Alzheimer’s disease (AD) is the most common form of neurodegenerative disorder to cause dementia in the elderly. It affects millions of people worldwide and puts tremendous emotional and economic burden on affected families. It is estimated that care for AD patients costs more than $200 billion annually in the United States [1]. Despite the aging global population and prevalence and effects of AD there is no effective treatment. Finding a cure or preventative is one of the biggest challenges of the 21st century and represents one of the highest priorities in life science research and drug discovery.
A hallmark of AD is the presence of plaques found between neurons in the brain. These mainly consist of insoluble β-amyloid protein fragments and are thought to be cytotoxic when aggregated. This can lead to neuron death and subsequent loss of memory and perception. The β-amyloid peptide has 40~42 residues and comes from the transmembrane (TM) segment of amyloid precursor protein (APP), which has a large extracellular domain (ECD) and a small intracellular domain (ICD). APP can be processed by two different pathways. It can be cleaved by α-secretase to release the APP ECD. This cleavage blocks production of β-amyloid and reduces plaque buildup. In the second pathway, APP is first cleaved by β-secretase at the extracellular side near the TM segment, and then by γ-secretase within the TM segment. This releases β-amyloid peptides with lengths of 37–43 residues. The two major forms of β-amyloid peptides have 40 (Aβ-40) and 42 residues (Aβ-42) and contain most of the TM segment. These peptides, especially the longer Aβ-42, are hydrophobic and can easily aggregate into large oligomers.
The production of β-amyloid could be blocked by inhibiting either β- or γ-secretase as an effective treatment for AD. However, over the last decade, there have been several unsuccessful attempts at this, including the costly withdrawal of three late stage clinical trials [2]. Mutations causing a loss of function in γ-secretase may also be the cause of AD, thus simple inhibition of γ-secretase would not offer an effective therapeutic solution [3]. In addition, γ-secretase processes the Notch receptors, which play an important role to regulate cell biology. As a result, full inhibition of γ-secretase could result in toxic side effects. There is now a need for better understanding of the biochemistry and structure of γ-secretase.
The γ-secretase intramembrane protease complex has four core subunits: presenilin, nicastrin (NCT), anterior pharynx-defective 1 (APH-1) and presenilin enhancer 2 (PEN-2). Presenilin is a catalytic subunit with a protease active site containing two aspartyl residues located in transmembrane helices 6 and 7 (TM6 and TM7). The accessary functions of transmembrane proteins APH-1, PEN-2 and NCT are required for enzyme activity. In addition to APP, γ-secretase processes a range of substrates that are vital to signaling pathways involved in cell fate, growth and development. Important details of substrate recognition and cleavage by γ-secretase are unknown because of the challenge to obtain a high resolution structure for this multi-subunit transmembrane protein complex. Until recently, the most detailed structure of γ-secretase was obtained by cryo-electron microscopy (EM) at a resolution of 12 Å [4].
Shi, Scheres and colleagues recently made a breakthrough by determining a 4.5 Å resolution structure of the human γ-secretase complex with cryo-EM single-particle reconstruction [5]. This achievement was made possible by two technical advances: one is the ability to obtain sufficient quality and quantity of pure γ-secretase through transient expression in mammalian cells and the other is the ability to collect and process high quality EM data using a recently developed direct electron detector and new computational methods. Similar approaches had been used previously to determine a 3.4 Å structure of the transient receptor potential vanilloid 1 (TRPV1) ion channel [6], which is considered a landmark of membrane protein structural biology [2]. The 4.5 Å map of γ-secretase reveals clear density for the NCT ectodomain, which can be modeled using a bacterial glutamate carboxypeptidase and density for the transmembrane core of 19 TM helices arranged into a horseshoe-shape (Figure 1). This structure reveals, for the first time, the location of individual TM helices, although the resolution limit prevents a detailed description of the spatial arrangement of each subunit in the complex assembly.
Figure 1.
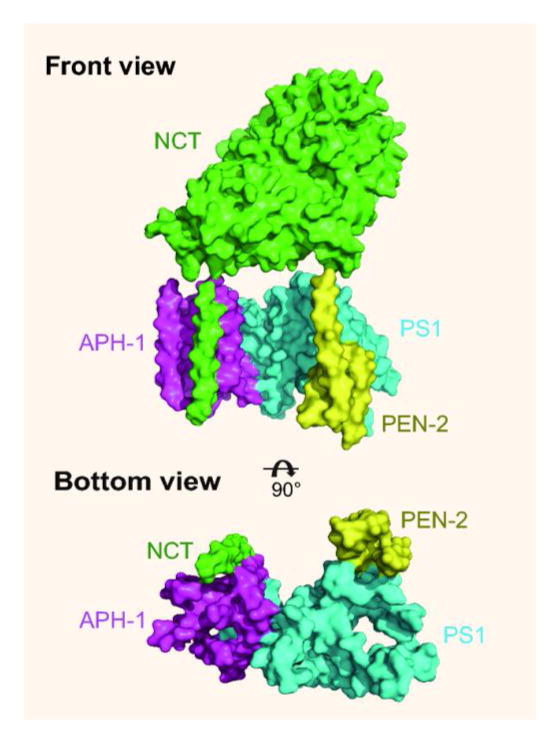
Front and bottom views of the overall architecture of γ-secretase. The ECD of NCT is removed in the bottom view for clarity.
Following this initial success, the Shi group solved the structure of the NCT ectodomain from Dictyostelium purpureum [7], an eukaryotic organism known to have a complete γ-secretase complex [8]. This allowed determination of a more complete model of the human NCT ectodomain and a more accurate revision of the subunit arrangement in the complex assembly. The C- and N-terminal PS1 subunits in this assembly form the central scaffold of a horseshoe, with the NCT and APH-1 TM helices forming the thick side of the horseshoe and PEN-2 forming the thin side (Figure 1, bottom view). Thus, for the first time, the overall architecture of γ-secretase could be visualized.
The new findings give rise to many new questions related to substrate recognition and proteolytic processing by γ-secretase. Addressing these questions will require validation of existing structures by alternative techniques such as double electron-electron resonance and disulfide crosslinking and to solve new structures with better resolution. This could be done through single particle cryo-EM or X-ray crystallography, which would provide residue-to-residue contact information related to complex assembly, substrate binding and catalytic mechanism when resolution approaches 3.5 Å.
The ultimate aim of a higher resolution structure of γ-secretase is to design better drugs for the treatment of AD. Given the expensive failures of recent clinical trials fresh new ideas are required. In the fields of G-protein coupled and nuclear receptors new drug discovery is mainly aimed at modulating a specific pathway, rather than full activation or blockage. Rational design of novel γ-secretase modulators could be guided by an atomic structure model of γ-secretase. The 4.5 Å cryo-EM structure is a breakthrough beginning towards this goal.
References
- 1.Association, A.s. 2014 Alzheimer’s Disease: Facts and Figures. Alzheimer’s & Dementia. 2014;10 doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.De Strooper B, Gutierrez LC. Learning by Failing: Ideas and Concepts to Tackle gamma-Secretases in Alzheimer Disease and Beyond. Annual review of pharmacology and toxicology. 2014 doi: 10.1146/annurev-pharmtox-010814-124309. [DOI] [PubMed] [Google Scholar]
- 3.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO reports. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osenkowski P, Li H, Ye W, Li D, Aeschbach L, Fraering PC, Wolfe MS, Selkoe DJ, Li H. Cryoelectron microscopy structure of purified gamma-secretase at 12 A resolution. Journal of molecular biology. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu P, Bai XC, Ma D, Xie T, Yan C, Sun L, Yang G, Zhao Y, Zhou R, Scheres SH, et al. Three-dimensional structure of human gamma-secretase. Nature. 2014;512:166–170. doi: 10.1038/nature13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie T, Yan C, Zhou R, Zhao Y, Sun L, Yang G, Lu P, Ma D, Shi Y. Crystal structure of the gamma-secretase component nicastrin. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13349–13354. doi: 10.1073/pnas.1414837111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMains VC, Myre M, Kreppel L, Kimmel AR. Dictyostelium possesses highly diverged presenilin/gamma-secretase that regulates growth and cell-fate specification and can accurately process human APP: a system for functional studies of the presenilin/gamma-secretase complex. Disease models & mechanisms. 2010;3:581–594. doi: 10.1242/dmm.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]


