. 3 and 4, these results support the conclusion that ricin-mediated pulmonary inflammation is dependent on the presence of IL-1.
FIGURE 5.
Exogenous IL-1β administered in combination with ricin restores ricin-mediated recruitment of neutrophils to the BAL fluid in both IL-1α/β−/− and macrophage-depleted MAFIA mice. Animals were treated with ricin and euthanized 48 h later. a, Total neutrophils counted in the BAL fluid of IL-1α/β−/− mice treated with ricin, 15 ng of IL-1β or ricin, and 15 ng of IL-1β (n= 3; *p < 0.05). b, Total neutrophils counted in the BAL fluid of MAFIA mice pretreated with ethanol vehicle (hatched bars) or AP20187 (white bars) for 5 days followed by administration of either 15 ng of IL-1β or ricin, or 15 ng of IL-1β and ricin (n= 3; *p < 0.05; ns, not significant). Error bars represent SEM.
To explore the possibility that reduced IL-1β production was a contributing factor in the suppressed inflammatory response observed in macrophage-depleted MAFIA mice, we examined the ability of macrophage-depleted animals to recruit neutrophils to the lungs when exposed to aerosolized ricin in conjunction with exogenous IL-1β. Co-administration of ricin and 15 ng of IL-1β to macrophage-depleted MAFIA mice resulted in a significant (p > 0.01) 10-fold increase in the number of neutrophils in the BAL fluid when compared with mice treated with either IL-1β or ricin alone (Fig. 5b). The data in Fig. 5 demonstrate that IL-1β can contribute to ricin-mediated recruitment of neutrophils in both IL-1α/β−/− and macrophage-depleted mice.
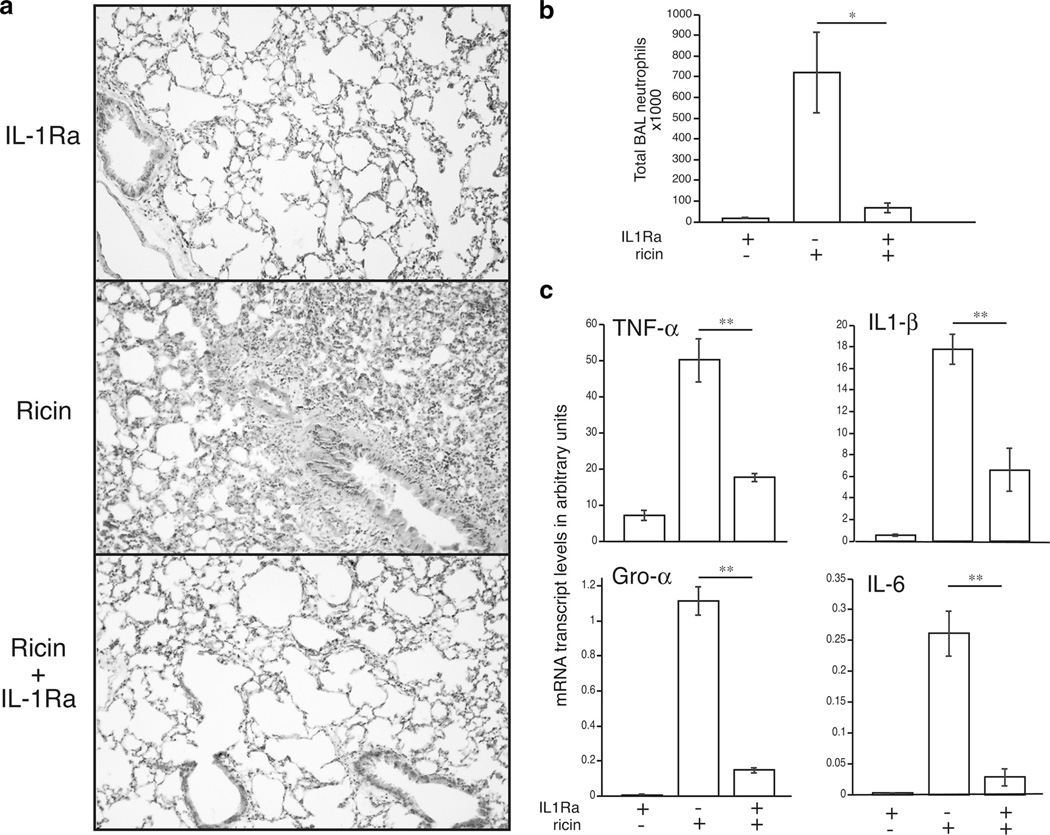
Experimental studies show that recombinant human IL-1R antagonist (IL-1Ra), a competitive inhibitor that interferes with binding of IL-1α and IL-1β to the IL-1R1, may limit the release of cytokines and the development of neutrophilia in animal models of lung inflammation (40–43). To determine the ability of IL-1Ra to block ricin-induced pathology, we administered IL-1Ra to wild-type mice at the time of ricin exposure and examined lung tissue sections after 48 h. Animals receiving IL-1Ra exhibited reduced vascular congestion, destruction of alveoli, and accumulation of neutrophils in the airways compared with animals receiving ricin alone (Fig. 6a). Lungs from IL-1Ra-treated mice had significantly lower expression of proinflammatory transcripts (p < 0.01; Fig. 6b and reduced appearance of neutrophils in the BAL fluid (Fig. 6c). These data confirm the central role of IL-1 signaling in the inflammatory response to ricin, and they suggest the possibility that blockade of the IL-1 pathway could be employed to suppress ricinmediated inflammation.
FIGURE 6.
Coadministration of IL-1Ra with ricin to wild-type mice prevents ricin-mediated inflammatory responses. Wild-type mice received ricin, 30 mg/kg IL-1Ra, or ricin plus 30 mg/kg IL-1Ra and were euthanized 48 h later. a, Representative photomicrographs of lung tissue sections stained with H&E at 10 × magnification. b, Total neutrophils counted in the BAL fluid (n= 3; p < 0.05). c, mRNA transcript levels of proinflammatory genes measured from lung homogenates by real-time PCR (n= 3; **p < 0.01). Error bars represent SEM.
Discussion
Here we show that acute inflammatory lung injury induced by aerosolized ricin was characterized by increased mRNA expression of proinflammatory genes, production of IL-1β, recruitment of neutrophils to the lungs, and elevated microvascular permeability, and that these responses were dependent on the presence of pulmonary macrophages and IL-1 signaling. Indeed, lung pathology was significantly reduced in animals depleted of macrophages or genetically deficient in IL-1α/β, as well as in wild-type animals injected with IL-1Ra to block IL-1 signaling at the time of ricin exposure. Furthermore, at a dose insufficient to produce inflammatory effects on its own, exogenous IL-1β, when co-administered with ricin to mice deficient in IL-1, recapitulated neutrophil recruitment to the BAL fluid in numbers consistently observed in wild-type animals exposed to ricin alone. Taken together, these results demonstrate that pulmonary IL-1 signaling is essential for the acute inflammatory response to aerosolized ricin.
There is increasing evidence that the inflammatory effects triggered by ricin are responsible for ricin’s lethality (12, 44). Since aerosolized ricin represents the most plausible means of exposure for human populations in the event of a bioterrorist attack, understanding the pulmonary immune response to inhaled ricin toxin is critical for developing therapies aimed at managing symptoms and reducing mortality in case ricin is used as an agent of warfare. In the lung, immune responses to inhaled substances are orchestrated by resident macrophages, which initiate inflammatory cascades through the production and release of immunomodulatory mediators like early response cytokines TNF-α and IL-1 (45). We previously demonstrated that ricin triggers the enhanced expression of genes encoding TNF-α and IL-1 (16) and the release of TNF-α from primary murine macrophages in culture (26). During microbial invasion, the release of cytokines and chemokines by macrophages serves to recruit and activate other leukocytes, mainly neutrophils, to help clear pathogens and resolve the infection. Inflammatory injury to local tissue by host immune cells is viewed as collateral damage in an infection (46). However, in the case of ricin exposure, in which there is no pathogen to clear, tissue damage occurs without benefit to the host. In view of this, we hypothesized that suppression of the innate immune response to ricin would serve to reduce inflammatory lung injury and lethality caused by ricin.
Depletion of macrophages resulted in diminished ricin-mediated expression of proinflammatory transcripts (Fig. 1), accumulation of neutrophils (Fig. 2, a and b), microvascular barrier permeability (Fig. 2c), and tissue IL-1β levels (Fig. 3), indicating that macrophages are required for these inflammatory processes. Not surprisingly, since macrophages are important for pulmonary homeostasis and constitute the cell type primarily responsible for the clearance of cell debris (47), the depletion of macrophages from mice caused some inflammation and weight loss that was independent of ricin treatment, consistent with studies published previously on MAFIA mice (33). We established 20% weight loss as a criterion for mortality and, to conform with ethical guidelines established by the Oregon Health and Science University Institutional Animal Care and Use Committee, sacrificed the animals that had reached this endpoint. For this reason, the weight loss consistently observed in MAFIA mice injected with AP20187 alone (10–15%) confounded our ability to perform survival studies. Both groups of macroph-age-depleted animals, saline and ricin treated, exhibited some alveolitis (Fig. 2a) and increased IL-1β levels (Fig. 3) over control animals, and these effects were observed only in MAFIA and not AP20187-treated wild-type C57BL/6 mice (data not shown), indicating that the effect was specific to the induction of Fas-mediated apoptosis of macrophages in the transgenic animals. It is known that ligation of Fas on tissue macrophages induces proinflammatory cytokine release that can initiate acute inflammatory responses and tissue injury (48, 49), which may explain the higher level of “basal” inflammation in macrophage-depleted animals. Despite these limitations, inflammatory responses were drastically induced after ricin treatment, and we were able to glean from these experiments that macrophages play an essential role in the development of ricin-mediated inflammatory disease.
Since the depletion of macrophages before ricin treatment resulted in an attenuation of inflammatory symptoms, we investigated the requirements for TNF-α and IL-1, the major proinflammatory mediators produced by macrophages. Early response cytokines such as TNF-α and IL-1 act on a variety of cells via cell membrane-bound receptors (50–52) to initiate a proinflammatory cascade resulting in chemokine production, up-regulation of adhesion molecules, transmigration of neutrophils into alveolar compartment and lung interstitium, and the release of proteases and reactive oxygen radicals, which are linked to tissue damage (36, 53). Mice deficient in both TNF-α and IL-1 signaling exhibit impaired neutrophilic inflammation in response to Streptococcus pneumoniae (54). To determine the requirement for these cytokines in the response to ricin, we obtained animals genetically deficient in TNF-α and IL-1α/β and administered aerosolized ricin. Notably, mice deficient in TNF-α showed no improvement over wild-type animals (data not shown), indicating that TNF-α, although induced by ricin (Fig. 1), is dispensable for this response. In contrast, animals lacking IL-1α/β had significant protection from ricin-mediated inflammatory indices (Fig. 4, a and b, and Table I) and improved survival over wild-type animals (Fig. 4c), although they all eventually succumbed after several days. Furthermore, a 10-fold higher dose of ricin administered by aerosol to the pulmonary system led to death of all animals within 48 h regardless of genotype (data not shown). Because ricin ultimately leads to apoptosis of pulmonary epithelium (27), the death observed in IL-1-deficient animals in the absence of pulmonary inflammation may have resulted from the loss of barrier function and the subsequent entry of ricin into the vascular system.
IL-1β is thought to be a major participant in the pulmonary inflammatory cascade in ARDS; IL1-β, rather than TNF-α, was found to be the major inflammatory mediator in BAL fluid of patients with ARDS (55). IL-1 signaling mediates inflammatory lung injury induced by a variety of stimuli including endotoxemia (42), subacute ozone exposure (56), thermal injury (57), bleomycin administration (43), and experimental ventilation (40). Administration of IL-1 is sufficient to induce chemokine expression and recruitment of neutrophils to the airways (42). Neutrophils responding to cytokine and chemokine gradients accumulate in the airways and contribute to injury and loss of epithelial integrity that characterize ARDS/ALI (35). Indeed, in the present study, mice lacking IL-1α/β or pulmonary macrophages (and thus the major producers of IL-1) showed drastically reduced numbers of neutrophils in the BAL fluid following ricin exposure (Figs. 2b and 4b), and this reduction in neutrophils coincided with reduced edema in IL-1α/β−/− mice (Table I) and diminished microvascular permeability in macrophage-depleted MAFIA mice (Fig. 2c). Additionally, mice treated with IL-1Ra to block IL-1R1 signaling at the time of ricin exposure exhibited reduced expression of proinflammatory transcripts (Fig. 6c) and accumulation of neutrophils in the BAL fluid (Fig. 6b), supporting a primary role for IL-1 signaling in ricin-mediated inflammation.
Precisely which cells respond to ricin-induced secretion of IL-1 remains unknown. Potential targets of IL-1 include pulmonary macrophages acting in an autocrine or paracrine fashion, microvascular endothelial cells, and alveolar epithelial cells. Type I (TI) and type II (TII) alveolar epithelial cells are known to be capable of responding directly to inflammatory stimuli as well as through inflammatory mediators produced by first-responders. For example, alveolar epithelial cells secrete chemokines MIP-2 (CINC-3/CXCL3) and MCP-1 (CCL2) in response to IL-1β and LPS (58) but require stimulation from macrophage-derived IL-1α to elicit chemokine secretion in response to ozone exposure (59). Similarly, human lung microvascular endothelial cells up-regulate the expression of adhesion molecules and release MCP-1, IL-8, and Gro-α/CXCL-1 in response to IL-1 (60), suggesting that these cells may also play an active role in the propagation of inflammation initiated by ricin.
The finding that IL-1Ra suppressed the transcription of other genes following ricin exposure (Fig. 6c) suggests that IL-1 signaling is situated upstream of other events in the inflammatory process. Regulation of IL-1-responsive genes is mediated in large part through activation of the ubiquitously expressed transcription factor NF-κB (61). Nuclear NF-κB activity is rapidly induced in the presence of IL-1 in human lung epithelial cells, and this activation is inhibited by the addition of IL-1 receptor antagonist (61). Activation of NF-κB in airway epithelial cells has been implicated in the development of neutrophilic inflammation by multiple stimuli (54, 62, 63), including ricin (27). We reported previously that NF-κB is activated in human epithelial cells after exposure to ricin, and that targeted inhibition of NF-κB by small interfering RNA results in an inhibition of the expression of proinflammatory genes (27). The data presented herein suggest that ricinmediated IL-1 signaling may contribute to the activation of NF-κB in the airways to promote the development of acute inflammatory disease.
Although many investigators have elucidated mechanisms of lung inflammation and injury following administration of pathogens and microbial products such as LPS, the mechanisms by which environmental xenobiotics induce lung inflammatory responses are not well understood. In addition to providing insight for development of therapeutic approaches to counteract the use of aerosolized ricin as a bioterrorist agent, an increased understanding of mechanisms underlying ricin’s toxicity could serve to provide greater understanding of how inhaled toxins can cause inflammation, injury, and death.
Acknowledgments
We thank the Oregon Health and Science University Department of Dermatology Molecular Profiling Resource of human tissue samples (IRB no. 809) and the Oregon Health and Science University Cancer Institute.
Footnotes
This work was funded by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5-T32-CA106195 from the National Cancer Institute and by National Institutes of Health Grant AI1059335 (to B.E.M.).
Abbreviations used in this paper: ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; EBD, Evans blue dye; qRT-PCR, quantitative real-time RT-PCR; SAPK, stress-activated protein kinase.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Shar L. Probe aims at sale of deadly bacteria. USA Today. 1995 Jul 11;:2–A. [Google Scholar]
- 2.Kifner J. Man is arrested in a case involving deadly poison. New York Times. 1995 Dec 23;:A–7. [Google Scholar]
- 3.Zilinskas RA. Iraq’s biological weapons. J. Am. Med. Assoc. 1997;278:418–424. [PubMed] [Google Scholar]
- 4.Mayor N. UK doctors warned after ricin poison found in police raid. Brit. Med. J. 2003;326:126. doi: 10.1136/bmj.326.7381.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franz DR, Jaax JK. Ricin toxin. In: Sidell FR, Takfuji ET, Franz DR, editors. Textbook of Military Medicine, Part 1, Warfare, Weaponry, and the Casualty: Medical Aspects of Chemical and biological Warfare. Washington, DC: Office of the Surgeon General, Department of the Army; 1997. pp. 631–642. [Google Scholar]
- 6.Greenfield RA, Brown BR, Hutchins JB, Iandolo JJ, Jackson R, Slater LN, Bronze MS. Microbiological, biological, and chemical weapons of warfare and terrorism. Am. J. Med. Sci. 2002;323:326–340. doi: 10.1097/00000441-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 8.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 9.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit. Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 10.Brown RF, White DE. Ultrastructure of rat lung following inhalation of ricin aerosol. Int. J. Exp. Pathol. 1997;78:267–276. doi: 10.1046/j.1365-2613.1997.300363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelmsen CL, Pitt ML. Lesions of acute inhaled lethal ricin intoxication in rhesus monkeys. Vet. Pathol. 1996;33:296–302. doi: 10.1177/030098589603300306. [DOI] [PubMed] [Google Scholar]
- 12.Wong J, Korcheva V, Jacoby DB, Magun B. Intrapulmonary delivery of ricin at high dosage triggers a systemic inflammatory response and glomerular damage. Am. J. Pathol. 2007;170:1497–1510. doi: 10.2353/ajpath.2007.060703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, van Deurs B, Iversen TG. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 2002;117:131–141. doi: 10.1007/s00418-001-0346-2. [DOI] [PubMed] [Google Scholar]
- 14.Saxena SK, O’Brien AD, Ackerman EJ. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28S RNA when microinjected into Xenopus oocytes . J. Biol. Chem. 1989;264:596–601. [PubMed] [Google Scholar]
- 15.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes: the site and the characteristics of the modification in 28S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 16.Korcheva V, Wong J, Corless C, Iordanov M, Magun B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am. J. Pathol. 2005;166:323–339. doi: 10.1016/S0002-9440(10)62256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 19.Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ. An osmo-sensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 22.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 23.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 24.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 25.Jandhyala DM, Ahluwalia A, Obrig T, Thorpe CM. ZAK: a MAP3 kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell. Microbiol. 2008;10:1468–1477. doi: 10.1111/j.1462-5822.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 26.Korcheva V, Wong J, Lindauer M, Jacoby DB, Iordanov MS, Magun B. Role of apoptotic signaling pathways in regulation of inflammatory responses to ricin in primary murine macrophages. Mol. Immunol. 2007;44:2761–2771. doi: 10.1016/j.molimm.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong J, Korcheva V, Jacoby DB, Magun BE. Proinflammatory responses of human airway cells to ricin involve stress-activated protein kinases and NF-κB. Am. J. Physiol. 2007;293:L1385–L1394. doi: 10.1152/ajplung.00207.2007. [DOI] [PubMed] [Google Scholar]
- 28.Kim KA, Park CY, Lim Y, Lee KH. Recent advances in particulate-induced pulmonary fibrosis; for the application of possible strategy experimentally and clinically. Curr. Drug Targets. 2000;1:297–307. doi: 10.2174/1389450003349146. [DOI] [PubMed] [Google Scholar]
- 29.Khalil N, Churg A, Muller N, O’Connor R. Environmental, inhaled and ingested causes of pulmonary fibrosis. Toxicol. Pathol. 2007;35:86–96. doi: 10.1080/01926230601064787. [DOI] [PubMed] [Google Scholar]
- 30.Glazer CS, Newman LS. Occupational interstitial lung disease. Clin. Chest Med. 2004;25:467–478. doi: 10.1016/j.ccm.2004.04.004. vi. [DOI] [PubMed] [Google Scholar]
- 31.Roche JK, Stone MK, Gross LK, Lindner M, Seaner R, Pincus SH, Obrig TG. Post-exposure targeting of specific epitopes on ricin toxin abrogates toxin-induced hypoglycemia, hepatic injury, and lethality in a mouse model. Lab. Invest. 2008;88:1178–1191. doi: 10.1038/labinvest.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zenilman ME, Fiani M, Stahl P, Brunt E, Flye MW. Use of ricin A-chain to selectively deplete Kupffer cells. J. Surg. Res. 1988;45:82–89. doi: 10.1016/0022-4804(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 33.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukocyte Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 34.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham E. Neutrophils and acute lung injury. Crit. Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 36.Shanley TP, Warner RL, Ward PA. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol. Med. Today. 1995;1:40–45. doi: 10.1016/1357-4310(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 37.Baluk P, Thurston G, Murphy TJ, Bunnett NW, McDonald DM. Neurogenic plasma leakage in mouse airways. Br. J. Pharmacol. 1999;126:522–528. doi: 10.1038/sj.bjp.0702323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukhopadhyay P, Ali MA, Nandi A, Carreon P, Choy H, Saha D. The cyclin-dependent kinase 2 inhibitor down-regulates interleukin-1/3-mediated induction of cyclooxygenase-2 expression in human lung carcinoma cells. Cancer Res. 2006;66:1758–1766. doi: 10.1158/0008-5472.CAN-05-3317. [DOI] [PubMed] [Google Scholar]
- 39.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 40.Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008;63:147–153. doi: 10.1136/thx.2007.079608. [DOI] [PubMed] [Google Scholar]
- 41.Abraham E, Allbee J. Effects of therapy with interleukin-1 receptor antagonist on pulmonary cytokine expression following hemorrhage and resuscitation. Lymphokine Cytokine Res. 1994;13:343–347. [PubMed] [Google Scholar]
- 42.Calkins CM, Bensard DD, Shames BD, Pulido EJ, Abraham E, Fernandez N, Meng X, Dinarello CA, McIntyre RC., Jr IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J. Endotoxin Res. 2002;8:59–67. [PubMed] [Google Scholar]
- 43.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mabley JG, Pacher P, Szabo C. Activation of the cholinergic anti-inflammatory pathway reduces ricin-induced mortality and organ failure in mice. Mol. Med. 2009;15:166–172. doi: 10.2119/molmed.2008.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fels AO, Cohn ZA. The alveolar macrophage. J. Appl. Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 46.Male D. Immunology. St. Louis: Mosby; 2006. [Google Scholar]
- 47.Gordon S. The macrophage: past, present and future. Eur. J. Immunol. 2007;37(Suppl. 1):S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 48.Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, Liles WC. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J. Immunol. 2003;170:6209–6216. doi: 10.4049/jimmunol.170.12.6209. [DOI] [PubMed] [Google Scholar]
- 49.Hohlbaum AM, Gregory MS, T Ju S, Marshak-Rothstein A. Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. J. Immunol. 2001;167:6217–6224. doi: 10.4049/jimmunol.167.11.6217. [DOI] [PubMed] [Google Scholar]
- 50.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 51.Dinarello CA. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002;20:S1–S13. [PubMed] [Google Scholar]
- 52.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 53.Holmes MC, Zhang P, Nelson S, Summer WR, Bagby GJ. Neutrophil modulation of the pulmonary chemokine response to lipopolysaccharide. Shock. 2002;18:555–560. doi: 10.1097/00024382-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-κB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proin-flammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am. J. Respir. Crit. Care Med. 1996;153:1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 56.Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am. J. Respir. Cell Mol. Biol. 2007;37:477–484. doi: 10.1165/rcmb.2006-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen LW, Chang WJ, Wang JS, Hsu CM. Interleukin-1 mediates thermal injury-induced lung damage through C-Jun NH2-terminal kinase signaling. Crit. Care Med. 2007;35:1113–1122. doi: 10.1097/01.CCM.0000259175.78174.B2. [DOI] [PubMed] [Google Scholar]
- 58.Manzer R, Wang J, Nishina K, McConville G, Mason RJ. Alveolar epithelial cells secrete chemokines in response to IL-1β and lipopolysaccha-ride but not to ozone. Am. J. Respir. Cell Mol. Biol. 2006;34:158–166. doi: 10.1165/rcmb.2005-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manzer R, Dinarello CA, McConville G, Mason RJ. Ozone exposure of macrophages induces an alveolar epithelial chemokine response through IL-1α. Am. J. Respir. Cell Mol. Biol. 2008;38:318–323. doi: 10.1165/rcmb.2007-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beck GC, Yard BA, Breedijk AJ, Van Ackern K, Van Der Woude FJ. Release of CXC-chemokines by human lung microvascular endothelial cells (LMVEC) compared with macrovascular umbilical vein endothelial cells. Clin. Exp. Immunol. 1999;118:298–303. doi: 10.1046/j.1365-2249.1999.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ray KP, Kennard N. Interleukin-1 induces a nuclear form of transcription factor NF-κB in human lung epithelial cells. Agents Actions. 1993;38:C61–C63. doi: 10.1007/BF01991138. Spec. no. [DOI] [PubMed] [Google Scholar]
- 62.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-κB activation in lipopolysaccharide-induced airway inflammation. J. Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 63.Haegens A, Barrett TF, Gell J, Shukla A, Macpherson M, Vacek P, Poynter ME, Butnor KJ, Janssen-Heininger YM, Steele C, Mossman BT. Airway epithelial NF-κB activation modulates asbestos-induced inflammation and mucin production in vivo. J. Immunol. 2007;178:1800–1808. doi: 10.4049/jimmunol.178.3.1800. [DOI] [PubMed] [Google Scholar]