Abstract
Elevated expression of neuroinflammatory factors in the central nervous system (CNS) contributes to the cognitive impairment in CNS disorders such as injury, disease and neurodegenerative disorders. However, information on the role of specific neuroimmune factors in normal and abnormal CNS function is limited. In this study, we investigated the effects of chronic exposure to the chemokine CCL2 on hippocampal synaptic function at the Schaffer collateral-CA1 synapse, a synapse that is known to play an important role in cognitive functions such as memory and learning. Synaptic function was measured in vitro using hippocampal slices obtained from transgenic mice that express elevated levels of CCL2 in the CNS through astrocyte expression and their non-transgenic littermate controls. Extracellular field potential electrophysiological recordings showed a significant reduction in the magnitude of synaptic responses in hippocampal slices from the CCL2 transgenic mice compared with slices from non-transgenic littermate controls. Two forms of short-term synaptic plasticity (post-tetanic potentiation and short-term potentiation) thought to be important cellular mechanisms of short-term memory were enhanced in hippocampal slices from CCL2 transgenic mice compared to non-transgenic hippocampal slices, whereas long-term synaptic plasticity (LTP), which is critical to long-term memory formation, was not altered. Western blot analysis of hippocampus from the CCL2 transgenic mice and non-transgenic mice showed no change in level of neuronal specific enolase, a neuronal specific protein, GFAP, an astrocyte specific protein, and several synaptic proteins compared with non-transgenic littermate controls. These results show that CCL2, which is known to be chronically produced at elevated levels within the CNS in a number of CNS disorders, can significantly alter hippocampal function and implicate a role for CCL2 in the cognitive dysfunction associated with these CNS disorders.
Keywords: chemokine, synaptic plasticity, short-term potentiation, long-term potentiation, post-tetanic potentiation, electrophysiology, hippocampal slice, Western blot, field potential
1. Introduction
It is now recognized that chemokines, a group of small signaling proteins that are members of the cytokine family of inflammatory factors, are produced within the CNS and can play an important role in normal CNS function and development as well as in CNS disease and injury (Cartier et al., 2005; Ubogu et al., 2006). The primary CNS cell types that produce chemokines are astrocytes and microglia, although neurons also produce chemokines under some conditions (Flugel et al., 2001; Rock et al., 2004). Chemokines are classified into four basic subfamilies, primarily based on the position of specific conserved cysteine residues in the N-terminal structure: α-(or CXC) chemokines, β-(or CC) chemokines (e.g. CCL2), γ-(or C) chemokines and δ-(or CX3C) (Murphy et al., 2000). Our studies focus on the β-chemokine CCL2 (CC chemokine ligand 2, previously known as monocyte chemoattractant protein-1 or MCP-1), a small secreted protein.
Chemokines were first described in the immune system where they play a role in host immune surveillance, directing leukocyte traffic to sites of inflammation or injury, a role that they also play in the CNS (Miller et al., 2008). Additional roles for chemokines as signaling molecules in the CNS are now emerging, although information is still limited. For example, recent studies show a physiological role for CXCL12 in CNS development. In these studies, selective deletion of either CXCL12 (SDF-1) or its receptor CXCR4, which have a monogamous interaction, disrupts the normal migration of cerebellar granule neurons and leads to abnormal formation of the cerebellum (Ma et al., 1998; Tran and Miller, 2003; Zhu et al., 2002). The CXCR4 deficient mice show numerous deficiencies (Ma et al., 1998). A similar role for CXCL12/CXCR4 was demonstrated for the migratory process occurring during morphogenesis of the dentate gyrus of the hippocampus (Lu et al., 2002). Evidence for a role of CXCL12 in memory function has also appeared. Thus, in studies of a mouse model of Alzheimer’s disease, CXCL12 levels were down-regulated, coincident with the expression of cognitive deficits (Parachikova and Cotman, 2007).
In contrast to CXCL12, relatively little is known about the actions of CCL2 in the CNS. CCL2 is expressed in the healthy CNS (Foresti et al., 2009; Little et al., 2006; Madrigal et al., 2010; Meng et al., 1999) but a physiological role for CCL2 in the CNS has yet to be established. However, recent studies show that elevated levels of CCL2 occur in the CNS parenchyma or cerebral spinal fluid (CSF) in CNS disease, injury, and neurological and behavioral disorders suggesting a role for CCL2 in these conditions. For example, elevated levels of CCL2 in the CNS were shown to occur in multiple sclerosis (Mahad and Ransohoff, 2003; Sorensen et al., 1999), CNS trauma (Little et al., 2002; Muessel et al., 2002; Rhodes et al., 2009; Stefini et al., 2008), stroke (Losy and Zaremba, 2001), epilepsy (Foresti et al., 2009; Wu et al., 2008), depression (Sutcigil et al., 2007), Alzheimer’s disease (Ishizuka et al., 1997; Sokolova et al., 2009), viral and bacterial infection (Dhillon et al., 2008; Klein et al., 2006; Ramesh et al., 2009; Tribouillard-Tanvier et al., 2009) and cancer (Kielian et al., 2002; Sato et al., 1995). Correlative studies indicate that CCL2 is an important factor in the cognitive dysfunction associated with several of these disorders. The increased levels of CCL2 in the CSF of HIV-infected individuals correlate with the level of viral load and severity of dementia (Kelder et al., 1998). Increased levels of CCL2 in the CSF also correlate with cognitive deficits in older Alzheimer’s patients (Galimberti et al., 2006). CSF levels of CCL2 were found to significantly increase with the age of patients with and without neuropsychiatric disease (Blasko et al., 2006), suggesting that CCL2 plays an important role in the detrimental effects of aging on the CNS. A correlation of CSF levels of CCL2 and neuropsychiatric syndromes has also been reported for conditions where the primary insult occurs outside of the CNS. For example, CSF levels of CCL2 were significantly higher in patients with systemic lupus erythematosus showing neuropsychiatric symptoms than those without neuropsychiatric symptoms (Iikuni et al., 2006; Okamoto et al., 2010).
Studies in experimental animals support a role for CCL2 in the impaired CNS function associated with CNS pathology. For example, neurological impairments such as abnormal gait and diminished righting reflex were reported for transgenic mice (7–15 months of age) that express elevated levels of CCL2 in the CNS under the control of the human GFAP promoter (Huang et al., 2005). Consistent with these results, injection of CCL2 into brain ventricles of adult rats produced altered motor activity (Banisadr et al., 2002). Studies of bigenic mice constructed by crossing an Aβ deposition mouse model (Tf2576) of Alzheimer’s disease with a CCL2 overexpressing mouse showed enhanced behavioral deficits, altered hippocampal synaptic transmission, and altered Aβ metabolism in the bigenic mice, suggesting that CCL2 accelerates the detrimental effects of Aβ on the brain (Kiyota et al., 2009a; Kiyota et al., 2009b).
Several studies have implicated a role for CCL2 in alcohol use disorders. Thus, in behavioral tests both CCL2 and CCR2 (the receptor for CCL2) null mice appear normal but show lower preference for alcohol and lower alcohol consumption than wildtype mice, suggesting a potential role for CCL2 in the motivational aspects of alcohol consumption (Blednov et al., 2005). The levels of mRNA and protein for CCL2 are increased in the CNS of normal mice subjected to either single or repetitive alcohol doses and the elevated levels persist for days after repetitive alcohol exposure, suggesting that mechanisms regulating levels of CCL2 in the CNS are particularly sensitive to alcohol (Qin et al., 2008). Intraperitoneal injections of alcohol increased the levels of CCL2 mRNA in the hippocampus but not the corpus striatum, suggesting regional sensitivity to this effect of alcohol (Breese et al., 2008). Increased levels of CCL2 have been observed in several regions of the brain of alcoholics, including the hippocampus (He and Crews, 2008). Research has identified that the hippocampus is one of several brain regions that play a central role in the cognitive deficits produced by alcohol abuse (Matsumoto et al., 2007; Ryabinin, 1998). Thus, actions of CCL2 in the hippocampus could play an important role in alcohol use disorders. In addition to a role in alcoholism, morphine exposure has been shown to up-regulate the expression of CCL2 in cultures of human brain neurons (Rock et al., 2006) but down-regulate the expression of CCL2 in human astrocytes (Mahajan et al., 2005), results that may forecast a potential role for CCL2 in opiate abuse disorders.
Although these and other studies provide strong evidence for a role of CCL2 in a variety of CNS disorders, relatively little is known about the effects of elevated levels of CCL2 on neuronal function in the CNS. To address this issue, we have investigated the effects of chronically elevated levels of CCL2 on synaptic function in the hippocampus, a CNS region that is essential for cognitive functions such as short- and long-term memory. For these studies we employed transgenic mice that express elevated levels of CCL2 in the CNS through astrocyte expression under the control of the human GFAP promoter (Huang et al., 2005). Results show that chronic in vivo exposure to CCL2 significantly alters synaptic transmission and plasticity in the hippocampus. These results are consistent with an important role for CCL2 in the impaired CNS function observed in CNS disorders associated with increased levels of CCL2 in the CNS.
2. Methods
2.1. Animals
The construction of the CCL2 transgenic mice (CCL2-tg) was carried out as described by Huang et. al. (Huang et al., 2005). Briefly, the murine CCL2 gene was placed under control of the huGFAP promoter and purified GFAP-CCL2 fusion gene fragment injected into fertilized eggs of the SWXJ (H-21g,s) mice using standard procedures. The transgenic animals and their progeny were identified by analysis of the tail DNA and used to develop huGFAP-CCL2-tg mice on a SJL background. Heterozygous mice of the CCL2-tg line (SJL background) were used for experiments; age matched littermates that did not express the transgene (non-tg) were used as controls.
The CCL2-tg mice have been extensively characterized in the unmanipulated state and in disease models (Bennett et al., 2003; Elhofy et al., 2005; Huang et al., 2002; Huang et al., 2005; Kiyota et al., 2009b). Characterization of the CCL2-tg mice in the unmanipulated state (Huang et al., 2005) showed that transgenic mice younger than 6 months of age appear normal. Transgenic mice from 7–15 show mild perivascular infiltrates and impaired blood brain barrier but no consistent evidence of cell, axon or synapse loss. At these older ages, microglial activation was evident morphologically, but levels of MHC-II, CD11b, CD11c, CD40 and CD45 were not upregulated indicating that CCL2 did not induce the microglia to express features of antigen presenting cells (APCs). Neurological impairment was observed in the older mice and consisted of weight loss, postural changes, difficulty in the righting reflex, limb weakness and hindlimb paralysis.
2.2. Genotyping
Genotyping was carried out as described previously (Huang et al., 2005) using standard protocols. Briefly, DNA was isolated using the Easy DNA kit (Invitrogen Life Technologies, Carlsbad, CA, USA) from tail samples obtained from individual animals at weaning. Expression of the human GFAP promoter-murine CCL2 transgene was identified by PCR of the genomic DNA with primers that identified a 150 bp DNA fragment from the transgene but not the endogenous murine CCL2 gene.
2.3. Protein assays
The right hippocampus was used for electrophysiological studies and the left hippocampus was snap frozen on dry ice and used for protein assays. All protein assays were done on samples from individual animals. The tissue was stored at −80°C until use. To prepare the protein samples, the hippocampus from each animal studied was thawed on ice and placed in cold lysis buffer containing 50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% NP-40, a Protease Inhibitor Cocktail Tablet (Boehringer Mannheim USA), and a cocktail of phosphatase inhibitors (4.5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1 mM sodium fluoride, 1 mM sodium orthovanadate). The hippocampus was sonicated to disrupt the cells and then incubated on ice for 30 minutes. After incubation, the homogenate was centrifuged at 15,000 rpm (30 min), the supernatant collected and protein concentration determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Typically 8 individual hippocampi were processed at one time.
CCL2 levels in the hippocampal samples were measured by ELISA using the Mouse CCL2 (MCP-1) ELISA Ready-SET-Go kit (eBioscience, San Diego, CA, USA). Statistical analyses of differences were performed using the unpaired t-test. Statistical significance was set at p < 0.05. Relative levels of cellular and synaptic proteins in the hippocampal samples were determined by Western blot. Proteins (20–50 µg of protein; in duplicate) were separated by SDS-PAGE using 4–12% Novex NuPage Bis-Tris gels (Invitrogen Life Technologies) and transferred overnight onto Immobilon-P membranes (Millipore, Billerica, MA, USA). Uniform transfer was confirmed by Ponceau S staining. After washing, the membranes were blocked with casein (Pierce Biotechnology, Rockford, IL, USA), transferred to phosphate buffered saline (PBS) containing 0.1% Tween-20, and incubated in primary antibody overnight at 4oC. After washing with 0.1% PBS/Tween-20, the membranes were incubated in secondary antibody coupled to horseradish peroxidase (HRP), washed and the immunoreactive bands visualized on photographic film (Kodak, VWR Scientific Products, San Diego, CA, USA) by chemiluminescence using the ECL system (Pierce Biotechnology Products, Rockford, IL USA). CCL2-tg and non-tg samples were run on the same gel. To determine if equal loading of protein was achieved, membranes were stripped with Pierce Restore Stripping buffer (Pierce Biotechnology Products), washed and reprobed for β-actin.
Protein signals were quantified from densitometry measurements of immunoreactive bands on photographic film using NIH Image software (available on the Internet at http://rsb.info.nih.gov/nih-image/). The density of each band was quantified and normalized against the corresponding density of β-actin in the same lane to adjust for possible loading errors. Normalized data from CCL2-tg animals were then normalized to values from non-tg animals run on the same gel. Data were combined according to treatment group. Summarized results are the combined normalized data and are reported as the mean ± SEM. Statistical analyses were performed using the one group t-test. Statistical significance was set at p < 0.05.
2.4. Antibodies
The following antibodies were used for Western blot analysis: a monoclonal antibody to β-actin (AC-15, 1:5000; Sigma, St. Louis, Missouri); a monoclonal antibody to glial fibrillary acidic protein (GFAP; MAB360, 1:10,000; Millipore, Billerica, MA, USA); a purified rabbit antibody raised against a synthetic protein made to an internal region of the mouse CD11b protein (NB110–89474, 1:500; Novus Biologicals, Littleton, CO, USA); a monoclonal antibody raised against neuron specific enolase (MAB314, 1:5000; Millipore); a purified rabbit polyclonal antibody raised against a synthetic peptide to the C-terminus of rat GAD 65/67 (AB1511, 1:1000; Millipore); a purified rabbit antibody raised against purified bovine synapsin 1 (51–5200, 1:5,000; Invitrogen Life Technologies); a purified rabbit polyclonal antibody raised against a synthetic peptide of the rat GluR1 subunit of the AMPA receptor conjugated to KLH with a cysteine added (07–660, 1:500; Millipore); a purified rabbit polyclonal antibody raised against a carboxy terminus peptide of the rat mGluR2/3 conjugated to BSA with gluteraldehyde (AB1553, 1:1000; Millipore); a purified goat polyclonal antibody raised against a peptide corresponding to an amino acid mapping the C-terminus of the human NMDAR1 subunit of the NMDA receptor (sc-1467, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
2 5. Preparation of hippocampal slices
All animal procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care. The CCL2-tg mice were studied at 7–12 months of age. Both females and males were used. The mice were weighed, anesthetized with halothane and decapitated. Brains were rapidly removed, immersed in ice-cold artificial cerebrospinal fluid (ACSF) and allowed to cool for 2–3 min. Slices of the dorsal hippocampus (400 µm) were cut using a McIlwain tissue chopper (Mickle Laboratory Engineering Co. Ltd., Surrey, UK) and placed in a gas-fluid interface chamber maintained at approximately 33°C and with an ACSF perfusion rate of 0.55 ml/min until use. The composition of the ACSF was (in mM): 130.0 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24.0 NaHCO3, 2.0 CaCl2, 1.3 MgSO4, and 10.0 glucose (all chemicals from Sigma). All solutions were gassed continuously with 95% O2/5% CO2 (pH 7.2–7.4). There was no significant difference in body or brain weight between the CCL2-tg and non-tg mice studied. Mean (±SEM) body weight was 23.7±0.8 g (n=16) for the CCL2-tg mice and 24.0±0.9 g (n=17) for the non-tg mice. Total brain weight was estimated from the unused half of the brain and was 0.46± 0.01 (n=11) g for CCL2-tg mice and 0.45 ± 0.01 g (n= 16) for non-tg mice.
2.6. Electrophysiological recordings
Hippocampal slices were transferred to a gas-fluid interface recording chamber and allowed to stabilize for 20–30 minutes. The slices were continuously superfused with ACSF at a rate of 2 ml/min (33°C). Synaptic responses were elicited by electrical stimulation (50 µs duration, 0.033 Hz)(S88 Square Pulse Stimulator and PSIU6 Stimulus Isolation Unit, Grass Technologies, West Warwick, RI, USA) of the Schaffer collateral-commissural afferent pathway (Schaffer collaterals) using a concentric bipolar stimulating electrode (FHC Inc., Bowdoin, ME, USA) placed at the border of the CA2 and CA1 regions of the hippocampus. Extracellular field potential recordings of synaptic responses were made using microelectrodes (1–3 MΩ) filled with ACSF placed within area CA1 in the stratum radiatum (dendritic region of pyramidal neurons) and stratum pyramidale (somatic region of pyramidal neurons) to record dendritic field excitatory postsynaptic potentials (fEPSPs) and somatic population spikes (PS), respectively. The signals were amplified with an Axoclamp-2A amplifier and acquired using the pCLAMP software program (both from Molecular Devices, Union City, CA, USA).
2.7. Experimental protocols for electrophysiological recordings
An input-output (I/O) protocol was performed to determine the synaptic response parameters for each slice. The Schaffer collaterals were stimulated (stimulus rate of 1 pulse per 30 sec) at a range of stimulus intensities (typically from 20 to 240 µA) starting at the threshold intensity required to elicit a presynaptic fiber volley (PSV) measured in the dendritic region of the CA1. The stimulus strength was increased in steps of 10–20 µA until the maximum population spike amplitude was reached.
Short-term synaptic plasticity at the dendritic synapses was assessed in each slice by measuring paired-pulse facilitation (PPF) using a standard paired-pulse stimulation protocol applied to the Schaffer collaterals. Paired-pulse intervals of 40–200 ms were used at a test stimulus intensity that elicited a fEPSP equal to 50% of the maximal fEPSP amplitude, as determined from I/O protocols. The functionality of the inhibitory influences in the somatic region was assessed using a paired-pulse stimulation protocol applied to the Schaffer collaterals at short intervals (10–20 ms). A test stimulus intensity was used that elicited a population spike equal to 50% of the maximal population spike amplitude, as determined from I/O protocols. For both protocols, three paired-pulse responses (1 minute between acquisitions) were averaged in each slice.
Three forms of synaptic plasticity induced by high frequency stimulation were assessed at the dendritic synapses and the somatic region in each slice. Long-term potentiation (LTP) was induced by theta-burst stimulation (TBS) consisting of 15 bursts (4 pulses) of high-frequency stimulation (100 Hz) applied to the Schaffer collaterals at the test stimulus intensity (50% half-maximal fEPSP) separated by a 200 ms inter-burst interval (theta frequency). Post-tetanic potentiation (PTP), another form of synaptic plasticity that occurs immediately following the induction stimulation and short-term synaptic plasticity (STP), which occurs after PTP and lasts for ~ 30 min, were also measured.
2.8. Analysis of electrophysiological data
The data were analyzed off-line with the AxoGraph software program (AxoGraph Scientific, Sydney, Australia). For each synaptic response, measurements were made of the maximum fEPSP slope, presynaptic volley amplitude, and population spike amplitude. The fEPSP slope was determined over a range that allowed reliable measurements without interference from the preceding presynaptic volley. The reflection of the somatic action potential that occurs on the falling phase of the fEPSP did not interfere with fEPSP slope measurements. Population spike amplitudes were measured between the peak of the first rising component and the peak of the subsequent downward deflection (i.e., the spike). Paired-pulse facilitation and inhibition were expressed as the ratio of the second response with respect to the first response in each slice. LTP data were expressed as a percentage of the mean value for five baseline (pre-TBS) test responses in each slice. Statistical analyses of I-O curves were done with either linear or nonlinear regression using ANCOVA or the F test, respectively, for comparison of fitted lines/curves between groups. Linear relationships were fitted to the raw data using the formula, Y=INTERCEPT+SLOPE x X. Sigmoidal curves were fitted to the raw data using the Boltzmann equation: Y=BOTTOM+(TOP-BOTTOM)/(1+exp((V50-X)/SLOPE)), in which BOTTOM is the minimum value of the response, TOP is the maximum value of the response, V50 is the potential at which conductance is halfway between the minimum and maximum response, and SLOPE is the steepness of the curve. Compiled data are expressed as the mean ± SEM. Statistical significance was set at P<0.05.
3. Results
3.1 Protein expression in the CCL2-tg hippocampus
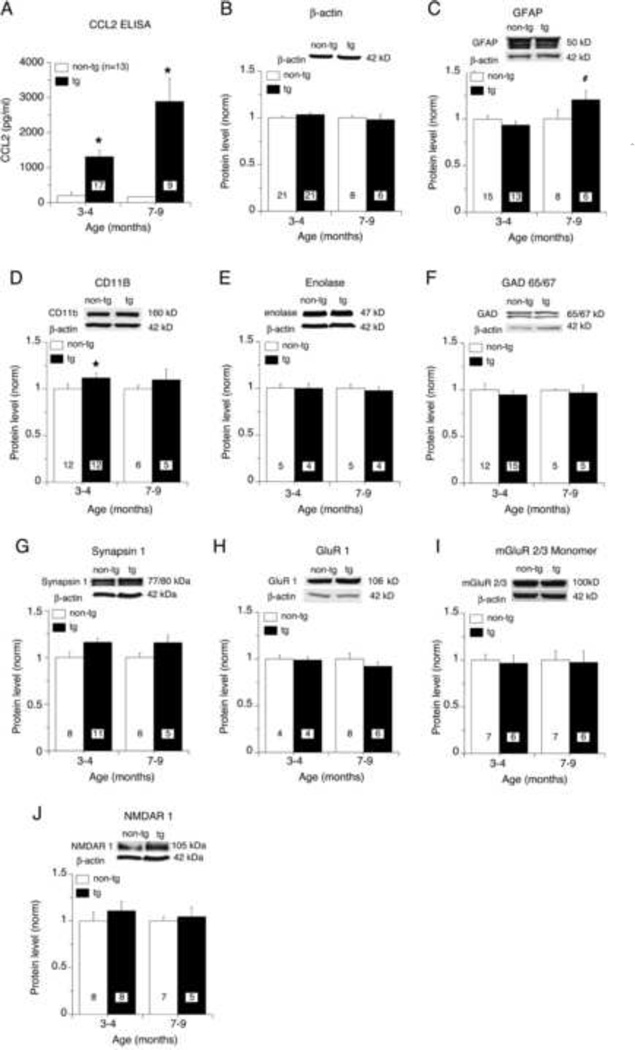
To determine the relative level of expression of CCL2 in the CCL2-tg and non-tg hippocampus, measurements of CCL2 were made in the hippocampus of individual animals by ELISA. Two age groups were examined, 3–4 months of age, when the CCL2-tg mice were noted to be free of neurological impairment in a previous study, and 7–9 months of age, when neurological changes were observed in a previous study (Huang et al., 2005). At both ages, CCL2 levels were significantly higher in the CCL2-tg hippocampus compared with the age matched non-tg littermate controls (Fig. 1A). CCL2 levels in the CCL2-tg were also significantly higher at 7–9 months of age compared to levels at 3–4 months of age (Fig. 1A).
Figure 1.
Protein expression in CCL2-tg and non-tg hippocampus. (A) Levels (mean±SEM) of CCL2 in CCL2-tg and non-tg hippocampus as determined by ELISA. (B–J) Levels of cellular and synaptic proteins determined by Western blot analysis at two age groups. Graphs show mean (±SEM) normalized values (see methods). Numbers of animals studied are shown in the boxes. Insets above graphs show representative Western blots. *= significant difference from the non-tg hippocampus of the same age (p>0.05); #= trend in the data (0.10>p>0.05).
To determine if the chronic exposure to CCL2 in vivo altered the expression of cellular or synaptic proteins in the hippocampus, the levels of selected proteins were determined by Western blot analysis. There were no significant differences between the CCL2-tg and non-tg hippocampus in the relative levels of several proteins at both 3–4 months of age and at 7–9 months of age. Thus, β-actin, a structural protein found in all cells, was expressed at similar levels in the CCL2-tg and non-tg hippocampus at both ages studied (Fig. 1B). GFAP, a structural protein found in astrocytes, was expressed at similar levels in the CCL2-tg and non-tg hippocampus at 3–4 months of age but there was a trend for increased expression in the CCL2-tg at 7–9 months of age (Fig. 1C). A small but significant increase in the level of CD11b, a marker for microglia, was observed in the CCL2-tg hippocampus at 3–4 months of age, but there was no difference between the CCL2-tg and non-tg hippocampus at 7–9 months age (Fig. 1D). The levels of neuron specific enolase, a marker for neurons, and GAD65/67, the synthetic enzyme for GABA and a marker for inhibitory interneurons, were also expressed at comparable levels in CCL2-tg and non-tg hippocampus (Fig. 1E,F). Levels of protein for synapsin I, a protein involved in synaptic transmission and a marker for presynaptic terminals, and several glutamate receptors (NMDAR1, GluR2, mGluR 2/3) were all comparable in CCL2-tg and non-tg hippocampus (Fig. 1G–J). Taken together, these results suggest that chronic in vivo exposure to CCL2 does not produce dramatic alterations in the cellular and molecular composition of the hippocampus, at least with respect to the proteins measured.
3.2. Altered basal synaptic function and excitability in CCL2-tg mice
Synaptic transmission at the Schaffer collateral to CA1 pyramidal neuron synapse was investigated in hippocampal slices from 7–9 month old CCL2-tg mice and their non-tg littermate controls to determine if exposure to chronically elevated levels of CCL2 altered hippocampal function. Measurements were made of input/output (I/O) relationships and short- and long-term plasticity. In the first series of experiments, I/O relationships were determined. Schaffer collaterals were electrically activated at a range of stimulus intensities and recordings were made of the resulting synaptic events: (a) the presynaptic volley (PSV), which reflects a summation of the action potentials occurring in the stimulated Schaffer collateral afferents, (b) the field excitatory postsynaptic potential (fEPSP), which reflects a summation of excitatory postsynaptic responses evoked by stimulation of the afferent fibers and occurring in the dendrites of CA1 pyramidal neurons, and (c) the population spike (PS), which represents a summation of the of action potentials evoked by the synaptic response and occurring in the somata of the pyramidal neurons. The PSV and fEPSP were recorded in the dendritic region and the PS was simultaneously recorded in the somatic region. To quantify these responses, measurements were made of the slope of the fEPSP and the amplitude of the PSV and PS. From the I/O data, the mean values for the PSV, fEPSP and PS for the population of hippocampal slices studied were plotted as a function of stimulus intensity and fitted curves for each parameter were compared (CCL2-tg vs. non-tg) using nonlinear regression (Boltzmann equation; see Methods).
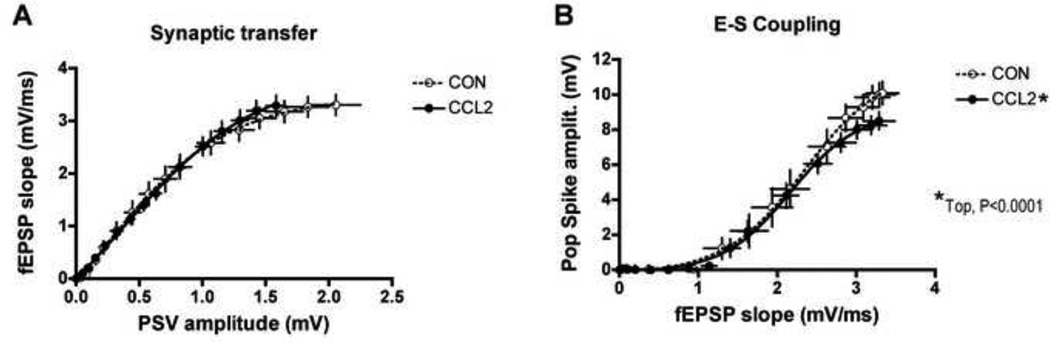
Comparison of the I-O curves for CCL2-tg and non-tg hippocampus showed that the magnitudes of the PSV, fEPSP and PS (Fig. 2A,B,C) were significantly smaller in CCL2-tg hippocampal slices compared with the non-tg (i.e., control) hippocampal slices. This reduction was characterized by a rightward shift in the I-O curves showing the relationship between PSV amplitude, fEPSP slope or PS amplitude and stimulus intensity (Fig. 2 A2,B2,C2). To determine if the smaller fEPSP in the CCL2-tg hippocampus was due to a reduced efficacy of synaptic transmission, the relationship between the fEPSP slope and PSV amplitude was determined for each slice (Dingledine, 1981). There was no significant difference in the mean values for efficacy of synaptic transmission between the CCL2-tg and non-tg hippocampus (Fig. 3A). These results suggest that the smaller fEPSP in the CCL2-tg hippocampus was due, at least in part, to the smaller PSV. To determine if the smaller PS in the CCL2-tg hippocampus was due to reduced E-S coupling (fEPSP-spike coupling), the relationship between the amplitude of the PS as function of fEPSP slope was determined for all slices studied (Dingledine, 1981). Hippocampal slices from CCL2-tg mice exhibited a significant decrease in the E-S coupling relationship relative to non-tg hippocampus slices at strong stimulus strengths, indicating a reduction in maximal somatic excitability in the CCL2-tg hippocampus compared to non-tg hippocampus (Fig. 3B).
Figure 2.
Baseline input-output (I/O) curves recorded in the CA1 of hippocampal slices. The presynaptic volley (PSV; A), fEPSP slope (B), and population spike (C) were significantly reduced in hippocampal slices from CCL2-tg mice (CCL2) compared with hippocampal slices from non-tg littermate control mice (CON) A1,B1,C1. Representative traces are shown of PSVs (A1), dendritic fEPSPs (B1) and somatic population spikes (C1) recorded in slices from control and CCL2 hippocampus in response to varying stimulus intensities (80, 160, 240 µA in A1, C1; 80, 140, 240 µA in B1; traces a–c, respectively). PSV and population spike peaks are indicated by upward arrowheads (trace c) in A1 and C1. Dashed lines indicate fEPSP slope (trace b) in B1. In all panels, the downward arrowheads indicate the time point of the stimulus artifact resulting from electrical stimulation of the Schaffer collaterals. A2. The slope of the linear relationship between stimulus intensity and PSV amplitude was significantly decreased in CCL2 hippocampal slices (n=27) compared with control hippocampal slices (n=19) (*lines of best fit compared using linear regression). B2. fEPSP magnitude, as determined by fEPSP slope, was significantly decreased in CCL2 hippocampal slices (n=26) compared with control hippocampal slices (n=16) resulting in a rightward shift in the I/O relationship between slope and stimulus intensity (*V50=stimulus intensity producing half-maximal slope, curves fitted with Boltzmann equation). Arrows indicate V50 for each I/O curve. C2. Population spike amplitude was significantly reduced in CCL2 hippocampal slices (n=26) compared with control hippocampal slices (n=15) resulting in a rightward shift in the I/O relationship between amplitude and stimulus intensity (*V50, Boltzmann) and a reduction in the mean maximum amplitude (#Top, Boltzmann). Arrows indicate V50 and dashed lines indicate Top for each I/O curve.
Figure 3.
Comparison of dendritic and somatic excitability in CA1 of CCL2-tg hippocampus (CCL2) compared with non-tg control (CON) hippocampus. A. Synaptic transfer, a measure of dendritic excitability expressed as the relationship between presynaptic activation (PSV) and the dendritic response (fEPSP), was unchanged in CCL2 hippocampus (n=26) compared with control hippocampus (n=16). B. EPSP-spike (E–S) coupling, a measure of somatic excitability expressed as the relationship between dendritic activation (fEPSP) and somatic responsiveness (population spike), was significantly suppressed in CCL2 hippocampus (n=18) compared with control hippocampus (n=22) (*Top, Boltzmann curve fitting).
3.3. Paired-pulse facilitation and paired-pulse inhibition are altered in CCL2-tg hippocampus
To determine if alterations in presynaptic neurotransmitter release contributed to the reduced synaptic transmission identified in the I/O relationship of the fEPSP slope for the CCL2-tg hippocampus, a standard paired-pulse protocol was used to examine paired-pulse facilitation (PPF). PPF results from a stimulation-induced increase in transmitter release and is quantified by calculating the ratio of the slope of the fEPSP elicited by the second (test) stimulation with respect to the slope of the fEPSP elicited by the first (conditioning) stimulation. The magnitude of PPF is inversely related to probability of transmitter release for the first stimulation. This inverse relationship between PPF and transmitter release reflects the relative availability of synaptic vesicles in the ‘readily releasable pool’ during the initial conditioning stimulus pulse and the subsequent test pulse (Andreasen and Hablitz, 1994; Creager et al., 1980; Debanne et al., 1996; Dobrunz and Stevens, 1997; Dunwiddie and Haas, 1985; Otmakhov et al., 1993). The increased transmitter release produced by the test stimulus is thought to result from residual Ca2+ in the synaptic terminal derived from Ca2+ flux produced by the conditioning (i.e., first) stimulus (Zucker and Regehr, 2002).
To determine PPF, the Schaffer collaterals were stimulated at a fixed stimulus intensity (half maximal fEPSP amplitude) at interpulse intervals of 40, 100 and 200 ms. PPF was observed at all intervals in both the CCL2-tg and non-tg hippocampus. PPF was significantly larger in the CCL2-tg hippocampus compared with the non-tg hippocampus at a 40 ms interpulse interval but not at the longer interpulse intervals (Fig. 4A). The longer interpulse intervals (100 and 200 ms) may be beyond the period that residual Ca2+ is present at sufficient levels to induce enhanced PPF in the synaptic terminal of the CCL2-tg hippocampus. The larger paired-pulse ratio in the CCL2-tg hippocampus indicates that the probability of transmitter release for the first Schaffer Collateral stimulus is lower in the CCL2-tg hippocampus. This result is consistent with the smaller fEPSP in the CCL2-tg hippocampus measured in the I/O protocol.
Figure 4.
Altered short-term synaptic plasticity and inhibitory influences in CCL2-tg hippocampal slices. A1,B1. Representative traces illustrating paired-pulse facilitation (PPF; A1) and inhibition (PPI; B1) in CCL2-tg (CCL2) and non-tg control (CON) hippocampal slices. Traces #1 and #2 are superimposed responses to paired stimuli (arrowheads) separated by a 40 ms (A1) or 10 ms (B1) interpulse interval. A2. PPF was enhanced in CCL2 hippocampal slices (n=28) compared with control hippocampal slices (n=28). Summarized results for 40, 100, and 200 ms PP intervals are expressed as the ratio of the fEPSP slopes of the second response (#2) with respect to the first (#1). Results were significantly different at the 40 ms PP interval only. B. PPI, which represents activation of inhibitory influences (synaptic and intrinsic) in the somatic region, was decreased (i.e., PP ratio was increased) in CCL2 hippocampal slices (n=29) relative to control hippocampal slices (n=26). Summarized results for 10 and 20 ms PP intervals are expressed as the ratio of the population spikes of response #2 with respect to response #1. Inhibition was not strong in either control or CCL2 hippocampal slices, as evidenced by the mean PP ratios > 1.0. Results were significantly different at both 10 and 20 ms intervals.
We also investigated inhibitory influences at the somatic region to determine if differences were observed that could contribute to the reduced PS observed in the I/O relationship of the CCL2-tg hippocampus compared with the non-tg hippocampus. For these studies we determined the paired-pulse ratio for the PS. A paired pulse stimulation was applied to the Schaffer collaterals at a fixed stimulus intensity (half maximum for PS amplitude) and short interpulse intervals (10–20 ms) and the paired pulse ratio was calculated as the ratio of the amplitude of the second PS with respect to the amplitude of the first PS. The amplitude of the second PS is modulated by feedback circuitry involving GABAergic interneurons (i.e., basket cells) that provide inhibitory control of CA1 pyramidal neuron excitability and by the intrinsic firing properties of the CA1 neurons. In both the CCL2-tg and non-tg hippocampus, inhibitory influences were not strong at the stimulus intensity used and the paired-pulse ratio showed facilitation rather than inhibition (Fig 4B). The paired-pulse ratio for the PS was significantly larger in the CCL2-tg hippocampus compared with the non-tg hippocampus at both 10 and 20 ms interpulse intervals (Fig. 4B), indicating smaller inhibitory influences in the somatic region of the CCL2-tg hippocampus. Because reduced inhibitory influences produces a larger PS, the smaller PS observed in the I/O relationship of the CCL2-tg hippocampus cannot be explained by alterations in inhibitory synaptic mechanisms in the somatic regions. Thus, the reduced PS in the CCL2-tg hippocampus is likely to be mediated by altered intrinsic excitability of the neurons.
3.4. Synaptic plasticity elicited by theta burst
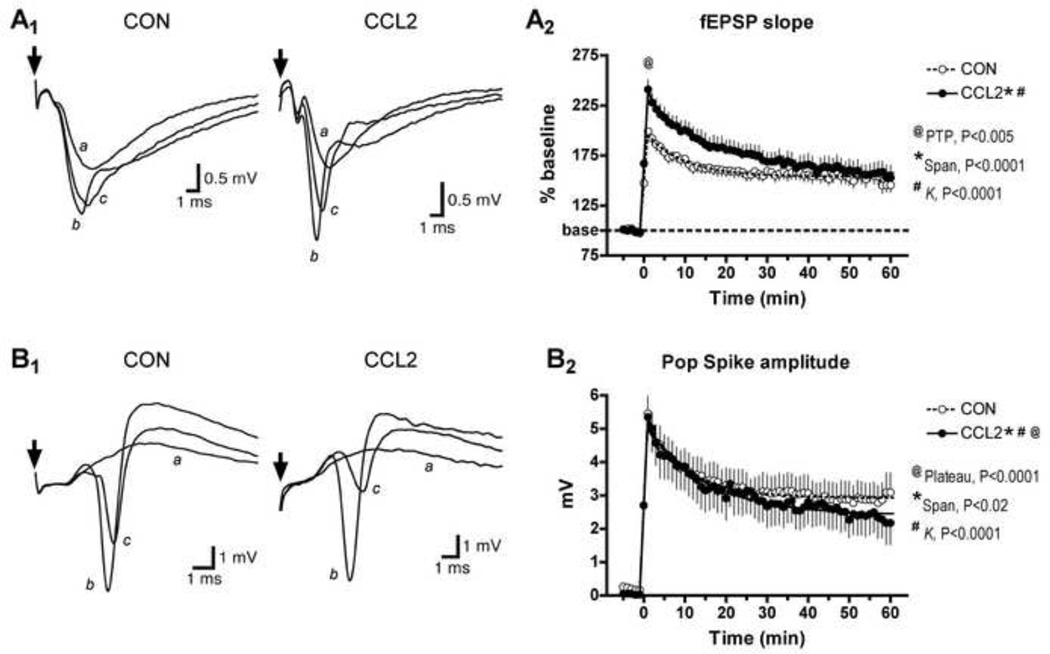
The above results show that chronic in vivo exposure to CCL2 results in decreased synaptic transmission and reduced excitability at Schaffer collateral-CA1 pyramidal neuron synapses when the synapse is subjected to low frequency stimulation. To determine if higher frequency activity-dependent synaptic function was altered by chronic in vivo exposure to CCL2, we compared the ability of a LTP induction stimulation paradigm to induce synaptic plasticity at this synapse in the CCL2-tg and non-tg hippocampal slices. A theta-burst stimulation (TBS) was used as the induction protocol (a single train of 15 bursts consisting of 4 pulses at 100 Hz, each burst separated by 200 ms). This induction protocol produced short-term plasticity (PTP and STP) and long-term plasticity (LTP) of the fEPSP in both the CCL2-tg and non-tg hippocampus. PTP was reflected by the initial peak in the magnitude of the fEPSP slope occurring after the termination of the induction protocol (TBS). The subsequent declining phase of the fEPSP amplitude following PTP, which lasts ~30 min, is referred to as STP. Both PTP and STP, short-term enhancements of synaptic strength, are thought play an important role in short-term memory (Erickson et al., 2009; Silva et al., 1996). The plateau phase reflecting a stabilized fEPSP slope occurring 50 to 60 min following the LTP induction protocol reflects LTP, a long-term change in synaptic strength that is considered to be an important cellular mechanism of long-term memory and learning (Silva, 2003). PTP is thought to result from a presynaptic enhancement of synaptic vesicle release that lasts for seconds to minutes after cessation of repetitive stimulation (Fisher et al., 1995; Zucker and Regehr, 2002). The mechanisms mediating STP have yet to be fully resolved, but both presynaptic and postsynaptic mechanisms appear to be involved (Erickson et al., 2009; Lauri et al., 2007; Schulz and Fitzgibbons, 1997). It is generally accepted that LTP is mediated primarily by postsynaptic mechanisms involving alterations in expression and/or function of glutamate receptors of the AMPA subtype (Miyamoto, 2006).
PTP of the fEPSP was significantly larger in the CCL2-tg hippocampus compared with non-tg hippocampus (Fig. 5A), suggesting that repetitive high frequency stimulation results in greater transmitter release in the CCL2-tg hippocampus compared with non-tg hippocampus. STP of the fEPSP was also significantly larger in the CCL2-tg hippocampus compared with non-tg hippocampus (Fig. 5A). These results show that short-term plasticity is significantly altered by chronic exposure to CCL2. The magnitude of LTP of the fEPSP was similar in CCL2-tg and non-tg hippocampus, indicating that exposure to elevated levels of CCL2 did not alter LTP (Fig. 5B). A comparison of I/O curves for the PSV (Fig 6A), fEPSP (Fig 6B) and PS (Fig 6C) generated before and 60 min after TBS confirmed that there was no difference in the degree of LTP between CCL2-tg and non-tg hippocampus.
Figure 5.
Post-tetanic potentiation (PTP)/short-term potentiation (STP), but not long-term potentiation (LTP), was altered in CCL2-tg (CCL2) hippocampal slices. A. PTP/STP, expressed as percent of baseline fEPSP slope, was significantly enhanced (@by unpaired t-test) in CCL2 hippocampal slices (n=19) compared to non-tg control (CON) hippocampal slices (n=20) until 14 min following theta-burst stimulation (TBS). Using nonlinear regression analysis (single exponential decay model), the peak PTP (as indicated by the Span of the fitted curve), and the decay phase of STP (indicated by the rate constant, K, of the fitted curve) were significantly altered in CCL2 hippocampal slices. However, the responses decayed to the same level of LTP by 60 min following TBS (Plateau, not significant). B. Population spike amplitude was not significantly different between CCL2 (n=20) and control (n=22) hippocampal slices at any time point following TBS (unpaired t-test). However, nonlinear regression analysis of the fitted curves (single exponential decay) indicated that PTP peak, STP decay, and LTP plateau phases were significantly different between CCL2 and control hippocampal slices.
Figure 6.
Comparison of I-O curves before and 60 min after LTP induction in CCL2-tg (CCL2) and non-tg (control) hippocampal slices. A. PSV amplitude was unchanged by TBS-induced LTP induction in both CCL2 (n=17) and control (n=18) hippocampal slices. Graph to the right shows mean values (±SEM) for the slope of the linear relationship between stimulus intensity and PSV amplitude. Consistent with Fig. 2 results, the slope was significantly reduced in CCL2 hippocampal slices both before and after LTP induction compared with control hippocampus (*2-factor ANOVA). B. TBS resulted in a leftward shift of the fEPSP slope I-O curve (Boltzmann equation, normalized to baseline maximum) that was similar in CCL2 (n=15) and control (n=14) hippocampal slices, resulting in a significant decrease in V50 between baseline and LTP time points (#2-factor ANOVA). Graph to the right shows mean values (±SEM) for V50. Consistent with Fig. 2 results, V50 was increased in CCL2 hippocampal slices compared with control hippocampal slices (*2-factor ANOVA). However, the increase in fEPSP V50 in CCL2 hippocampal slices was similar at baseline and LTP. C. TBS resulted in a leftward shift of the population spike amplitude I-O curve (Boltzmann equation, normalized to baseline maximum) that was similar in CCL2 (n=15) and control (n=14) hippocampal slices, resulting in a significant decrease in V50 between baseline and LTP time points (Inset#2-factor ANOVA). Graph to the right shows mean values (±SEM) for V50. Consistent with Fig. 2 results, V50 was increased in CCL2 slices compared to control slices (*2-factor ANOVA). However, the increase in population spike V50 in CCL2 slices was similar at baseline and LTP.
The amplitude of the PS also showed PTP, STP and LTP phases following TBS in both the CCL2-tg and non-tg hippocampus, as expected from the increased size of the fEPSP that evokes the PS. However, there was no significant difference in the PS amplitude between CCL2-tg and non-tg hippocampal at any time point following TBS despite the larger fEPSP in the CCL2-tg hippocampus (Fig. 5B). Moreover, nonlinear regression analysis of the fitted curves (single exponential decay) for the time-dependent decline of the PS amplitude following TBS showed a significantly faster decay time course for the PS in the CCL2-tg hippocampus (Fig 5B). These results are consistent with altered excitability in the somatic region of the CCL2-tg hippocampus.
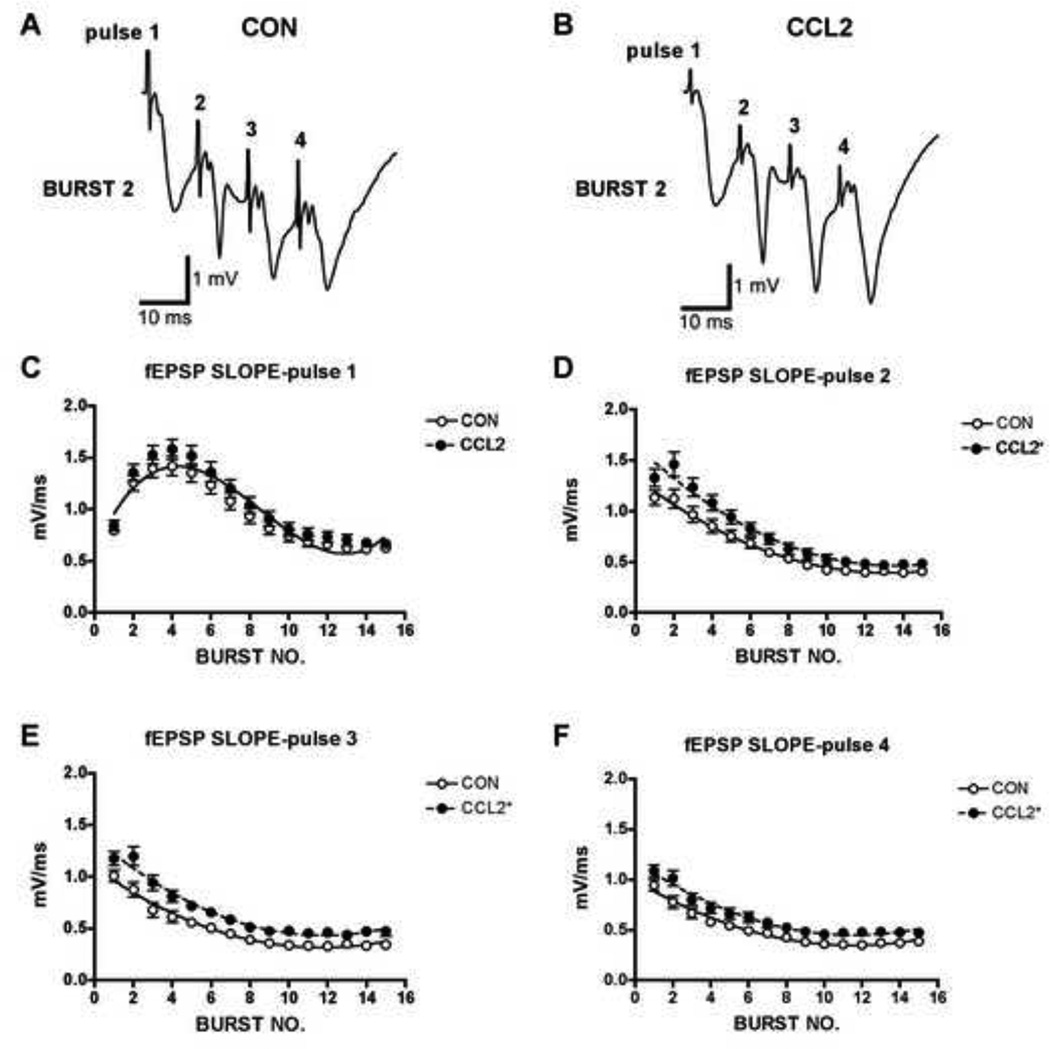
To identify potential differences in the effects of the LTP induction protocol on the magnitude of the fEPSP slope and PS amplitude, these responses were measured during TBS. For all pulses of the TBS protocol (4 pulses of 15 bursts), the fEPSP slope showed short-term plasticity that consisted of an initial facilitation followed by depression (Fig. 7). The extent of facilitation declined from the 1st to the 4th pulse, leading to primarily depression during the 4th pulse. This short-term depression of synaptic transmission (STD) is know to occur at the Schaffer collateral to CA1 synapse (Dobrunz and Stevens, 1997) when afferent fibers are repetitively activated and is thought to result from a decrease in transmitter release due to depletion of the readily releasable pool of presynaptic vesicles (Dittman et al., 2000; Zucker and Regehr, 2002). During the first pulse there was no significant difference in fEPSP slope between the CCL2-tg and non-tg hippocampus at any time point. In contrast, during pulses 2–4 the amplitude of the fEPSP slope was consistently larger in the CCL2-tg hippocampus compared with the non-tg hippocampus, consistent with enhanced transmitter release and a lower level of transmitter depletion (Fig. 7). The PS amplitude was also examined during the LTP induction protocol. As for the fEPSP, the PS amplitude showed an initial facilitation followed by depression that changed in magnitude according to pulse number. However, there was no significant difference in the amplitude of the PS between CCL2-tg and non-tg hippocampus at any time point during the four pulses in spite of the larger fEPSP (Fig. 7). Taken together, these results indicate that the fundamental mechanisms underlying PTP and STP of the fEPSP slope and PS are significantly altered by chronic in vivo CCL2 exposure, whereas mechanisms mediating LTP are largely unaffected by the chronic in vivo exposure to CCL2.
Figure 7.
Field excitatory postsynaptic potentials (fEPSPs) in hippocampal slices from CCL2 transgenic mice were facilitated compared to control hippocampal slices during theta burst stimulation (TBS) protocols. TBS consisted of 15 bursts (200 ms interburst interval, or 5 Hz) of 4 stimulus pulses (10 ms interpulse interval, or 100 Hz). A,B. Representative recordings of fEPSPs in CCL2 and control hippocampal slices during TBS. C–F. Graphs of mean±SEM values for fEPSP slopes in CCL2 (n=35) and control (n=43) hippocampal slices during TBS. fEPSP slope for each pulse of the four-pulse theta burst was measured independently and plotted across the 15 burst repetitions (i.e., burst no.). Pulse 1 data were best fitted with a third-order polynomial. Pulses 2–4 data were best fitted with a second-order polynomial. For each pulse, fitted curves in CCL2 slices were significantly different from control curves as determined by nonlinear regression analysis. This difference is indicated by a significant upward shift in the curves from CCL2 hippocampal slices relative to control hippocampal slices (CCL2 Y-intercept increased in D-E, P<0.0001, and in F, P<0.0005; F test).
4. Discussion
The studies described here show that chronic in vivo exposure to elevated levels of CCL2 in the CNS can result in alter synaptic function at the Schaffer collateral to CA1 pyramidal neuron synapse in the hippocampus. These effects include reduced synaptic transmission, reduced neuronal excitability and enhance short-term synaptic plasticity. The studies were carried out in hippocampus of transgenic mice that express elevated levels of CCL2 in the CNS through increased astrocyte production. Astrocytes are a primary source of CCL2 in the CNS during neuroinflammatory states (Babcock et al., 2003; Berman et al., 1996; Geppert, 2003; Glabinski et al., 1997; Madrigal et al., 2009). Therefore, the consequences of the increased levels of CCL2 in the transgenic hippocampus are likely to reflect those normally involved in pathological conditions associated with elevated CCL2 expression in the CNS. Measurement of CCL2 levels by ELISA showed that CCL2 was detectable in the hippocampus of the CCL2-tg and non-tg mice but that the concentration was significantly higher (10 fold or more) in the CCL2-tg mice.
Several aspects of synaptic transmission were altered by chronic in vivo exposure to CCL2 in the transgenic hippocampus including the PSV, fEPSP, and PS. The PSV is generated by the summation of action potentials occurring in the stimulated Schaffer collateral afferents. Therefore, the smaller PSV in the CCL2-tg hippocampus suggests that presynaptic alterations play a role in the reduced synaptic transmission observed in the CCL2-tg hippocampus. The smaller PSV could reflect a smaller number of activated afferent fibers or reduced excitability of the afferent fibers in the CCL2-tg hippocampus. Measurement of synaptic transfer, which characterizes the relationship between the magnitude of the PSV and the fEPSP, showed that there was no significant difference in the synaptic transfer between the CCL2-tg and non-tg hippocampus. This result indicates that the smaller PSV in the CCL2-tg hippocampus is an important contributing factor to the smaller fEPSP in the CCL2-tg hippocampus. In addition, an alteration in presynaptic mechanisms involved in transmitter releases in the CCL2-tg hippocampus was indicated by results from paired-pulse studies. These results showed that PPF of the fEPSP at the 40 ms interpulse interval was significantly larger in the CCL2-tg hippocampus compared with the non-tg hippocampus. It is generally accepted that a larger PPF reflects a lower probability of basal transmitter release, resulting in a smaller response to the first stimulus pulse of the paired-pulse protocol as compared to the response to the second stimulus pulse (Zucker and Regehr, 2002). A lower level of basal transmitter release is consistent with the smaller fEPSPs observed in the I/O relationship of the CCL2-tg hippocampus compared with the non-tg hippocampus. In a recent study, acute exposure to CCL2 enhanced excitatory synaptic responses in rat CA1 hippocampal neurons, an action that was found to involve presynaptic mechanisms (Zhou et al., 2010). Thus, both acute and chronic exposure of CA1 pyramidal neurons to CCL2 can result in altered synaptic responses, although in an opposite manner, and the actions of both acute and chronic exposure appear to involve presynaptic mechanisms that influence transmitter release.
Presynaptic effects of chronic in vivo exposure to CCL2 in the CCL2-tg hippocampus were also indicated by the enhanced PTP and STP of the fEPSP evoked by TBS and the reduced short-term depression of fEPSP during TBS in the CCL2-tg hippocampus compared with the non-tg hippocampus. These results are consistent with enhanced transmitter release during high frequency repetitive stimulation in the CCL2-tg hippocampus compared with the non-tg hippocampus. In contrast, LTP of the fEPSP, which is thought to be primarily mediated by postsynaptic mechanisms, was comparable in the CCL2-tg and non-tg hippocampus.
The PS, which represents a summation of the action potentials evoked by the fEPSP, was also reduced in the CCL2-tg hippocampus compared with the non-tg hippocampus. The smaller PS in the CCL2-tg hippocampus is due in part to a smaller fEPSP. However, analysis of E-S coupling, which shows the relationship between the magnitude of the fEPSP and the magnitude of the PS, identified that at strong stimulations the PS was smaller than could be accounted for by the smaller size of the fEPSP. Similar discrepancies between the magnitudes of the fEPSP and PS were observed in the CCL2-tg hippocampus during TBS and LTP. These results suggest that the elevated levels of CCL2 resulted in alterations in basic mechanisms of neuronal excitability involved in action potential generation. An involvement of neuronal excitability in the effects of chronic exposure to CCL2 is consistent with the smaller PSV observed in the CCL2-tg hippocampus.
A number of studies have shown that exposure to CCL2 can alter neuronal excitability. However, CCL2 exposure in these studies was short-term (i.e., acute application; min) compared with the chronic in vivo exposure used in our studies. For example, in a recent report by Zhou et al (Zhou et al., 2010), acute application of CCL2 depolarized the membrane potential and increased spike firing of CA1 hippocampal neurons recorded in a slice preparation. In our studies of cultured Purkinje neurons, acute application of CCL2 reduced current evoked spiking (van Gassen et al., 2005). In nigrostriatal dopamine neurons, acute application of CCL2 decreased membrane resistance through an action on K+ channels thereby resulting in an increased neuronal firing (Guyon et al., 2009). In injured dorsal root ganglion neurons, acute application of CCL2 depolarized the neurons and induced increased neuronal firing (Sun et al., 2006; White et al., 2005). Thus, mechanisms mediating neuronal excitability appear to be a target of CCL2 in a variety of preparations, although effects of CCL2 on neuronal excitability appear to vary among neuronal types.
Actions of CCL2 on ion channel function may play a role in the effects of CCL2 on neuronal excitability. For example, studies show that acute application of CCL2 inhibits N-type Ca2+ channels in transfected cells (tsA-201) via CCR2, the receptor for CCL2, and a voltage-dependent pathway, and inhibits the Ca(v)3.2 subtype of Ca2+ channels through a direct action at the channel (You et al., 2010). The Ca(v)3.2 subtype of Ca2+ channels is abundantly expressed in the hippocampus (McKay et al., 2006). N-type Ca2+ channels are also expressed in hippocampal neurons and are involved in transmitter release (Wheeler et al., 1994). CCL2 has also been reported to activate transient receptor potential (TRP) channels in dorsal root ganglia neurons (Jung et al., 2008) and in cultures of rodent midbrain neurons (Yao et al., 2009). TRP channels are Ca2+ permeable non-selective cation channels that play a role in a variety of physiological processes including neuronal excitability (Song and Yuan).
CCL2 induced changes in Ca2+ channel function could play a role in the effects of CCL2 on transmitter release, which is triggered by an increase in intracellular Ca2+. Other mechanisms involved in Ca2+ signaling or Ca2+ homeostasis could also be a target of CCL2 and contribute to changes in intracellular Ca2+ and consequently transmitter release. For example, in our previous studies of cultured Purkinje neurons acute application of CCL2 elicited in a small, prolonged increase in resting Ca2+ levels and enhanced the Ca2+ signal produced by activation of metabotropic glutamate receptor 1 (mGluR1). In contrast, more prolonged application (24 hr) of CCL2 to cultured cortical neurons reduced Ca2+ influx induced by glutamate activation of NMDA receptors (Madrigal et al., 2009).
Taken together, our results show that elevated levels of CCL2 in the CNS can produced alterations in several aspects of synaptic function in the CCL2-tg hippocampus. Although a number of studies have shown that CCL2 can directly alter neuronal function (see above), it is unknown if the actions of chronic CCL2 exposure in our studies were due to direct effects of CCL2 on hippocampal neurons. Indirect effects involving other cell types, other neuroimmune factors induced by CCL2, or other CNS changes induced by CCL2 could be involved. For example, it is known that CCL2-tg expression in this genetic model leads over time to modest perivascular monocyte infiltrates with consequent blood-brain-barrier dysfunction and abnormal microglial function (Huang et al., 2005). These changes could be important factors in our observed results. Further studies will be necessary to address this issue. “
The actions of elevated levels of CCL2 on synaptic function in the CCL2-tg hippocampus raise the possibility that similar actions could occur in human conditions in which elevated levels of CCL2 are produced in the CNS. For example, the elevated levels of CCL2 could contribute to the impaired CNS function characteristic of neurodegenerative diseases such as Alzheimer’s disease (Conductier et al., 2010) or chronic drug abuse such as alcohol use disorders (He and Crews, 2008). The Schaffer collateral-CA1 synapse is known to play a central role in cognitive functions such as short-term and long-term learning and memory. Our studies show that LTP of the fEPSP, believed to be a cellular model of mechanism mediating long-term memory, was not altered in the CCL2-tg hippocampus, whereas PTP and STP of the fEPSP, cellular mechanisms involved in short-term memory and learning, were enhanced in the CCL2-tg hippocampus. Moreover, PTP and STP of the PS in the CCL2-tg hippocampus were smaller than expected based on the magnitude of the fEPSP and a decline in LTP of the PS was observed. The PS plays a critical role in the output of the CA1 neurons to other brain regions. Such CCL2-induced alterations in synaptic function if they occur in human diseases could impair short-term memory processes and contribute to cognitive dysfunction. Behavioral studies are in progress to determine if these alterations in hippocampal synaptic function in the CCL2-tg mice are translated into changes in memory processes at the behavioral level.
Figure 8.
Population spike (PS) in CCL2 and control hippocampal slices were of similar magnitude during theta burst stimulation (TBS) protocols. TBS consisted of 15 bursts (200 ms interburst interval, or 5 Hz) of 4 stimulus pulses (10 ms interpulse interval, or 100 Hz). A,B. Representative recordings of PS in CCL2 and control hippocampal slices during TBS. C–F. Graphs of mean±SEM values for PS amplitude in CCL2 (n=35) and control (n=43) hippocampal slices during TBS. PS amplitude in each pulse of the four-pulse theta burst was measured independently and plotted across the 15 burst repetitions (i.e., burst no.). Pulse 1 data were best fitted with a third-order polynomial. Pulses 2–4 were best fitted with a second-order polynomial (the first pulse was not used in the analysis). For each pulse, fitted curves in CCL2 slices were not significantly different from control curves as determined by nonlinear regression analysis.
Acknowledgements
Supported by NIAAA Grant AA019261. We thank Floriska Chizer for administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen M, Hablitz JJ. Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J. Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Elhofy A, Canto MC, Tani M, Ransohoff RM, Karpus WJ. CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45(high)CD11b(+) monocytes and enhanced Theiler's murine encephalomyelitis virus-induced demyelinating disease. Journal of Neurovirology. 2003;9:623–636. doi: 10.1080/13550280390247551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. Journal of Immunology. 1996;156:3017–3023. [PubMed] [Google Scholar]
- Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, Marksteiner J, Humpel C. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dement Geriatr Cogn Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2008;33:867–876. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Creager R, Dunwiddie T, Lynch G. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J Physiol. 1980;299:409–424. doi: 10.1113/jphysiol.1980.sp013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Front Biosci. 2008;13:3913–3918. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. Possible mechanisms of enkephalin action on hippocampal CA1 pyramidal neurons. J Neurosci. 1981;1:1022–1035. doi: 10.1523/JNEUROSCI.01-09-01022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhofy A, Wang J, Tani M, Fife BT, Kennedy KJ, Bennett J, Huang D, Ransohoff RM, Karpus WJ. Transgenic expression of CCL2 in the central nervous system prevents experimental autoimmune encephalomyelitis. Journal of Leukocyte Biology. 2005;77:229–237. doi: 10.1189/jlb.0804465. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Maramara LA, Lisman J. A single brief burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J Cogn Neurosci. 2009;22:2530–2540. doi: 10.1162/jocn.2009.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SN, Vanguri P, Shin HS, Shin ML. Regulatory mechanisms of MuRantes and CRG-2 chemokine gene induction in central nervous system glial cells by virus. Brain, Behavior, and Immunity. 1995;9:331–344. doi: 10.1006/brbi.1995.1031. [DOI] [PubMed] [Google Scholar]
- Flugel A, Hager G, Horvat A, Spitzer C, Singer GM, Graeber MB, Kreutzberg GW, Schwaiger FW. Neuronal MCP-1 expression in response to remote nerve injury. J Cereb Blood Flow Metab. 2001;21:69–76. doi: 10.1097/00004647-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Foresti ML, Arisi GM, Katki K, Montanez A, Sanchez RM, Shapiro LA. Chemokine CCL2 and its receptor CCR2 are increased in the hippocampus following pilocarpine-induced status epilepticus. J Neuroinflammation. 2009;6:40. doi: 10.1186/1742-2094-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- Geppert AM. Constitutive patterns of RANTES, MCP-1 and MIP-1 alpha expression at the mRNA and protein level during postnatal development of the rat brain. Folia Neuropathol. 2003;41:79–88. [PubMed] [Google Scholar]
- Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. American Journal of Pathology. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovere C, Conductier G, Trocello JM, Dauge V, Kitabgi P, Rostene W, Nahon JL, Melik Parsadaniantz S. Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience. 2009;162:1072–1080. doi: 10.1016/j.neuroscience.2009.05.048. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Tani M, Wang J, Han Y, He TT, Weaver J, Charo IF, Tuohy VK, Rollins BJ, Ransohoff RM. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci. 2002;22:10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wujek J, Kidd G, He TT, Cardona A, Sasse ME, Stein EJ, Kish J, Tani M, Charo IF, Proudfoot AE, Rollins BJ, Handel T, Ransohoff RM. Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. Faseb J. 2005;19:761–772. doi: 10.1096/fj.04-3104com. [DOI] [PubMed] [Google Scholar]
- Iikuni N, Okamoto H, Yoshio T, Sato E, Kamitsuji S, Iwamoto T, Momohara S, Taniguchi A, Yamanaka H, Minota S, Kamatani N. Raised monocyte chemotactic protein-1 (MCP-1)/CCL2 in cerebrospinal fluid of patients with neuropsychiatric lupus. Ann Rheum Dis. 2006;65:253–256. doi: 10.1136/ard.2005.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci. 1997;51:135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56:1–12. doi: 10.1023/a:1014495613455. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Schroder B, Jacobsen MT, Swan RJ, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. AAV1/2-mediated CNS gene delivery of dominant-negative CCL2 mutant suppresses gliosis, beta-amyloidosis, and learning impairment of APP/PS1 mice. Mol Ther. 2009a;17:803–809. doi: 10.1038/mt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Xiong H, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009b;4:e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Paul R, Angele B, Popp B, Pfister HW, Koedel U. Protein expression pattern in experimental pneumococcal meningitis. Microbes Infect. 2006;8:974–983. doi: 10.1016/j.micinf.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Palmer M, Segerstrale M, Vesikansa A, Taira T, Collingridge GL. Presynaptic mechanisms involved in the expression of STP and LTP at CA1 synapses in the hippocampus. Neuropharmacology. 2007;52:1–11. doi: 10.1016/j.neuropharm.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Little AR, Benkovic SA, Miller DB, O'Callaghan JP. Chemically induced neuronal damage and gliosis: enhanced expression of the proinflammatory chemokine, monocyte chemoattractant protein (MCP)-1, without a corresponding increase in proinflammatory cytokines(1) Neuroscience. 2002;115:307–320. doi: 10.1016/s0306-4522(02)00359-7. [DOI] [PubMed] [Google Scholar]
- Little AR, Sriram K, O'Callaghan JP. Corticosterone regulates expression of CCL2 in the intact and chemically injured hippocampus. Neurosci Lett. 2006;399:162–166. doi: 10.1016/j.neulet.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Losy J, Zaremba J. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke. 2001;32:2695–2696. doi: 10.1161/hs1101.097380. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Garcia-Bueno B, Hinojosa AE, Polak P, Feinstein DL, Leza JC. Regulation of MCP-1 production in brain by stress and noradrenaline-modulating drugs. J Neurochem. 2009;113:543–551. doi: 10.1111/j.1471-4159.2010.06623.x. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Garcia-Bueno B, Hinojosa AE, Polak P, Feinstein DL, Leza JC. Regulation of MCP-1 production in brain by stress and noradrenaline-modulating drugs. J Neurochem. 2010;113:543–551. doi: 10.1111/j.1471-4159.2010.06623.x. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005;115:323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Alexander-Kaufman K, Iwazaki T, Kashem MA, Matsuda-Matsumoto H. CNS proteomes in alcohol and drug abuse and dependence. Expert Rev Proteomics. 2007;4:539–552. doi: 10.1586/14789450.4.4.539. [DOI] [PubMed] [Google Scholar]
- McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24:2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Oka A, Takashima S. Developmental expression of monocyte chemoattractant protein-1 in the human cerebellum and brainstem. Brain Dev. 1999;21:30–35. doi: 10.1016/s0387-7604(98)00065-5. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J. Chemokine action in the nervous system. J Neurosci. 2008;28:11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Muessel MJ, Klein RM, Wilson AM, Berman NE. Ablation of the chemokine monocyte chemoattractant protein-1 delays retrograde neuronal degeneration, attenuates microglial activation, and alters expression of cell death molecules. Brain Res Mol Brain Res. 2002;103:12–27. doi: 10.1016/s0169-328x(02)00158-4. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Okamoto H, Kobayashi A, Yamanaka H. Cytokines and chemokines in neuropsychiatric syndromes of systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:268436. doi: 10.1155/2010/268436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Shirke AM, Malinow R. Measuring the impact of probabilistic transmission on neuronal output. Neuron. 1993;10:1101–1111. doi: 10.1016/0896-6273(93)90058-y. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28:143–153. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Borda JT, Gill A, Ribka EP, Morici LA, Mottram P, Martin DS, Jacobs MB, Didier PJ, Philipp MT. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflammation. 2009;6:23. doi: 10.1186/1742-2094-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JK, Sharkey J, Andrews PJ. The temporal expression, cellular localization, and inhibition of the chemokines MIP-2 and MCP-1 after traumatic brain injury in the rat. J Neurotrauma. 2009;26:507–525. doi: 10.1089/neu.2008.0686. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Hu S, Sheng WS, Peterson PK. Morphine stimulates CCL2 production by human neurons. J Neuroinflammation. 2006;3:32. doi: 10.1186/1742-2094-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE. Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology (Berl) 1998;139:34–43. doi: 10.1007/s002130050687. [DOI] [PubMed] [Google Scholar]
- Sato K, Kuratsu J, Takeshima H, Yoshimura T, Ushio Y. Expression of monocyte chemoattractant protein-1 in meningioma. J Neurosurg. 1995;82:874–878. doi: 10.3171/jns.1995.82.5.0874. [DOI] [PubMed] [Google Scholar]
- Schulz PE, Fitzgibbons JC. Differing mechanisms of expression for short- and long-term potentiation. J Neurophysiol. 1997;78:321–334. doi: 10.1152/jn.1997.78.1.321. [DOI] [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Sudhof TC, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MY, Yuan JX. Introduction to TRP channels: structure function, and regulation. Adv Exp Med Biol. 661:99–108. doi: 10.1007/978-1-60761-500-2_6. [DOI] [PubMed] [Google Scholar]
- Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. Journal of Clinical Investigation. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefini R, Catenacci E, Piva S, Sozzani S, Valerio A, Bergomi R, Cenzato M, Mortini P, Latronico N. Chemokine detection in the cerebral tissue of patients with posttraumatic brain contusions. J Neurosurg. 2008;108:958–962. doi: 10.3171/JNS/2008/108/5/0958. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tribouillard-Tanvier D, Striebel JF, Peterson KE, Chesebro B. Analysis of protein levels of 24 cytokines in scrapie agent-infected brain and glial cell cultures from mice differing in prion protein expression levels. J Virol. 2009;83:11244–11253. doi: 10.1128/JVI.01413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. Eur J Neurosci. 2005;21:2949–2957. doi: 10.1111/j.1460-9568.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang X, Mo X, Xi Z, Xiao F, Li J, Zhu X, Luan G, Wang Y, Li Y, Zhang J. Expression of monocyte chemoattractant protein-1 in brain tissue of patients with intractable epilepsy. Clin Neuropathol. 2008;27:55–63. doi: 10.5414/npp27055. [DOI] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Altier C, Zamponi GW. CCR2 receptor ligands inhibit Cav3.2 T-type calcium channels. Mol Pharmacol. 2010;77:211–217. doi: 10.1124/mol.109.059022. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]