Abstract
The medial prefrontal cortex (mPFC) is responsible for executive functions such as abstract rule coding. strategy switching, and behavioral flexibility; however, there is some debate regarding the extent to which mPFC is involved in reversal learning, especially in complex multisensory tasks such as conditional discrimination. Therefore, we investigated the effects of mPFC inactivation on the acquisition, retention. and reversal of a visuospatial conditional discrimination (CD) task. In experiment 1. muscimol was infused through bilateral cannulae on days 1,2, and 3 to rest the effects of mPFC inactivation on task acquisition and days 19,. 20, and 21 to test the effects on retention of the task. For experiment 2, rats were trained on the CD task for 21 days with no infusions given, after which the reward contingency was reversed, with infusions given during the first six days of reversal. The results of experiment 1 showed that the muscimol and saline groups did not differ on acquisition or retention. However, experiment 2 showed that the muscimol group displayed significantly more performance errors than the control group during reversal. Compared to the control group, the muscimol group also showed a decreased tendency to use a side-bias strategy during the intermediate stages of reversal. The failure of the muscimol group to exhibit a side bias suggests that the mPFC is necessary for sampling strategies necessary for the reversal of a visuospatial CD task.
Keywords: Medial prefrontal cortex, Behavioral flexibility, Reversal, Conditional discrimination, T-maze, Muscimol, Configural learning
1. Introduction
The medial prefrontal cortex (mPFC) is thought to be responsible for higher executive functions, such as behavioral flexibility, which requires the suppression of a previously successful behavior in response to an unexpected change in the environment to favor a more suitable behavior [1.2]. Damage to the mPFC in rodents has been shown to impair performance of tasks that require behavioral flexibility, such as rule or strategy switching in spatial navigation tasks [3–6] and extinction of a conditioned response in appetitive Pavlovian conditioning [7].
Conditional discrimination (CD) learning requires a subject to learn that a particular response will be rewarded and another response not rewarded in one context and that the opposite reward contingency will be applied in a different context. This type of learning involves associating each unique context-response configuration with a reinforcement value (rewarded or not) and is thus considered to be a type of configural learning [8]. It has been established that the formation of configural associations depends on the hippocampal formation based on evidence that rats with hippocampal lesions cannot solve configural tasks such as negative patterning and transverse patterning [9] or visuospatial conditional discrimination [10]. However, the hippocampus is part of a larger system of anatomically-connected neural structures, including the medial prefrontal cortex (mPFC). The role of the mPFC in configural learning in general and conditional discrimination learning in particular has been virtually unexplored. Moreover, the fact that the mPFC is known to be crucial for executive functioning, specifically task switching, suggests that the mPFC would be particularly important for conditional discrimination reversal, in which the context previously associated with one response-reward contingency is switched to the opposite context. Additionally, examination of intermediate strategy selection, such as the temporary development of a side bias, can serve as a measure of behavioral flexibility in this type of task.
Our laboratory has recently developed a visuospatial CD task that requires a two-choice spatial discrimination in response to a multisensory conditional cue. The involvement of the mPFC in the acquisition, retention and reversal of this type of learning is unknown. Therefore, the present study examined the necessity of mPFC integrity in the acquisition, retention (experiment 1), and reversal (experiment 2) of a visuospatial CD task that required rats to use intramaze cues (floor inserts that varied in texture and color) as a conditional cue for goal-arm selection on a T-maze.
2. Methods
2.1. Subjects
Male Long-Evans Hooded rats (Harlan. Indianapolis). 3–6 months old, were housed in standard laboratory cages maintained in a temperature and humidity controlled colony room on a 12:12-h light /dark cycle. After a 1-week acclimation period, rats were kept at 90% if their free-feeding body weight and given ad libitum access to water throughout and the experiment.
2.2. Apparatus
All training was performed on a T-maze constructed of wood and painted black. The maze consisted of a central arm (117 × 6 cm), two goal arms (79 × 6 cm) and two return arms (120 × 6 cm) (Fig. 1). A plastic cup at the end of the goal arm was used to place a chocolate sprinkle food reward. There was a start box located at the base of the maze stem, where the rat was confined between trials. The experimental room was illuminated by a single 60 W bulb and surrounded by a black curtain with various visual cues attached. Experiments 1 and 2 were carried out in the same room and on the same maze. The floor of the maze was covered with black contact paper.
Fig. 1.
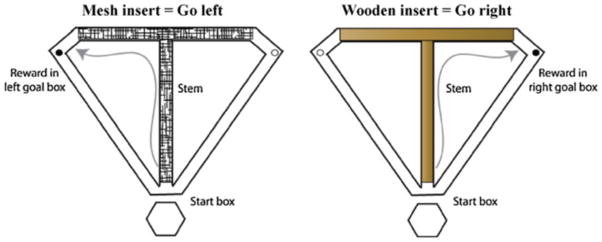
Schematic of the conditional discrimination task. Wooden floor inserts were placed in the stem and goal arms of the T-maze prior to each trial. The inserts were covered on one side with black plastic mesh and the other side was left as plain wood. The texture and appearance of the insert acted as a conditional cue signaling which goal arm to visit in order to obtain a food reward. Trials were presented in a pseudorandom sequence. During the intertrial interval, the rats were confined to the start box using a large wooden barricade. Half of the rats in each experiment learned the “left on wood, right on mesh” rule: and the other half learned the “right on wood, left on mesh” rule. For the reversal phase of experiment 2, the rats that learned the “left on wood, right on mesh” rule were switched to the “right on wood, left on mesh rule” and vice versa.
2.3 Maze acclimation and pretraining
Prior to maze acclimation, rats wore handled by the experimenter for ~10 min a day for one week Rats were then acclimated to the testing room and maze for 6–8 days. The first 2 acclimation sessions consisted of goal-zone training in which rats were confined to the right and left goal zone on alternating trials and learned to consume chocolate sprinkles from reward cups. The next 4–6 sessions were 12-trial forced-run sessions in which one of the two goal arms was blocked (in a pseudorandom sequence) so that rats learned to run up the maze stem into the open goal arm to the reward cup, where they received a chocolate sprinkle reward. Floor inserts (see next section) were not introduced during the pretraining stage of the experiment.
2.4. Conditional discrimination (CD) training
Prior to each trial, the experimenter placed wooden floor inserts covered on one side with black plastic mesh into the central stem and both goal arms of the T-maze with either the wood or mesh side facing up. Rats learned to select either the left or right goal arm to obtain a food reward contingent upon the texture/color (black mesh or bare wood) of the floor insert (Fig. 1). Half of the rats were trained on the rule ‘left on wood/right on mesh’ and the other half was trained on the ‘right on wood/left on mesh’ rule. During the inter-trial interval (ITI), a black wooden barricade was placed between the pedestal and the maze to obstruct the rats’ view while the experimenter prepared for the next trial. In order to prevent the rat from using auditory cues to anticipate the next trial, the experimenter flipped the insert during every ITI. The unrewarded goal zone was sham baited on each trial. Each ITI lasted for 8–10s. Rats were given 24 trials (12 mesh, 12 wood) in a pseudorandom sequence [11].
2.5. Surgery
Rats were given a pre-anesthetic subcutaneous dose of atropine (0.05 mg/kg) and anesthetized with continuous-flow isoflurane (1.5–3% in oxygen), mounted on a stereotaxic frame. The scalp was shaved, anesthetized with a subcutaneous injection of lidocaine and sterilized with Nolvasan® After the skull was exposed and cleaned, four small holes were drilled near the skull ridge using a sterotaxic-mounted drill (Fine Science Tools). Bone screws (shaft length 4.0 mm. shaft diameter 0.85 mm) were fixed into the holes and affixed to the skull with dental acrylic (Patterson Dental). Circular holes were drilled using a 1.8-mm-diameter trephine (Fine Science Tools) in each hemisphere at the following coordinates: 3. 0 mm anterior to bregma, ±1.8 mm lateral to bregma [12]. Dorsal-ventral coordinates were taken from dura mater for more accurate placement of the cannulae. Once dura was removed, the exposed brain was kept moist using gel foam soaked in sterile saline A 26-gauge stainless steel guide cannulae, held in a stereotaxic arm at a 14° angle, was lowered into the respective hemisphere 2.0 mm ventral to dura. The guide cannulae were affixed to the skull with dental acrylic. A subcutaneous injection of Banamine (2.5 mg/kg) was given approximately 30min prior to the end of surgery and children’s ibuprofen (20mg/mL) was given in the drinking water two days postoperatively for analgesia. Rats were allowed to recover for five days prior to behavioral training. All procedures were carried out in accordance with the University of Delaware Institutional Animal Care and Use Committee.
2.6. Infusions
Muscimol, a GABAA agonist, was dissolved in sterile saline and the solution was infused bilaterally via a 31-gauge injector connected to a 10-μl. Hamilton syringe by a polyethylene tube. The injector extended 1.5 mm beyond the tip of the guide cannula. The infusion volume and rate was controlled by an infusion pump (World Precision lnstruments) programmed to deliver the infusate, either muscimol (0.1 μg/μL) or sterile saline, at a rate of 0.25 μL/min for 2 min, for a total volume of 0.50 μL for each hemisphere. Infusion cannulae were left in place for 2 min after the infusion to allow for diffusion. Rats were lightly anesthetized with isoflurane during each infusion and given 30 min in their home cages after the infusion to recover from the anesthesia. Anesthesia was necessary due to the small size of the internal cannula and has been shown by other investigations to have no effect on subsequent behavioral performance [6].
2.7. Histology
After completion of behavioral training, rats were anesthetized with isoflurane and infused bilaterally with 0.5 μL of a neutral red solution (dissolved in saline) for determination of the spread of the infusate. Rats were then given an overdose of sodium pentobarbital (200mg/kg.ip) and perfused using 0.9% saline followed by 10% buffered formalin to fix the tissue. The brains were then removed and placed in 10% buffered formalin. After at least 24h in formalin, the brains were placed in 30% buffered sucrose solution. After sinking, the brains were frozen and sectioned (40 μm) using a cryostat. The sections were mounted on slides, stained using cresyl violet and photographed using a camera mounted on a microscope. Cannulae placements were verified by overlaying the photograph of the section with atlas plates from Paxinos and Watson [12] in Adobe Illustrator. Because it was often difficult to see the injector cannulae tracks, placements were determined from the guide cannula tracks, so the actual placement of the injectors was 1.5mm ventral to the placement.
2.8. Experiment I: effects of mPFC inactivation on CD acquisition and retention
After recovery from surgery, rats were trained on the CD task for 21 days. To examine whether mPFC inactivation impaired CD acquisition, muscimol infusions were given prior to training on the first 3 days of CD training. Infusions were again given on days 19,20 and 21 to test the effects of mPFC inactivation on retention of the CD task. Groups of rats were counterbalanced so that half of the rats that received muscimol during acquisition would receive either saline or muscimol during the retention and vice versa. To control for any effects due to anesthesia that was used for the infusions, acquisition and retention of the CD task was measured in a third group of rats. These rats were implanted with bilateral mPFC cannulae but did not receive anesthesia or infusions during the 21 days of task acquisition and performance. This group of rats was then used in experiment 2 to test for effects of mPFC inactivation on CD reversal (sec below). The acquisition and retention of the visuospatial CD task were compared across the no-infusion, saline, and muscimol groups.
2.9. Experiment 2: effects of mPFC inactivation on reversal of the CD task
Rats were trained on the CD task for 21 days without infusions and randomly assigned to either the saline or muscimol group. Before entering the reversal phase of the experiment, all of the rats included in experiment 2 met a criterion of 2 consecutive days performing 75% correct on the CD task Starting on day 22, the reward contingency was reversed. For instance, if the previous rule was ‘left on wood’, the rats were then trained on the rule ‘right on wood’ from days 22 to 42. In order to examine the effects mPFC inactivation on CD reversal, for the first six days of reversal, infusions of either muscimol or saline were given prior to training using the same procedures as those described above for experiment 1.
2.10. Statistical analysis
The training sessions were grouped into 3-session (72-trial) blocks for comparison of the learning curves across training. The number of errors was then compared between the no-infusion, muscimol, and saline groups using mixed design (3 group × 6 block) ANOVA for experiment 1. Also for experiment 1, choice accuracy (percentage of correct trials) was compared between the no-infusion, muscimol, and saline groups using a 3 (group) × 4 (session) mixed-design ANOVA. For both experiment 1 and 2, learning and reversal rates were compared between groups by counting the number of sessions in which the choice accuracy was below chance (50%) and comparing the number of below-chance sessions between the groups using a one way ANOVA. The reversal training sessions were grouped into 3-session (72-trial) blocks for comparison of the learning curves across training. The number of errors was then compared between the muscimol and saline groups using mixed design (2 group × 6 block) ANOVA. Side bias for each reversal session was calculated by the following formula: bias index =|(NR-NL)|/(NR + NL), where NR = number of right-turn error trials and NL = number of left-turn error trials. The bias index ranges from zero, meaning no side bias to 1.0, meaning that the rat chose the same goal arm on every trial of that session. For experiment 2, the side bias index was compared between the muscimol and saline groups using a 2 (group) × 21 (session) mixed design ANOVA. Post hoc t-tests with Bonferroni corrections were then used to identify sessions in which the groups were significantly different.
3. Results
3.1. Experiment 1
3.1.1. Histology
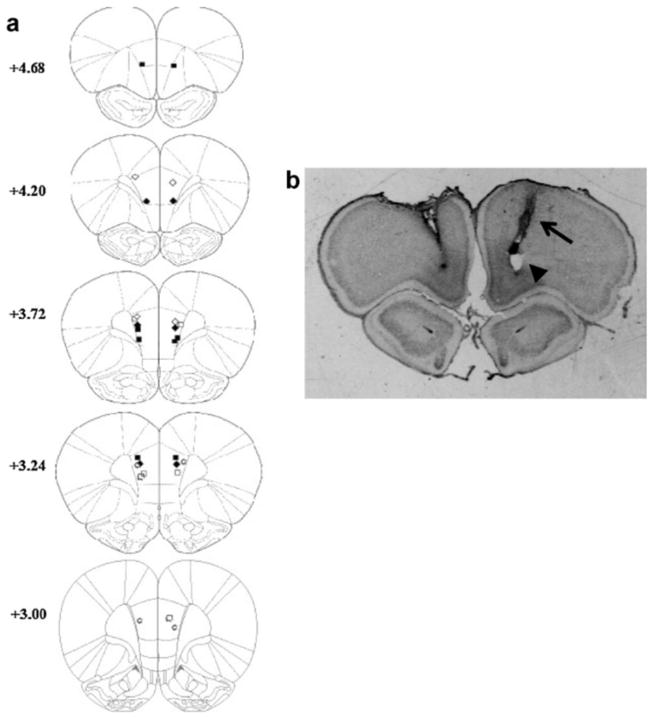
Fig. 2 displays the placements of the bilateral guide cannulae tips for experiment 1. Cannulae placements were restricted to the prelimbic region of the mPFC. Data from rats with incorrect placements were excluded from further analysis, leaving a total of 14 rats, 8 in the saline group and 6 in the muscimol group for acquisition and 7 in each group, saline and muscimol, for retention. From examining the placements, it is possible that the infusion spread to neighboring regions including the infralimbic region of mPFC and the medial portions of OFC in some of the rats.
Fig. 2.
Experiment 1: cannula tip locations. (a) Coronal sections +4.68 to +3.00mm from bregma adopted from Paxinos and Watson [12]. Open diamonds, rats that received saline for both acquisition and retention; filled squares, rats that received saline during acquisition and muscimol during retention; filled diamonds, rats that received muscimol during acquisition and saline during retention; open squares, rats that received muscimol for both acquisition and retention. (b) Representative section through the mPFC stained with cresyl violet, showing tracks from the guide cannula (arrow) and tip of the injection cannula (arrowhead).
3.1.2. Behavior
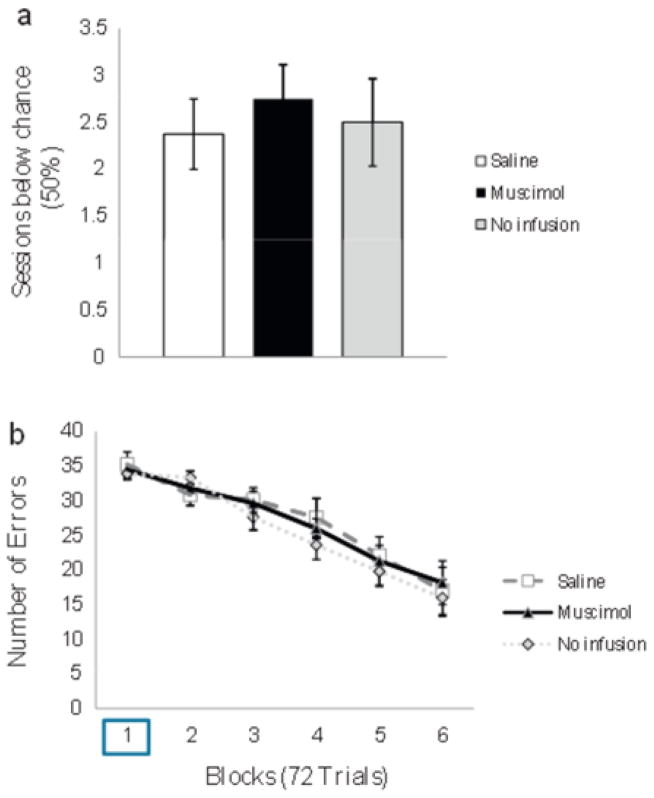
In experiment 1, we set out to determine whether the mPFC is necessary for the acquisition of the CD task. A previous experiment from our laboratory found that intact rats initially perform the CD task below chance (50%) on the first two training sessions, presumably due to the use of an alternation strategy, bur group performance rises above chance on the third day of training [13]. Therefore, infusions of muscimol or saline were given prior to training on the first 3 sessions. A group of animals that received surgery but no anesthesia and no infusions was compared to the saline and muscimol groups to control for any effects due to anesthesia administration during infusions. The average number of sessions in which choice accuracy was below 50% was compared between groups using a oneway ANOVA. There were no significant differences between groups, F(2.29)=0.128. p=0.881 (see Fig. 3a). A 3 (group) by 6 (block) ANOVA on the number of errors per block revealed a significant main effect of block. F(5.135) = 35.347. p=0.000, but no significant main effect of group, F(2.27)=0.310, p=0.736, and no group × block interaction, F( 10.135)=0.465, p=0.910 (See Fig. 3b).
Fig. 3.
Experiment 1: effect of mPFC inactivation on acquisition of the CD task (a) Number of sessions (SEM) in which choice accuracy was below chance (50%) for the saline (white), muscimol (black) and no-infusion (gray) groups, (b) Number of errors (SEM) across six 72-trial blocks of acquisition for the saline, muscimol and no-infusion groups. Infusions were given during the first block (box).
To assess the effects of mPFC inactivation on asymptotic performance, rats were given 3 additional infusions immediately prior to the training session on days 19, 20 and 21. The percentage of correct trials was then compared between the no-infusion, muscimol, and saline groups for days 18–21 (No infusion. Infusion 1, Infusion 2 and Infusion 3, respectively; Fig. 4) using a 3 (group) × 4 (session) ANOVA. There was no significant main effect of session, F(3.18)=1.562, p=0.233, no significant main effect of group, F(1.6)=0.850, p=0.392 and no group × session interaction, F(3.18)=0.699, p=0.565 (see Fig. 4). Together, these results show that the acquisition and retention of visuospatial CD are not dependent upon the mPFC.
Fig. 4.
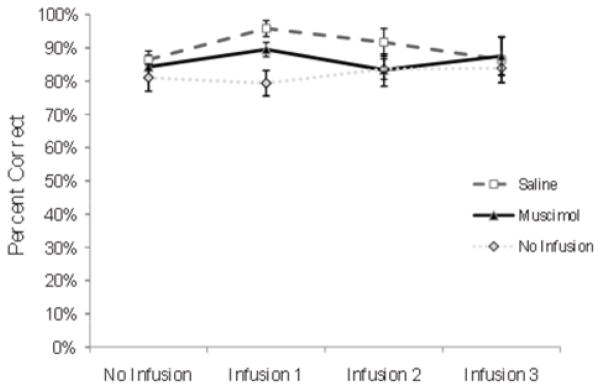
Experiment 1: effect of mPFC inactivation on asymptotic performance of the CD task Percentage of correct trials (SEM) for the last day of CD training and 3 subsequent infusion sessions. The no-infusion, saline, and muscimol groups were not significantly different on the no-infusion day or the 3 subsequent infusion days.
3.2. Experiment 2
3.2.1 Histology
Fig. 5 shows the guide cannula placements for the rats included in experiment 2. Guide cannula tips were located in the prelimbic (PL; N=7 in both the muscimol and saline groups)or dorsal anterior cingulate (ACd; N=2 in the muscimol group) regions of the mPFC. The 2 rats with ACd placements were included in the study because the injector extended 1.5 mm beyond the tip of the guide cannula (see Section 2), into the prelimbic region of mPFC. After excluding rats with incorrect placements, there were 7 rats in the saline group and 9 rats in the muscimol group included in the behavioral analysis.
Fig. 5.
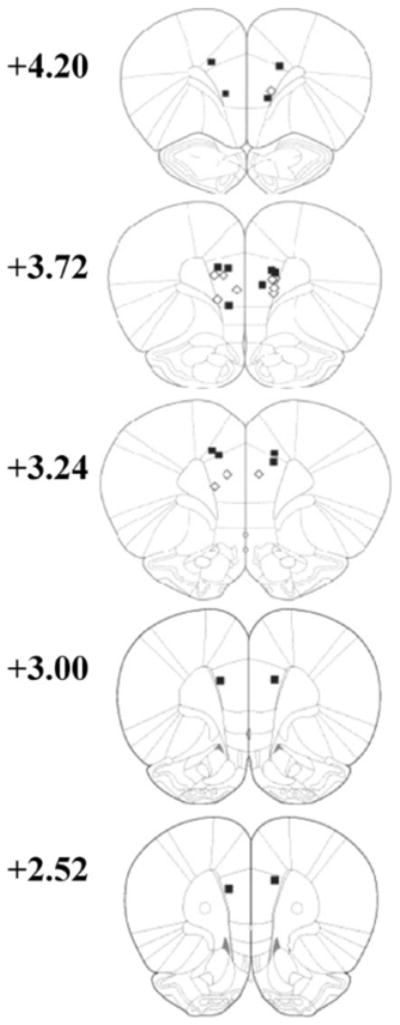
Experiment 2: cannulatip locations. Coronal sections +4.20 to +2.52 mm from bregma adopted from Paxinos and Watson [12] Filled squares, muscimol group: open diamonds, saline group.
3.2.2. Behavior
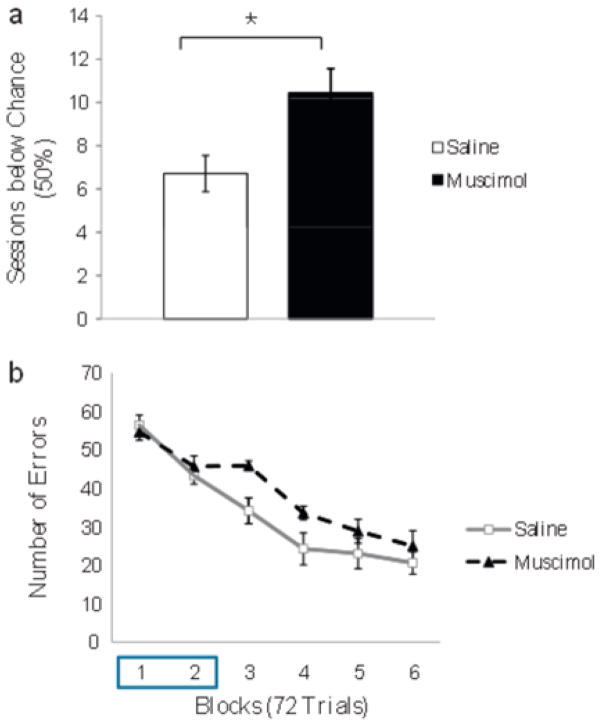
For experiment 2. we investigated whether mPFC inactivation would impair reversal of the CD task. The muscimol group had a significantly greater number of reversal sessions in which performance accuracy was below chance (M = l0.4, SD=3.35) than the saline group (M=6.7, SD = 2.21, t(12)=2.58, p=0.011; Fig. 6a). The learning curves of the saline and muscimol groups were then compared across the 6 blocks of reversal. A 2 group × 6 block ANOVA revealed a significant main effect of group. F(1.14)=5.270, p=0.038 (See Fig. 6b), a significant main effect of block, F(1.994, 27.918)=44.056, p=0.000, and no group × block interaction, F(1.994, 27.918)=1.617, p=0.217.
Fig. 6.
Experiment 2: effects of mPFC inactivation on reversal learning. (a) Number of sessions (SEM) below chance (50%) for the saline (white) and muscimol groups (black). *p <0.05. (b) Number of errors (SEM) across all 6 reversal blocks for the saline and muscimol groups. Infusions were given in the first 2 blocks of reversal (box).
In order to further investigate the effects of mPFC inactivation on CD reversal, the tendency to use a side bias strategy was compared between groups. Side bias is the degree to which a rat favors either the left or right goal arm. The values range from 0, meaning no side bias, to 1, meaning that every error in a session was either exclusively to the left or exclusively to the right. A 2 (group) × 21 (session) ANOVA on the side bias index during reversal revealed a significant main effect of session, F(16.745,234.431)=3.027, p=0.000, no significant main effect of group, F(1.14)=1.640, p=0.221, and a significant group × session interaction, F(16.745,234.431)=1.95, p=0.015. Post hoc t-tests with Bonferroni corrections revealed that the saline group showed a significantly higher bias index on sessions 7 (the first infusion-free reversal session), 8, 11, and significantly lower bias index on sessions 15 and 21 than the muscimol group (p < 0.05; see Fig. 7). Rats were then categorized based on whether they showed a side bias index at or above 75% on 3 or more consecutive sessions. This analysis revealed 4 out of 7 rats in the saline group and no rats in the muscimol group consistently used a side-bias strategy sometime during the course of reversal learning. The difference in the utilization of a side bias was significantly different between the saline and muscimol groups (X2 (1) = 6.85, p= 0.008). The four rats from the saline group that were categorized as showing a side bias all showed a common pattern. Side bias was low early in reversal, when all of the rats were following the initial rule. Starting on session 5 or 6, the 4 rats showed a robust side bias, which was maintained between sessions 8 and 12 and dramatically dropped over subsequent sessions (see Fig. 7). In contrast to the performance results described above, the side bias index for the muscimol group was consistently low throughout all reversal sessions, with the exception of the final reversal session. Together, these different patterns of side bias across reversal sessions suggest that the development of a side bias is a common strategy used by intact rats during the intermediate stages of reversal learning. The fewer number of rats exhibiting a side bias in the muscimol group suggests that the mPFC may be critical for the development of the side bias strategy and that the transient use of this strategy can facilitate reversal learning.
Fig. 7.
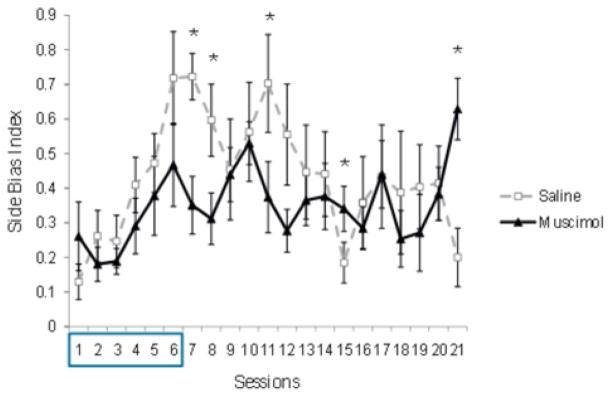
Experiment 2: side bias during reversal learning. The side bias index was calculated by taking the absolute value of the difference between number of left trial errors and the number of right trial errors and dividing the result by the total number of errors. Infusions were given during the first 6 sessions (box). Error bars indicate SEM. *p< 0.05.
4. Discussion
Our results show that inactivation of the mPFC using microinfusions of muscimol does not impair the acquisition or retention of a visuospatial CD task. However, reversal of the task was significantly disrupted by mPFC inactivation. Interestingly, the saline group developed a strong side bias in the intermediate stages of reversal learning that was nor evident in the muscimol group. Together, these findings suggest that the mPFC is crucial for strategy selection that may aid in subsequent successful task reversal.
4.1. Inactivation of the mPFC does not impair CD acquisition or retention
Our findings suggest that the prelimbic region of the mPFC is not critical for the acquisition or retention of the CD task. The mPFC has been established as an important component of the neural system involved in working memory [14–17]. Working memory tasks such as delayed spatial alternation involve a delay period over which the rat has to remember trial-unique information. In contrast, the CD task does not rely on working memory. In fact, if rats use a spatial alternation strategy on the CD task, they will only be rewarded on 50% of the trials due to the pseudorandom sequence of cue presentation. Because of their natural tendency to alternate, rats may initially use an alternation strategy to solve the CD task [18], a strategy which needs to be abandoned in order to adopt the rules needed to perform a conditional discrimination. This notion is supported by the fact that for both muscimol and control groups in both experiment 1 and experiment 2, the percentage of correct trials is slightly below chance during the first few sessions of CD training (data not shown, see Griffin et al. [13]).
The acquisition of a visuospatial CD task similar to the task used in the current investigation has been shown to be impaired by excitotoxic lesions of the entire hippocampus [10] and ischemia-induced hippocampal CA1 neuronal loss [19]. Because the ventral hippocampus sends a strong monosynaptic projection to mPFC [20–22] we suspected that the deficits seen in previous investigations after hippocampal lesions might be a secondary result of the loss of crucial hippocampal input to the mPFC. Instead, our results suggest that the deficits in the visuospatial CD task after hippocampal ablation arise either because the hippocampus itself performs a critical role in acquiring the task, perhaps due to its putative role in configural learning [9], or because the hippocampus sends critical input to another downstream structure (i.e. the striatum) that is crucial for the acquisition and/or retention of the CD task.
4.2. Inactivation of the mPFC disrupts reversal of the CD task
Our results show that mPFC inactivation by muscimol infusions impairs reversal learning, which is consistent with previous investigations that have found deficits in reversal learning after mPFC lesions or disruptions. Although the requirement of the mPFC has been consistently shown in studies that have used a strategy switch. there are also studies that found impairments after mPFC disruption in tasks that do not require a shift in strategy. Kinoshita et al. [38] found that mPFC lesions impaired the serial reversal of a two-choice olfactory discrimination. The mPFC was not required for the initial reversal but was required for the second reversal. The mPFC has also been shown to be required for reversal learning when stimuli are difficult to discriminate [26]. Moreover, excitotoxic lesions of the mPFC have been shown to impair spatial reversal in the Morris Water maze [13,19] and reversal, but not acquisition, of an aversively-motivated visual discrimination in a rotating T-maze [27]. Future studies could examine whether mPFC inactivation results in an impairment of serial reversal learning of the CD task and examine the effects of mPFC inactivation on CD task performance and reversal using conditional cues that vary in discriminability and motivational valance. Different subregions of the rodent prefrontal cortex (PFC) have been shown to mediate dissociable types of behavioral flexibility [28]. The two subregions of interest for the reversal of the CD task are orbitofrontal cortex (OFC) and mPFC. Both the OFC and mPFC consist of large functional networks making many connections to a heterogeneous mix of brain regions and are anatomically connected to one another which often results in functional overlap [28]. Recent experiments have been parsing out the specific roles of the OFC and mPFC in learning, memory, and behavioral flexibility. Studies have found a double dissociation between OFC and mPFC in reversal and extradimensional set-shifting in rats [9,10,29,30] marmosets [31] and mice [32], with OFC lesions causing deficits in reversal, but not set-shifting, and mPFC lesions causing deficits in set-shifting, but not reversal. Together with previous studies, our results indicate that, with the exception of very complex discrimination tasks, the mPFC is only required during a shifting of strategy, cues, or modalities. Future studies could examine whether the integrity of the OFC is critical for the successful reversal of the CD task.
4.3. mPFC inactivation impairs the use of a side-bias strategy, which could be advantageous to reversal learning
Our results show that rats in the saline group were more likely than rats in the muscimol group to use a side-bias strategy during the intermediate stages of reversal learning. Similar to results reported in a previous investigation in which rats switched from a win-shift strategy to a win-stay strategy in a T-maze [3], the saline group showed a 3-stage pattern of reversal learning, beginning with perseverative responding to the old rule, the adoption of a side-bias strategy (in which the rat gets rewarded on half of the trials), and finally successful reversal. The muscimol group, on the other hand, did not develop a consistent side-bias strategy at any point during reversal learning. This lack of consistent use of a side bias strategy in the muscimol group compared to the saline group suggests that the mPFC is responsible for the behavioral flexibility required to sample alternative strategies when reward contingencies change. The fact that saline rats were more likely to exhibit a side bias and showed faster learning rates suggests that the development of a side bias is an optimal (albeit not essential) intermediate strategy for eventual reversal learning.
In a series of experiments. Delat our and Gisquet-Virrier [33–35] have demonstrated a dissociation between behavioral effects of lesions of the dorsal subregion of the mPFC (the dorsal anterior cingulate (ACd)), and the ventral subregions of mPFC (the prelimbic (PL) and infralimbic cortices (IL)). These experiments suggest that the ACd, which is anatomically connected with “premotor” brain regions [36] is involved in temporal behavioral sequencing whereas the more ventral PL/IL regions, which receive strong projections from the ventral hippocampus [21], are involved in attention and behavioral flexibility. The low number of placements at the ACd/PL border precluded a systematic analysis of the differences in behavioral consequences of inactivation of ACd vs. PL. However, examination of the behavioral data revealed that the 2 rats in the muscimol group with ACd/PL placements performed similarly to the rats with PL placements during the first two-thirds of the reversal sessions. Interestingly, these two rats showed a profound deficit (compared to the rats in the muscimol group with PL placements and the saline group) in the last third of the training sessions, with performance hovering around chance throughout the duration of the reversal sessions. Future studies could specifically target the PL and ACd in separate groups to investigate if these mPFC subregions indeed show differential participation in CD reversal.
The advantages of using the microinfusion technique over permanent excitotoxic, radiofrequency, or aspiration lesions include minimizing destruction of fibers of passage, the ability to use repeated measures designs to increase statistical power, and a lower likelihood of compensatory plasticity that can occur after brain damage. These advantages are tempered by two major limitations. One problem is that multiple infusions in the same infusion site over the course of several days are likely to cause mechanical tissue damage. To minimize this risk, we restricted the number of infusions per rat to 6. In experiment 1, infusions were given in the first 3 sessions to test for acquisition deficits and the last 3 sessions to test for retention deficits and in experiment 2, infusions were given in the first 6 days of CD reversal. Now that we have established that the mPFC is not crucial for retention, a logical next step would be to inactivate the mPFC during the first 6 days of acquisition to see if a longer period of mPFC inactivation during CD acquisition would cause disrupt learning of the CD task. Another challenge inherent to the microinfusion technique is selecting infusion parameters that manage the trade off between effectively inactivating a discrete region and spreading to neighboring regions, which could lead to global sensorimotor or motivational deficits. We used a muscimol concentration and volume identical to those shown by Rich and Shapiro [6| to induce deficits in strategy switching. The fact that we observed a disruption in learning of the reversal suggests that the concentration and volume of muscimol was sufficient to inactivate the mPFC. Moreover, examination of the spread of neutral red dye injected into the mPFC prior to perfusion showed approximate 1.0-mm spread of the infusate around the tip of the injection cannula, as shown in previous investigations [37]. Therefore, we believe that we adequately inactivated the mPFC.
5. Conclusions
Consistent with its purported role in executive function, the mPFC was shown to be crucial for both reversal learning and strategy selection. This investigation sets the stage for future studies that utilize the CD task and its reversal to further investigate brain mechanisms underlying behavioral flexibility.
HIGHLIGHTS.
We examined the effects of mPFC inactivation on conditional discrimination learning.
Acquisition/retention of conditional discrimination task does not require the mPFC.
mPFC inactivation impaired conditional discrimination reversal learning.
The rat mPFC plays a crucial role in strategy selection during task reversal.
Acknowledgments
The authors would like to thank Monica Patel for collecting portions of behavioral data that were used in this manuscript.
References
- 1.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19:4585–894. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy Science. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 3.Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. European Journal of Neuroscience. 2000;12:4457–66. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 4.Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behavioral Neuroscience. 2003;117:1054–65. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- 5.Salazar RF, White W, Lacroix L, Feldon J. White IM NMDA lesions in the medial prefrontal cortex impair the ability to inhibit responses during reversal of a simple spatial discrimination. Behavioural Brain Research. 2004;152:413–24. doi: 10.1016/j.bbr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. Journal of Neuroscience. 2007;27:4747–55. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin AL, Berry SD. Inactivation of the anterior cingulate cortex impairs extinction of rabbit jaw movement conditioning and prevents extinction-related inhibition of hippocampal activity. Learning and Memory. 2005;11(5):604–10. doi: 10.1101/lm.78404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudy JW, Keith JR, Georgen K. The effect of age on children’s learning of problems that require a configural association solution. Developmental Neurobiology. 1993;26(3):171–84. doi: 10.1002/dev.420260304. [DOI] [PubMed] [Google Scholar]
- 9.Rudy JW. Sutherland RJ The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behavioural Brain Research. 1989;34(1–2):97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- 10.Murray TK, Ridley RM. The effect of excitotoxic hippocampal lesions on simple and conditional discrimination learning in the rat. Behavioural Brain Research. 1999;99:103–13. doi: 10.1016/s0166-4328(98)00077-1. [DOI] [PubMed] [Google Scholar]
- 11.Fellows BJ. Chance stimulus sequences for discrimination tasks. Psychological Bulletin. 1967;67:87–92. doi: 10.1037/h0024098. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos J, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier; 2004. [Google Scholar]
- 13.Griffin AL, Owens CB, Peters GJ, Adelman PC, Cline KM. Spatial representations in dorsal hippocampal neurons during a tactile-visual conditional discrimination task. Hippocampus. 2012;22(2):299–308. doi: 10.1002/hipo.20898. [DOI] [PubMed] [Google Scholar]
- 14.Aura J, Riekkinen P., Jr Blockade of NMDA receptors located at the dorsomedial prefrontal cortex impairs spatial working memory in rats. NeuroReport. 1999;10:243–8. doi: 10.1097/00001756-199902050-00008. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behavioural Brain Research. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- 16.Rios Valentim SJ, Jr, Gontijo AV, Peres MD, Rodrigues LC, Nakamura Palacios EM. D1 dopamine and NMDA receptors interactions in the medial prefrontal cortex: modulation of spatial working memory in rats. Behavioural Brain Research. 2009;2004:124–8. doi: 10.1016/j.bbr.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Taylor CL, Latimer MP, Winn P. Impaired delayed spatial win-shift behaviour on the eight arm radial maze following excitotoxic lesions of the medial prefrontal cortex in the rat. Behavioural Brain Research. 2003;147:107–14. doi: 10.1016/s0166-4328(03)00139-6. [DOI] [PubMed] [Google Scholar]
- 18.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neuroscience and Biobehavioral Reviews. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Modo M, Sowinski P, Hodges H. Conditional discrimination learning in rats with global ischaemic brain damage. Behavioural Brain Research. 2000;111(1–2):213–21. doi: 10.1016/s0166-4328(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 20.Ferino F, Thierry A, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon’s horn to the medial prefrontal cortex in the rat. Experimental Brain Research. 1987;65(2):421–6. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- 21.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. Journal of Comparative Neurology. 1991;313:574–86. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 22.Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMP Areceptors in hippocampo-prefrontal cortex transmission. European Journal of Neuroscience. 1992;4:1285–95. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 26.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;111:920–36. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Shao J. Restricted lesions to ventral prefrontal subareas block reversal learning but not visual discrimination learning in rats. Physiology and Behavior. 1998;65:371–9. doi: 10.1016/s0031-9384(98)00216-9. [DOI] [PubMed] [Google Scholar]
- 28.Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 32.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. Journal of Neuroscience. 2008;28(44):11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delatour B, Gisquet-Verrier P. Prelimbic cortex specificlesions disrupt ‘delayed-variable response tasks’ in the rat: possible interpretations. Behavioral Neuroscience. 1996;110(6):1282–98. doi: 10.1037//0735-7044.110.6.1282. [DOI] [PubMed] [Google Scholar]
- 34.Delatour B, Gisquet-Verrier P. Lesions of the prelimbic and infralimbic cortices in rats do no disrupt response selection but induce delay-dependent deficits: evidence for a role in working memory? Behavioral Neuroscience. 1999;113(5):941–55. doi: 10.1037//0735-7044.113.5.941. [DOI] [PubMed] [Google Scholar]
- 35.Delatour B, Gisquet-Verrier P. Involvement of the dorsal anterior cingulate cortex in temporal behavioral sequencing: subregional analysis of the medial prefrontal cortex in rat. Behavioural Brain Research. 2001;126:105–14. doi: 10.1016/s0166-4328(01)00251-0. [DOI] [PubMed] [Google Scholar]
- 36.Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Progress in Brain Research. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 37.Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171(1):30–8. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinoshita S, Yokoyama C, Masaki D, Yamashita T, Tsuchida H, Nakatomi Y, et al. Effects of rat medial prefrontal cortex lesions on olfactory serial reversal and delayed alternation tasks. Neuroscience Research. 2008;60(2):213–8. doi: 10.1016/j.neures.2007.10.012. [DOI] [PubMed] [Google Scholar]