Abstract
A single-institution pilot clinical trial was performed combining non-myeloablative chemotherapy and the adoptive transfer of tumor-infiltrating lymphocytes with IL-2 in patients with metastatic melanoma. Nineteen patients were enrolled with 13 patients (68%) successfully completing treatment. An overall response rate (partial and complete responses) of 26% by intention to treat was achieved with a median follow-up time of 10 months. Of the 13 treated patients, there were 2 complete responses and 3 partial responses (38% response rate among treated patients), along with 4 patients with stable disease ranging from 2+ to 24+ months. Three of the four patients with stable disease have had disease control without additional therapy, including one at 24+ months. Adoptive therapy with TIL is labor-intensive but feasible and has a high response rate in treated patients.
Keywords: metastatic melanoma, adoptive cell therapy, tumor-infiltrating lymphocytes, lymphodepletion
Introduction
Adoptive cell therapy (ACT) with ex vivo expanded tumor-infiltrating lymphocytes (TIL) from resected tumors is a promising T cell-based immunotherapy for melanoma (1, 2). In 1988, it was first shown that TIL could be expanded in vitro from tumor-bearing mice and used to mediate regression of established poorly immunogenic tumors in murine models (3). ACT TIL treatment comprises the combination of lymphodepletion with chemotherapy, TIL transfer and the systemic administration of IL-2. Initial ACT TIL clinical trials conducted at the Surgery Branch, National Cancer Institute (NCI) resulted in a reported ~50% overall response rate; with >20% of treated patients achieving durable complete responses (4). In an effort to extend the ACT TIL approach to additional melanoma patients and to provide independent verification of its reported clinical outcome results, we conducted and report herein on both a pre-trial TIL growth analysis and an ACT TIL clinical trial employing laboratory procedures and clinical protocols developed and used by the Surgery Branch, NCI.
Methods
Patient Subjects
Appropriate institutional review board-approved informed consent was obtained for all patients. For the pre-trial validation analysis, patients older than 18 years of age with stage III or IV melanomas at least 2 cm in greatest dimension and scheduled for surgical resection were eligible. For the clinical trial, a separate cohort of patients older than 18 years of age with stage IV melanoma, who were unresectable for cure, had ECOG performance status of 0 or 1, were negative for hepatitis B and C and HIV infection, and were deemed to have an acceptable risk for high-dose IL-2 by the treating oncologist were eligible. All patients had measurable disease after tumor harvest in accordance with RECIST criteria version 1.1. Patients underwent surgical resection of at least one melanoma nodule of at least 2 cm in greatest dimension for subsequent TIL growth. Patients with adequate TIL growth received non-myeloablative lymphodepleting chemotherapy consisting of 2 days of inpatient cyclophosphamide (60 mg/kg) followed by 5 days of outpatient fludarabine (25 mg/m2). One day later, patients received cell infusion with TIL and high-dose IL-2 therapy consisting of 720,000 IU/kg intravenously every 8 hours up to 5 days or until tolerance as described previously (5, 6). Hematologic parameters were monitored daily by obtaining complete blood counts and by flow cytometric analysis of peripheral mononuclear cells. Patient response was assessed using standard radiographic studies and physical examination at 6 weeks after ACT and at regular intervals thereafter.
Preparation of Tumor Infiltrating Lymphocytes
Melanoma tumors were minced into 1–2 mm3 fragments and placed in culture with medium containing 6000 IU/mL IL-2. Fragments were monitored for growth every two to three days for up to five weeks. Wells were split when they became 90% confluent, keeping TIL derived from each fragment separately. The fastest-growing TIL from individual fragments were assessed for tumor reactivity by overnight co-culturing with autologous (when available) and HLA-matched and HLA-mismatched tumor cells at a 1:1 ratio. IFN-γ in culture supernatants was measured by ELISA. TIL were determined to be reactive if HLA-matched tumor co-culture yielded at least 200 pg/mL of IFN-γ and was at least 2 fold higher compared to medium alone or HLA-mismatched tumor co-culture.
Up to three of the highest IFN-γ-producing TIL numbering 3–6×107 were selected and pooled for rapid expansion. TIL were cultured in T175 flasks (1×106 cells per flask) at a 1:200 ratio with irradiated allogeneic PBMC feeder cells. IL-2 (Prometheus, Inc., San Diego, CA) at a concentration of 6000 IU/mL and OKT3 antibody (Ortho Biotech, Inc., Bridgewater, NJ) at a concentration of 30 ng/mL were added to the flasks. After seven days, flasks were pooled into 3-liter culture bags (American Fluoroseal, Gaithersburg, MD) so that a minimum TIL concentration of 3×105/mL was added to each bag. Bags were monitored for the next seven days and split as needed to maintain the TIL concentration at 2×106/mL. The cells were harvested, washed, and concentrated to less than 1.5 liters. The final product was tested for sterility and then intravenously infused into the patient at a rate of 300 mL per hour by gravity drip. For flow cytometry, cells from the final product were stained for CD3, CD4 and CD8 markers and analyzed on a FACSCalibur.
Results
Pre-Trial Experience
We performed a pre-trial analysis to establish the feasibility of TIL growth at our institution. Resected melanomas from 20 separate patients were utilized. TIL were successfully grown (as defined by the growth of 2×107 cells by 5 weeks of culture) from the melanomas of 18 of 20 patients (90%). Of the 425 total fragments cultured, positive TIL growth was detected by light microscopy in 130 fragments (30.6%). Fragments successfully yielding TIL reached the target number of 2×107 cells at mean of 30.2 days. TIL derived from 91 distinct fragments were separately co-cultured with autologous (when available), HLA-matched, and HLA-mismatched tumor cells. Specific IFN-γ production was detected in 31 fragments (40.7%).
Clinical Trial
To date, an additional cohort of 19 patients have been enrolled in our clinical trial to validate the feasibility and efficacy of ACT TIL therapy. Table I shows TIL growth. Resected melanoma from one patient produced no growth of TIL. Resected melanomas did not yield the initial target number for rapid expansion of 2×107 TIL from an individual fragment in 3 patients; in these cases, TIL were pooled from several fragments to reach >2×107 cells. Of the 815 fragments cultured, 34.4% generated the target number of 2×107 TIL. Of TIL generated from 158 distinct fragments tested for IFN-γ secretion against autologous or HLA-matched tumor cells, 75.7% were positive.
Table I.
TIL Growth
| Patient # |
Site of Resection |
# Fragments Cultured |
# Fragments Grown to 20e61 |
# Fragments Tested2 |
# Fragments IFN-γPos3 |
Comments4 |
|---|---|---|---|---|---|---|
| 1 | flank s.c. | 24 | 18 | 18 | 17 | not treated, progression |
| 2 | intramuscular latissimus | 24 | 6 | 6 | 6 | |
| 3 | back s.c. | 24 | discontinued | not treated, patient death |
||
| 4 | axillary LN, arm s.c. | 24 | 05 | TIL pooled | ||
| 5 | abdominal wall | 24 | 2 | 2 | 1 | |
| 6 | leg s.c. | 24 | 1 | 1 | 1 | not treated, progression |
| 7 | arm intramuscular | 48 | 05 | TIL pooled | ||
| 8 | gluteal subcut, back s.c. | 71 | 39 | 12 | 12 | |
| 9 | neck LN, hip s.c. | 48 | 30 | 14 | 9 | |
| 10 | gluteus s.c. | 72 | 50 | 16 | 15 | not treated, chemotherapy toxicity |
| 11 | abdominal s.c. x2 | 48 | 9 | 9 | 4 | not treated, progression |
| 12 | lung | 48 | 05 | TIL pooled | ||
| 13 | mesenteric and small bowel | 48 | 37 | 12 | 12 | |
| 14 | chest subcut x2 | 48 | 0 | no TIL growth | ||
| 15 | chest subcut, backsubcut x2 | 48 | 34 | 12 | 1 | |
| 16 | gluteal s.c. | 48 | 23 | 10 | 10 | |
| 17 | gluteal s.c. | 48 | 8 | 8 | 4 | |
| 18 | leg s.c., axillary LN | 48 | 17 | 14 | 14 | |
| 19 | epigastrium s.c., groin LN | 48 | 6 | 6 | 0 | Ocular primary |
| Total | 815 | 280 | 140 | 106 |
Abbreviations: s.c. subcutaneous; LN lymph node
Number of individual fragments that reached a final count of 20e6 cells within 5 weeks of culture
Number of individual fragments that reached a final count of 20e6 cells within 5 weeks of culture and were co-cultured with autologous or HLA-matched tumor cells for IFN-γ production
Number of individual fragments that produced IFN-γ in response to autologous or HLA-matched tumor cells
All patients had cutaneous primary tumors unless alternate primary site is designated.
No individual fragments reached a final count of 20e6 cells within 5 weeks of culture and were pooled for rapid expansion.
Of the 19 patients enrolled, 6 patients (32%) were unable to receive ACT TIL therapy. One patient had no TIL growth, one patient died from disease progression shortly after tumor harvest, and three patients’ disease progressed during TIL growth to the point that they were unable to be treated. One additional patient developed the syndrome of inappropriate anti-diuretic hormone (SIADH) immediately after cyclophosphamide administration and was unable to be treated with TIL.
Table II shows the demographics of patients included in our trial. Of the 13 patients receiving ACT TIL therapy, 4 patients demonstrated progressive disease (PD), 4 had stable disease (SD), 3 had a partial response (PR), and 2 had a complete response (CR). An overall response rate in treated patients (PR + CR) of 38% was achieved with a median follow-up time of 10 months; the objective response rate for all patients based on intention to treat was 26%. Notably, 3 of the 4 patients with stable disease have exhibited disease control without additional treatment, including one patient who has had stable disease ongoing for 24+ months. Figure 1 shows CT scan images of a patient who demonstrated a complete response. Figure 2 shows CT scan images of a patient who demonstrated a near-complete regression of all lesions and was classified as a partial responder. Table III shows the viability and composition of TIL from 13 treated patients. The median time from initial surgery to TIL infusion was 47 days. A median number of 5.2×1010 cells were infused.
Table II.
Patient Characteristics
| Patient # | Age | Gender | Performnce Status |
M stage | LDH level |
Previous Therapy1 | Response2 | Follow-Up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | 0 | M1c | 1027 | surgery, chemo, immuno, targeted inhib | NT | |
| 2 | 40 | F | 1 | M1c | 418 | surgery, immuno, XRT | PD | |
| 3 | 64 | F | 1 | M1c | 680 | surgery, immuno | NT | |
| 4 | 55 | M | 1 | M1c | 380 | surgery, chemo, immuno | PD | |
| 5 | 66 | M | 1 | M1c | 978 | surgery, chemo | SD | 24+ |
| 6 | 61 | M | 1 | M1c | 476 | surgery, immuno, XRT | NT | |
| 7 | 49 | M | 1 | M1c | 816 | surgery, chemo, radiotherapy, targeted inhib | PR | 19+ |
| 8 | 49 | M | 1 | M1c | 545 | surgery, chemo, XRT, immuno | CR | 16+ |
| 9 | 47 | F | 1 | M1c | 428 | surgery, immuno | CR | 14+ |
| 10 | 68 | F | 1 | M1c | 573 | surgery | NT | |
| 11 | 49 | M | 1 | M1c | 1345 | surgery, XRT, chemo, immuno | NT | |
| 12 | 41 | M | 1 | M1b | 456 | surgery, XRT | PD | |
| 13 | 33 | M | 1 | M1c | 896 | surgery, targeted inhib, XRT | SD | 10 |
| 14 | 55 | M | 0 | M1c | 631 | surgery, immuno | NT | |
| 15 | 49 | F | 1 | M1c | 530 | surgery, chemo, immuno, XRT | PR | 9+ |
| 16 | 26 | M | 0 | M1c | 371 | surgery, immuno | PD | |
| 17 | 67 | F | 1 | M1c | 549 | surgery, chemo | SD | 4+ |
| 18 | 54 | M | 1 | M1c | 513 | surgery, immuno | PR | 3+ |
| 19 | 25 | F | 0 | M1c | 1016 | surgery, chemo | SD | 2+ |
Therapy received prior to enrollment. Chemo= chemotherapy; immuno = immunotherapy; XRT = radiation therapy; targeted inhib = targeted inhibitor therapy
NT = not treated; PD = progressive disease; SD = stable disease; PR = partial response; CR = complete response
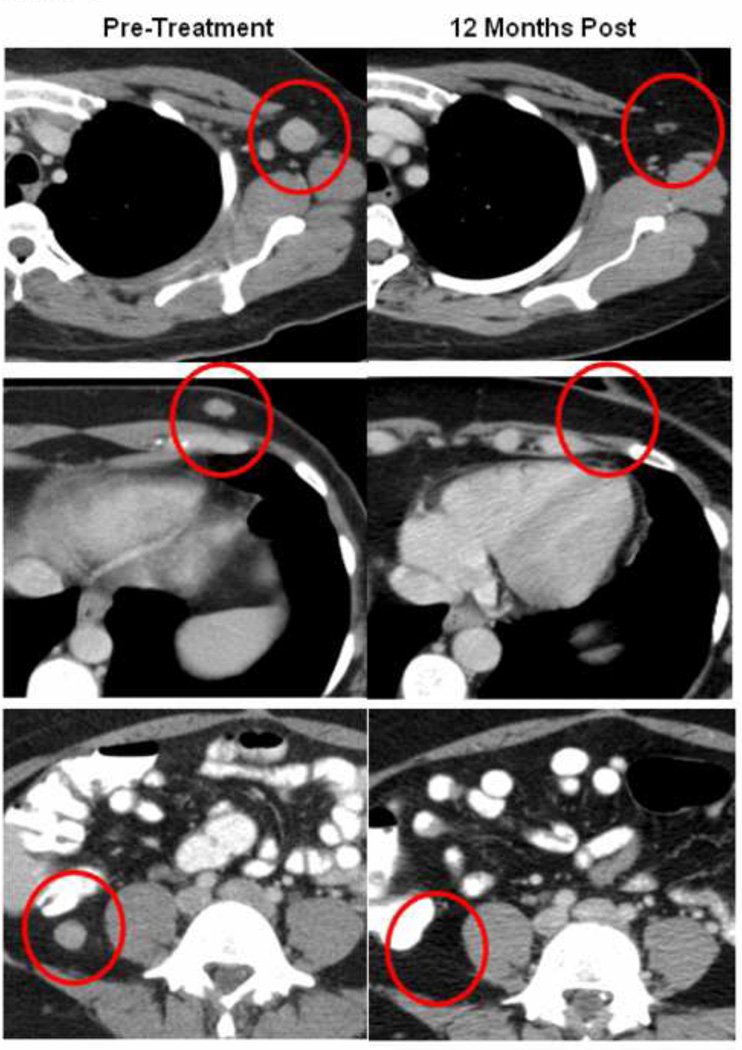
Figure 1.
Lymphodepletion and ACT induced regression of metastatic disease in multiple sites in one patient. Complete regressions were measured in (Top) a 2.0 X 1.7 cm lesion in the axilla, (Middle) a 1.4 X 0.8 cm lesion in the subcutaneous chest, and (Bottom) a 1.5 X 1.5 cm intraperitoneal lesion.
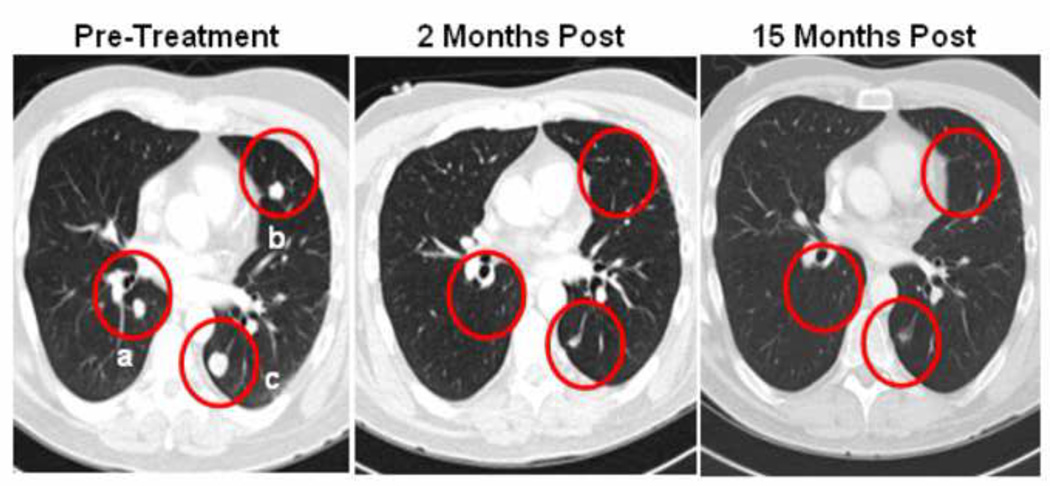
Figure 2.
Lymphodepletion and ACT induced regression of metastatic disease in the lungs, with a small degree of radiographic residua indicative of a partial response. In the pretreatment scans, (a) measured 1.3 cm × 1.0 cm, (b) measured 1.1 × 0.8, and (c) measured 2.0 × 1.5 cm. In the post-treatment scans, all lesions were less than 1.0 cm.
Table III.
Infused TIL Characteristics
| Patient # | Days1 | Expansion2 | # TIL Infused | Viability | %CD4 / %CD8 |
|---|---|---|---|---|---|
| 2 | 52 | 1567 | 4.7×1010 | 93% | 42 / 56 |
| 4 | 45 | 1133 | 3.4×1010 | 93% | 37 / 57 |
| 5 | 192 | 1433 | 4.3×1010 | 83% | 1 / 94 |
| 7 | 101 | 1040 | 5.2×1010 | 84% | 88 / 6 |
| 8 | 45 | 1340 | 6.3×1010 | 82% | 7 / 88 |
| 9 | 39 | 1480 | 7.4×1010 | 80% | 1 / 96 |
| 12 | 110 | 400 | 2.0×1010 | 85% | 66 / 33 |
| 13 | 47 | 1217 | 7.3×1010 | 79% | 4 / 82 |
| 15 | 38 | 1100 | 6.6×1010 | 91% | 5 / 89 |
| 16 | 45 | 1100 | 11.0×1010 | 95% | 9 / 80 |
| 17 | 53 | 630 | 3.8×1010 | 82% | 68 / 30 |
| 18 | 40 | 1300 | 7.8×1010 | 84% | 10 / 90 |
| 19 | 53 | 550 | 3.3×1010 | 83% | 66 / 23 |
| Mean | 66 | 1099 | 5.6×1010 | 85% | 31 / 63 |
Days from surgical resection to TIL infusion
Fold-expansion of TIL during Rapid Expansion
Toxicity
There were no treatment-related deaths. Hematologic toxicities were transient and included anemia and thrombocytopenia requiring transfusion in 12 patients. All patients experienced non-hematologic grade 3 and 4 toxicities during the IL-2 phase of the treatment. All toxicities decreased to grade 2 or less prior to discharge from the hospital. Long-term adverse events developed in 3 of 13 patients (23%). All 3 of these patients had some evidence of treatment-related clinical benefit. One patient with stable disease ongoing for 24+ months experienced hearing loss sufficient to require hearing aids. One patient with a complete response experienced vitiligo with complete loss of pigmentation of the hair, eyebrow and eyelashes, as well as uveitis lasting several months that required intraocular and systemic corticosteroids. Finally, one patient with stable disease has had an unresolved adverse event of hemorrhagic cystitis that started after cyclophosphamide therapy and has required multiple transfusions and cystoscopies with cauterization.
Discussion
Combination therapy of ACT with TIL and high dose IL-2 following lymphodepleting chemotherapy has resulted in up to 50% objective response rate in patients with metastatic melanoma (4). The purpose of our trial was to demonstrate the feasibility of performing TIL growth and the efficacy of ACT TIL therapy at the Moffitt Cancer Center using laboratory techniques and clinical protocols developed and used at the Surgery Branch, NCI. Nineteen patients were enrolled in our clinical trial. In 13 treated patients, a 38% objective response rate was obtained, which comprised 2 complete responses and 3 partial responses. On an intention-to-treat basis, 26% of all patients had an objective response.
For the clinical trial, TIL were successfully grown from the tumors of 17 patients (89%). Three of these patients demonstrated relatively slow TIL growth that did not reach the target number of 2×107 cells from any single fragment within 5 weeks. For these three patients, TIL from all fragments were pooled in order to obtain sufficient numbers to initiate the rapid expansion. One patient achieved a durable partial response (19+ months). Progressive disease was observed in two of these patients. While additional patients are required for verification, pooling TIL from multiple fragments in order to achieve a sufficient number of TIL for treatment appears to be an acceptable approach. Overall, similar responses were measured whether patients received TIL that contained predominantly CD4+ or CD8+ T cells. The contribution of CD4+ T cells in TIL has been recently reported (7).
Although our clinical study successfully met its goal of demonstrating that ACT TIL therapy could be offered to advanced melanoma patients at the Moffitt Cancer Center, strategies to improve upon its feasibility and efficacy are underway. Of 19 patients enrolled, 4 experienced disease progression during TIL production and were unable to be treated. Our center is implementing additional ACT TIL trials that will examine whether incorporation of strategies to control disease in the interim between TIL culture initiation and administration increases the number of patients that can be treated with ACT TIL. In one trial, patients whose melanomas harbor a BRAF V600 mutation will be treated with a selective BRAF inhibitor (8) during the ex vivo TIL growth phase of the protocol. In an additional trial, patients with wild-type BRAF melanomas will be treated with ipilimumab (9) during the TIL growth phase.
In addition, strategies to enhance the proliferation of TIL in vitro are currently being explored. Activated T cells can lose expression of CD28, a T cell co-stimulatory molecule, leading to anergy and apoptosis (10). It has been shown that although TIL cells down-regulate CD28, they maintain expression of the co-stimulatory molecule, 4-1BB. This molecule can be targeted to prevent activation-induced cell death (11, 12). We are currently exploring the use of anti-4-1BB agonistic antibody in initial TIL cultures to enhance proliferation, cytolytic activity, and increase the anti-tumor activity of TIL. Our initial results indicate an increased viability and activity in tumor-specific TIL (13).
Finally, it has been shown that persistence of adoptively transferred TIL correlates with tumor regression (14). Addition of post-transfer agents that enhance the persistence of TIL after ACT are currently being explored. We have shown that treatment with anti-PD-L1 antibody after ACT can enhance the persistence of TIL in a murine model of melanoma (15). Such a combination therapy may be considered in future clinical trials as well.
Acknowledgements
This study was supported by The Donald A. Adam Comprehensive Melanoma Research Center, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, a Team Science Award from the Melanoma Research Alliance, and generous contributions by private donors through the Moffitt Foundation.
References
- 1.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6:S11–S14. [PubMed] [Google Scholar]
- 6.Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 7.Friedman KM, Prieto PA, Devillier LE, et al. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35:400–408. doi: 10.1097/CJI.0b013e31825898c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Liu S, Hernandez J, et al. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184:452–465. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Chacon JA, Li Y, Wu RC, et al. Co-stimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating 13 lymphocytes from activation-induced cell death and enhances anti-tumor effector function. J Immunother. 2011;34:236–250. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarnaik A, Bilotto A, Jure-Kunkel M, et al. Costimulatory effect of agonistic 4-1BB antibody on proliferation and effector phenotype of tumor-infiltrating lymphocytes in melanoma. J Clin Oncol. 2012;(suppl) abstr 2511. [Google Scholar]
- 14.Zhou J, Shen X, Huang J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175(10):7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilon-Thomas SA, Mackay A, Vohra N, et al. Blockade of Programmed Death Ligand-1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]