Abstract
Myelodysplastic syndromes (MDSs) are a group of heterogeneous clonal bone marrow disorders characterized by ineffective hematopoiesis, peripheral blood cytopenias, and potential for malignant transformation. Lower/intermediate-risk MDSs are associated with longer survival and high red blood cell (RBC) transfusion requirements resulting in secondary iron overload. Recent data suggest that markers of iron overload portend a relatively poor prognosis, and retrospective analysis demonstrates that iron chelation therapy is associated with prolonged survival in transfusion-dependent MDS patients. New data provide concrete evidence of iron’s adverse effects on erythroid precursors in vitro and in vivo. Renewed interest in the iron field was heralded by the discovery of hepcidin, the main serum peptide hormone negative regulator of body iron. Evidence from β-thalassemia suggests that regulation of hepcidin by erythropoiesis dominates regulation by iron. Because iron overload develops in some MDS patients who do not require RBC transfusions, the suppressive effect of ineffective erythropoiesis on hepcidin may also play a role in iron overload. We anticipate that additional novel tools for measuring iron overload and a molecular-mechanism–driven description of MDS subtypes will provide a deeper understanding of how iron metabolism and erythropoiesis intersect in MDSs and improve clinical management of this patient population.
Introduction
Diseases associated with iron deficiency and iron overload affect a large fraction of the world’s population. Maintaining iron balance is critical for hemoglobin synthesis. No physiologic mechanism for iron excretion exists in humans. Furthermore, diseases requiring regular red blood cell (RBC) transfusions are hampered by iron overload that, if untreated, leads to significant morbidity and mortality. The purpose of this review is to present the current state of knowledge regarding the impact of iron overload and the benefits of iron chelation on survival and erythropoiesis in patients with low-risk myelodysplastic syndrome (MDS).
Role of RBC transfusion in different MDS subtypes
MDS is a heterogeneous group of disorders characterized by ineffective hematopoiesis. Multiple classifications are available to group MDS into prognostic subtypes based on clinical and pathologic characteristics. The current dogma suggests that all members of these classifications are clonal hematopoietic stem cell disorders with a progressively increasing propensity toward transformation to acute leukemia. Refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), and 5q− subtypes of the World Health Organization (WHO) classification and the low or intermediate-1 subtypes of the International Prognostic Scoring System have a longer median survival and the lowest rate of progression to acute leukemia. The common biological characteristic of low-risk MDS includes a defect in hematopoietic stem and progenitor cell self-renewal and differentiation, resulting in cytopenias.
Approximately 60% to 80% of patients with MDS experience symptomatic anemia, and 80% to 90% require RBC transfusions as supportive therapy.1,2 The WHO classification-based prognostic scoring system defines RBC transfusion dependence as requiring ≥1 RBC unit every 8 weeks averaged over 4 months,3 and RBC transfusion dependence correlates strongly with decreased survival in MDS patients.4 The prolonged exposure to RBC transfusions makes low-risk MDS patients most susceptible to iron overload and its clinical consequences.
Iron storage, recycling, and secondary iron overload
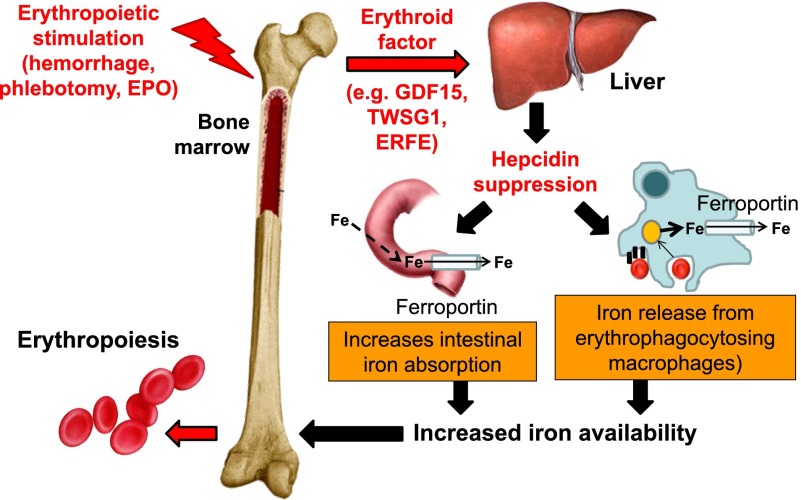
A unit of RBCs for transfusion contains 200 to 250 mg of iron released from hemoglobin as the RBCs are cleared from circulation. Iron released from reticuloendothelial cells (ie, bone marrow and spleen macrophages and Kupffer cells in the liver) is bound for storage in cytosolic ferritin or exported to plasma proteins (eg, transferrin). Present on all cells involved in iron flows (eg, macrophages, duodenal enterocytes, and hepatocytes), ferroportin is the only known iron exporter. In macrophages, ferroportin expression is transcriptionally regulated by heme5 and under translational regulation (ie, iron response elements on messenger RNA bound to iron response proteins) by iron.5-8 However, ferroportin expression is mainly regulated at the cell surface where hepcidin, a liver-derived peptide hormone, binds and leads to ferroportin internalization and degradation.9 Increased circulating hepcidin and consequent ferroportin degradation inhibit the absorption of dietary iron in the duodenum and iron release from erythrophagocytosing macrophages (Figure 1), making hepcidin the master negative regulator of iron homeostasis.10
Figure 1.
Hepcidin regulation by erythropoiesis and its effects on iron efflux from cells involved in iron metabolism. Hepcidin plays a central role in the maintenance of iron homeostasis and regulation of plasma iron concentrations by controlling ferroportin concentrations on iron-exporting cells, including duodenal enterocytes, recycling macrophages of the spleen and liver, and hepatocytes (involved in iron storage). The bone marrow has the highest iron requirements for hemoglobin synthesis, and thus, increased erythropoietic activity suppresses hepcidin production. Several potential candidate erythroid regulators of hepcidin (eg, GDF15 and TWSG1) in β-thalassemia have been reported. Recently, a non–disease-specific mechanism has been proposed (eg, ERFE). EPO, erythropoietin; Fe, iron; TWSG1, twisted gastrulation 1.
Iron released into circulation binds transferrin with high affinity. Transferrin saturation is calculated as a ratio of serum iron to total iron-binding capacity, approximately 30% under normal conditions. When transferrin’s iron-binding capacity is exceeded, non–transferrin-bound iron (NTBI) is produced. NTBI is found after transferrin saturation exceeds 80%.11,12 Labile plasma iron is redox-active NTBI, permeates cell membranes, and causes cellular damage via production of reactive oxygen species (ROS).13,14 ROS oxidize lipids, proteins, and nucleic acids, resulting in premature apoptosis, cell death, tissue and organ damage (eg, iron-overload–associated liver cirrhosis, diabetes and other endocrinopathies, and cardiomyopathy), and, if untreated, death. Transfusion-dependent β-thalassemia patients have a natural history of transfusion iron overload resulting in significant morbidity and mortality.12,15-18 Evidence of iron overload in MDS patients is mounting.19-23 However, because MDS patients present in adulthood (when propensity for comorbid diseases is high) and have a significantly shorter life expectancy, the impact of iron overload and benefit from iron chelation may be somewhat different relative to β-thalassemia patients.24
Iron requirement for and hepcidin regulation by erythropoiesis
In circulation, 2.4 g of iron is present at all times (5 L blood volume × hematocrit of 48% = 2.4 L of RBCs = 2.4 g of iron). Approximately 20 mg of iron is required daily for hemoglobin synthesis, the majority of which is recycled from senescent RBCs under the regulation of hepcidin (Figure 1). Since hepcidin’s discovery in 2000, convincing evidence suggests that erythroid suppression of hepcidin is a direct consequence of increased erythropoietic activity itself, irrespective of anemia, hypoxia, and increased erythropoietin.25,26 The observed finding of insufficient hepcidin (relative to the degree of iron overload) in diseases of expanded and/or ineffective erythropoiesis supports the predicted existence of an “erythroid factor” regulating iron metabolism.27
Multiple pieces of data suggest strongly that this erythroid factor is secreted by erythroid precursors, functioning as a hormone to suppress hepcidin expression in the liver. Several candidates have been proposed. Circulating growth differentiation factor 15 (GDF15) is increased in patients with several congenital and acquired anemias and correlates with concurrent low hepcidin.28 However, studies in phlebotomized mice29 and in MDS patients30 have shown poor correlation between GDF15 and hepcidin. Thus, mechanisms of hepcidin suppression may be disease specific. Recently, a potential physiological regulator of hepcidin, erythroferrone (ERFE), has been identified31; the authors demonstrate the loss of hepcidin suppression after phlebotomy in ERFE knockout mice, increased ERFE in mouse models of β-thalassemia, and relatively increased hepcidin expression and decreased iron overload in β-thalassemic/ERFE knockout relative to β-thalassemic mice.31 Circulating ERFE concentration in MDS patients and mouse models thereof have not yet been evaluated.
Hepcidin and iron overload in different MDS subtypes
Hepcidin levels are heterogeneous across different MDS subtypes, with a 10-fold difference between the lowest (1.43 nM in RARS) and the highest (11.3 nM in RA with excess blasts) (P = .003) concentrations. MDS subtypes remain independent predictors of hepcidin in multivariate analyses (adjusted for serum ferritin [SF] and transfusion history), suggesting that the relationship between erythropoiesis and iron metabolism differs between MDS subtypes.30
Another recent study reveals an increased hepcidin concentration in transfusion-dependent MDS patients relative to controls.32 A strong positive correlation between hepcidin and SF (r = 0.976) is observed in those receiving <9 RBC units, no correlation is observed in those receiving 9 to 24 RBC units, and a negative correlation is observed (r = −0.536) in those receiving >24 RBC units. Thus, despite the dominant regulation of hepcidin by erythropoiesis, hepcidin remains sensitive to regulation by iron in patients with relatively low RBC transfusion requirements; similar findings have been observed in β-thalassemic mice.33 RBC transfusions derepress circulating hepcidin but fall short of levels expected in response to iron overload (as measured by SF); these findings reflect prior evidence in transfusion-dependent β-thalassemia patients.34 Furthermore, hepcidin concentration is again suppressed prior to the next RBC transfusion, suggesting that erythropoiesis-mediated hepcidin suppression is proportional to the degree of erythroid expansion, highest immediately posttransfusion to lowest immediately prior to the next transfusion (Figure 2).
Figure 2.
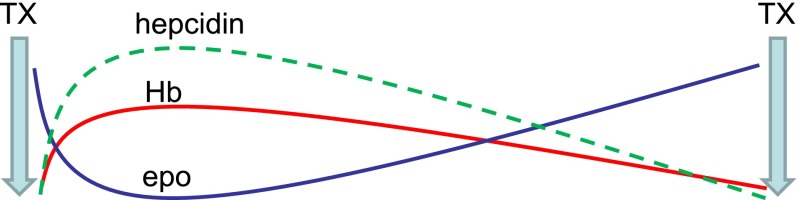
Model effect of erythropoiesis on hepcidin expression between RBC's transfusions. Ultimate hepcidin concentrations are the sum of effects from multiple regulators. RBC transfusions both suppress endogenous erythropoiesis and ultimately result in the accumulation of iron, released from transfused RBCs at the end of their life cycle. Thus, hepcidin is initially derepressed after RBC transfusion and progressively decreases to pretransfusion levels, mirroring hemoglobin and endogenous erythropoietin concentrations (modified from data in Ghoti et al15). epo, erythropoietin; Hb, hemoglobin; TX, transfusion.
RBC transfusions are the main source of progressive iron overload in transfusion-dependent MDS patients. A person requiring 4 RBC units per month acquires approximately 9.6 g of iron per year. Because maximal daily iron absorption is 4 mg (1.4 g per year), the amount of iron acquired from RBC transfusion exceeds >sixfold that gained from gastrointestinal absorption. In contrast to other iron-overload diseases, the literature presents only 1 case report of iron overload in a transfusion-independent MDS patient with RARS,35 consistent with RARS having the lowest hepcidin levels of all MDS subcategories.
Erythroid expansion and ineffective erythropoiesis suppress hepcidin and result in iron overload in RARS patients, evidenced by their highest levels of NTBI relative to other MDS subtypes.30 In RARS patients, the magnitude of ineffective erythropoiesis is higher than in MDS-RA patients because mitochondrial iron trapping prevents normal iron incorporation into heme, resulting in low tissue oxygen tension, higher endogenous erythropoietin levels, and hepcidin suppression.30 SF3B1 gene mutations (resulting in aberrant RNA splicing) in MDS patients are associated with ringed sideroblasts, low hepcidin, and parenchymal iron overload.36-38 Between 60% and 80% of RARS patients have the SF3B1 mutation.39 Furthermore, HFE gene polymorphisms that predispose to iron overload are detected in up to 21% of MDS-RARS patients, significantly higher than in other MDS subtypes (9%).40,41 These data suggest that multiple factors, including hepcidin, increase the burden of iron overload in RARS patients and that this MDS subtype may be different from other low-risk transfusion-dependent MDS patients in whom transfusional iron is the main cause of iron overload.
Effects of iron overload on erythropoiesis
Iron chelation results in improved hemoglobin and reduced RBC transfusion requirements,42,43 suggesting that iron overload impedes erythropoiesis. However, the mechanisms by which this occurs are not completely understood. Iron overload inhibits burst-forming unit colony formation and erythroblast differentiation of both murine and human hematopoietic progenitors in vitro, and cells exposed to excess iron exhibit dysplastic changes with increased intracellular ROS and decreased BCL-2 (antiapoptotic gene) expression.44 Furthermore, the addition of ferrous ammonium sulfate to peripheral blood mononuclear cells derived from MDS patients resulted in increased ROS, oxidation of 8-oxoguanine, and an abnormal comet assay consistent with DNA damage.45 Reduced BCL-2 and increased ROS in the cell together cause the leakage of cytochrome c from the mitochondria into the cytoplasm, triggering apoptosis by activated caspase-9.46
MDS patients with elevated SF have significantly fewer burst-forming units,47 even when SF is minimally elevated (>250 μg/L). Granulocyte-macrophage colony-forming units showed no significant difference between the 2 groups, suggesting that erythroid progenitors are more susceptible to iron overload than myeloid progenitors.47 Erythroid precursor susceptibility likely results from impaired heme synthesis and mitochondrial iron trapping, observed in RARS patients, and is reminiscent of primary diseases associated with impaired heme synthesis (eg, porphyria and congenital sideroblastic anemia). Because of this similarity and the known disease exacerbation in the presence of iron overload and improvement with iron chelation, it is reasonable to expect that iron depletion is beneficial for erythropoiesis in iron-overload diseases.48 Data from multiple sources, including our laboratories, reveal that iron restriction improves erythropoiesis in β-thalassemia, a disease characterized by ineffective erythropoiesis similar to MDS.49-53 Recent data suggest that in addition to causing relative iron deficiency and reversal of ineffective erythropoiesis in mouse models of β-thalassemia, the extracellular domain of activin receptor IIa results in ligand trapping of GDF11.54 Furthermore, the extracellular domain of activin receptor IIb also results in ligand trapping of GDF11 and has been shown to reverse anemia and ineffective erythropoiesis in a mouse model of MDS.55 Thus, the impact of iron overload on erythropoiesis may be mediated through GDF11, a member of the transforming growth factor β (TGF-β) superfamily.
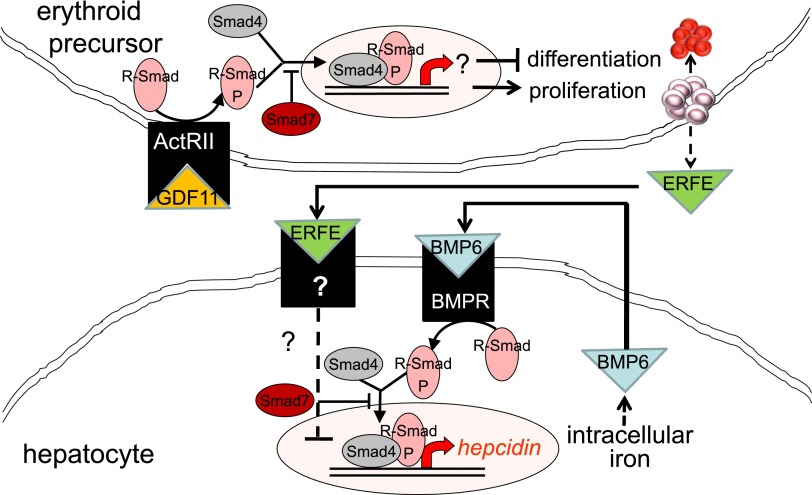
Increased TGF-β has been implicated in the pathophysiology of MDS. In a recent study, the authors report increased serum GDF11 concentration in MDS relative to age-matched controls.55 As signal transduction following TGF-β receptor binding results in the phosphorylation of Smad proteins, Smad2 is heavily phosphorylated in MDS bone marrow progenitors and is found to be upregulated in CD34+ cell from MDS patients.56 Furthermore, Smad7, a negative regulator in the Smad complex, is markedly decreased in MDS and leads to further increased TGF-β signal transduction (Figure 3).57 Consequently, more complete understanding of the importance of TGF-β is beginning to emerge.
Figure 3.
Model of crosstalk between erythropoiesis and iron metabolism involving TGF-β family member GDF11. Erythroid precursor proliferation and differentiation is regulated in part by multiple members of the TGF-β family. GDF11 binding to ActRII results in Smad2,3 phosphorylation and leads to the expansion of erythroid precursors and suppresses differentiation, resulting in ineffective erythropoiesis and iron overload. Hepcidin expression in hepatocytes is stimulated through the iron pathway (through bone morphogenic protein receptor signaling and Smad1,5,8 phosphorylation) and suppressed through the erythropoiesis pathway (possibly through ERFE binding and signaling through a yet-unidentified receptor). ActRII, activin receptor IIa; BMP6, bone morphogenic protein 6; BMPR, bone morphogenic protein receptor; GDF11, growth differentiation factor 11; R-Smad, receptor-mediated decapentaplegic protein; R-Smad-P, phosphorylated R-Smad.
Comparison of available methods to measure iron overload
Various noninvasive methods of measuring iron overload exist, including SF, NTBI, labile plasma iron, and liver iron concentration (LIC) as determined by magnetic resonance imaging (MRI) R2*, biomagnetic liver susceptometry, and cardiac T2* MRI. Invasive methods include liver and heart biopsies. In general, ubiquitous access to noninvasive methods has replaced biopsy as the standard method for measuring tissue iron concentrations in most centers. However, due to lack of consensus on the timing and utility of measuring the degree of, screening for, and diagnosing iron overload in MDS, the pros and cons of these markers and methods are discussed.
SF
A study in transfusion-naive newly diagnosed MDS patients reveals that patients with SF <500 ng/mL survive longer than those with SF >500 ng/mL (118.8 vs 10.2 months; P = .002),58 with significantly longer leukemia-free survival. In addition, SF independently predicts survival and risk of leukemia in MDS in multivariate analyses when transfusion dependence and iron overload are used as corrected time-dependent covariates.59 These studies indicate that excess iron is potentially harmful in MDS patients. Although some authors have questioned the utility of SF because of its increase in systemic inflammatory conditions as an acute-phase reactant, others assert that determining an individual’s SF/LIC ratio enables its use for further follow-up and monitoring of iron overload, especially when other markers of inflammation (eg, C-reactive protein or erythrocyte sedimentation rate) are also assessed.60 Despite its limitation, SF remains a valuable tool for monitoring iron overload given its ubiquitous availability, low cost, and well-standardized measures. However, more direct measures of iron stores are now available and required to establish diagnosis, estimate prognosis, and assess impact of treatment in patients with complex iron-overload syndromes (ie, transfusion-dependent MDS patients). These are discussed below.
LIC
LIC is the reference standard to estimate body-iron stores.61 Although normal LIC is estimated at 1.5 to 2 mg/g dry weight, high LIC (>15-20 mg/g dry weight) correlates with hepatic dysfunction,20 hepatic fibrosis,62 and worse overall prognosis.63 As high accuracy of noninvasive measures has been achieved, LIC is measured by noninvasive methods (eg, MRI), and biopsy use is secondary at most centers. Among the noninvasive methods, MRI is widely available and less expensive. The MRI R2 and R2* techniques demonstrate an average sensitivity of >85% and specificity of >92% and can be applied at any center with a reasonably new MRI machine, providing a rapid and accurate method for estimating LIC to diagnose and manage iron overload.64 MDS patients have a high prevalence of liver iron loading, with R2* >158.7 Hz in 35 out of 43 (81%) patients.23 In one study, liver R2* was positively correlated with RBC transfusion frequency (r = 0.72, P < .0001) and SF (r = 0.53, P < .0001),23 but in another study, liver T2* showed no correlation with RBC transfusion frequency (r = 0.19; P = .57) or with SF (r = −0.09; P = .77).20 Chelated MDS patients had a trend toward lower LIC (median 5.9 mg/g dry weight; range 3.0-9.3 mg/g dry weight) than unchelated MDS patients (median 9.5 mg/g dry weight; range 3.0-14.4 mg/g dry weight) (P = .17).20 Although all patients received RBC transfusions, not all those analyzed were low/intermediate-1–risk MDS, and because some received iron chelation and only a small number of patients was assessed in total, reliable conclusions regarding the natural history of iron overload or effectiveness of iron chelation in low/intermediate-1–risk MDS patients is not possible in this study.
NTBI and labile plasma iron
NTBI, measured by spectrophotometry, is significantly higher in low-risk relative to high-risk MDS.65 Tissue damage in iron-overload diseases is thought to result from the increased uptake of labile plasma iron leading to increased intracellular labile iron.66 Labile plasma iron >0.4 μM is highly correlated with iron overload.13 Labile plasma iron in β-thalassemia patients is undetectable during the course of deferoxamine infusion and peaks prior to the next infusion.67 These data suggest that labile plasma iron levels may aid in determining the efficacy of iron chelation therapy and provide more immediate evidence about iron overload to support decisions to change iron chelation dosing. Several means of measuring labile plasma iron have been proposed, but few laboratories have established an accurate and reproducible methodology at this time.
Cardiac T2* MRI
Heart failure is the primary cause of death in severe iron overload.68,69 Cardiac T2* MRI provides an accurate assessment of cardiac iron overload,70 correlating well with left ventricular function71 but poorly with SF and LIC, indicating that iron loading and clearance are differently regulated in the heart relative to the liver.70 Although cardiac complications account for the majority of nonleukemic death in low-risk MDS patients,4 there is insufficient evidence that these complications result from cardiac iron overload.20-22 Only one study using cardiac T2* MRI in MDS patients found that, despite 13% on iron chelation therapy, 19% (8/43) of patients demonstrated evidence of myocardial iron overload (R2* >50 Hz), which correlates with longer transfusion history.23 Although these retrospective studies include low-risk transfusion-dependent MDS patients with significantly elevated SF and evidence of iron overload in the liver, most patients evaluated were already receiving chelation therapy and had no symptoms or signs of heart failure. Prospective evaluation of cardiac T2* MRI in low-risk transfusion-dependent MDS patients is needed to clarify the relationship of frequent transfusion, systemic iron overload, and heart failure in this patient population (Table 1).
Table 1.
Diagnostic parameters for multiple methods used to evaluate clinical iron overload
| Method | Normal level | Mild/moderate iron overload | Severe iron overload |
|---|---|---|---|
| Serum ferritin (ng/mL) | <400 | 1000-2500 | >2500 |
| Transferrin saturation (%) | 20-40 | 55-70 in men; 50-70 in women | >70 |
| LIC (mg Fe/g dry weight) | <1.2 | 3-15 | >15 |
| Labile plasma iron (µM) | <0.4 | >0.4 | >0.4 |
| Liver T2* MRI (ms) | >6.3 | <6.3 | <1.4 |
| Cardiac T2* MRI (ms) | >20 | 8-20 | <8 |
Taken together, evaluation of iron overload in MDS is complex and requires multiple assessment methods to properly evaluate potential clinical risk and impact of therapy. SF is relatively nonspecific and, like transferrin saturation, is only clinically relevant for diagnosis of iron overload at very high values (ie, SF >2500 ng/mL and transferrin saturation >70%). However, using changes in these easily available methods to evaluate the efficacy of iron chelation remains appropriate. Finding labile plasma iron present in transfusion-dependent MDS patients is always pathological, warranting iron chelation therapy. The end-point goal of all therapy is to return patients to undetectable labile plasma iron; 0.4 µM is the lower limit of detectable in the standardized currently commercially available FeROS assay (Afferix, Ashkelon, Israel) previously used in studies with iron-overloaded patients.72 Elevated LIC (measured by liver T2* MRI) and below-normal cardiac T2* MRI strongly suggest parenchymal iron overload, warranting iron chelation therapy when cardiac iron overload is identified even in mild forms (eg, cardiac T2* <20 ms) because of the length of time it takes to remove iron from the heart. Trigger to initiate iron chelation in response to LIC measurement is more flexible, and although normal LIC is <1.2 mg Fe/g dry weight, iron chelation can be initiated when LIC reaches 3 mg Fe/g dry weight. As multiple novel methods for measuring iron-related parameters become standardized and applied to MDS in larger and prospective studies, a more robust understanding of differences between patients will further aid clinicians managing iron overload in MDS patients.
Clinical impact of iron chelation in MDS patients
Iron overload reduction and survival benefit
Uni- and multivariate analyses of MDS patients demonstrate that every 500-μg/L increase in SF >1000 μg/L is associated with a 1.36-fold increase in death.4 The increased mortality extended to low-risk MDS patients with RA, RARS, and 5q−, with a trend toward statistical significance between SF and mortality in RCMD patients and poor correlation in patients with RA with excess blasts. Additional studies corroborate these findings.73 However, one recent retrospective study showed that neither the number of RBC transfusions nor SF has a statistically significant effect on overall survival in RARS patients.74 We anticipate that serum hepcidin, particularly in RARS, and liver T2* MRI, more broadly in MDS, will provide more clarity to the association between iron overload and overall survival.
By demonstrating survival benefit, retrospective studies of iron-overloaded MDS patients receiving iron chelation therapy further support the premise that iron overload is an independent predictor of mortality. A study in low-risk MDS demonstrates a significant difference in median survival with iron-chelated vs nonchelated patients (160 months vs 40.1 months, respectively). Significantly more low-risk MDS patients receiving iron chelation survive up to 4 years (80% vs 44%; P < .03).75 Another study in low-risk MDS patients shows a median survival of 124 vs 53 months in the chelated and nonchelated groups, respectively (P < .0003).76 However, as in all retrospective studies, selection bias is an important potential confounder.
Multiple studies suggest that iron chelation inhibits the consequences of iron overload in MDS patients. Deferasirox (Exjade) decreases SF, labile plasma iron, and LIC in a dose-dependent manner despite ongoing RBC transfusions77-79 in transfusion-dependent MDS patients.80 Furthermore, treatment with deferasirox in iron-overloaded MDS patients increases serum and urinary hepcidin, likely by reducing iron overload and the inhibitory effect of ineffective erythropoiesis on hepcidin.81 Lastly, deferasirox also reduced cardiac iron concentration and cardiac myocyte superoxide levels.82
The EPIC (Evaluation of Patients’ Iron Chelation with Exjade) trial, the largest cohort of MDS patients using deferasirox, demonstrates a significant decrease in median SF at 1 year, but of significant concern is the 49% discontinuation rate due to renal and gastrointestinal side effects.83 Additional studies corroborate this relatively low rate of compliance due to side effects.84,85 An ongoing multicenter, randomized, double-blind, placebo-controlled clinical trial, TELESTO, to evaluate the effect of deferasirox on low-risk MDS patients with iron overload, is underway. This trial intends to evaluate death and nonfatal events related to cardiac and liver function,86 but, due to sustained difficulties with patient recruitment, the results may not be sufficiently powered to definitively answer these questions.
Prior to the approval of deferasirox, deferiprone (L1) was the only oral iron chelator available for clinical use outside of the United States; recent Food and Drug Administration approval of deferiprone has stimulated discussion and data analysis on its relative effectiveness. Profound granulocytopenia has been reported as the most severe adverse effect and thus limited the use of deferiprone to treat iron overload in MDS patients.87-89 Furthermore, use of deferiprone in low-risk MDS patients reveals the greatest utility in patients with relatively low SF and RBC transfusion requirements.90 A retrospective study comparing deferasirox with deferiprone in transfusion-dependent low-risk MDS patients with iron overload confirmed the superior efficacy and milder side effect profile of deferasirox in this patient population.89 However, several important details are missing, namely the degree of compliance with iron chelation therapy as well as the fraction of patients receiving the indicated dose of deferiprone (75 mg/kg per day), to determine deferiprone’s efficacy and properly interpret these results.
Although no universally accepted guidelines currently exist, both National Comprehensive Cancer Network (NCCN) and MDS Foundation recommendations are available (Table 2).91,92 The guidelines focus on avoiding harm from iron overload in otherwise-healthy, “low-risk,” transfusion-dependent MDS patients with elevated SF or evidence of organ iron overload (eg, liver T2* MRI). The recommendations for monitoring iron load and initiation of iron chelation include a baseline SF and liver T2* MRI and then follow-up studies (eg, every 3-4 months in transfusion-dependent patients) based on the rate of RBC transfusion. Although MRI is a well-established measure of organ iron deposition, it is not yet routinely used the way we use elevated SF, which by itself is insufficiently sensitive to inform when to initiate or how to monitor therapeutic effectiveness in MDS patients.
Table 2.
Recommendations for management of iron overload in MDS patients
| Characteristic | NCCN92 | MDS Foundation91 |
|---|---|---|
| Transfusion status | Received >20 RBC transfusions; continuing transfusions | Transfusion dependent, requiring 2 U/mo for >1 y |
| Serum ferritin concentration | >2500 μg/L | >1000 μg/L |
| MDS risk category | IPSS: low or intermediate-1 | IPSS: low or intermediate-1; WHO: RA, RARS, and 5q− |
| Patient profile | Candidate for allograft | Candidate for allograft; need to preserve organ function; life expectancy >1 y without comorbidities limiting progress |
IPSS, International Prognostic Scoring System.
These guidelines suggest that although benefit from iron chelation has not been clearly delineated, a strong consensus on the need to avoid iron overload is evident and needs to be balanced against potential side effects of iron chelation therapy. Deferoxamine, which because of its short half-life needs to be given parenterally, is relatively inconvenient, negatively impacting compliance,72 and is associated with injection site reactions as well as potential ocular and otic toxicity requiring monitoring. Side effects of the oral iron chelators mentioned above have resulted in cessation of treatment in a significant proportion of patients.84,87-89 Lastly, the benefit of iron chelation on quality of life may be more difficult to assess in light of the multiple comorbidities in many MDS patients.94 However, because transfusion dependence decreases quality of life,94-96 reversal of transfusion-induced iron overload or transfusion dependence as a consequence of iron chelation may result in quality-of-life improvements.
Iron chelation and improved erythropoiesis in MDS
The question remains whether iron chelation prevents iron-induced ROS, consequent oxidation of nucleotides (eg, 8-oxoguanine), and resultant mutations.97 In vitro experiments in trisomy 8 MDS patient cells demonstrate that iron chelation induces cell differentiation.98 In another report, MDS patients’ peripheral blood mononuclear cells treated with ferrous ammonium sulfate increased ROS and 8-oxoguanine oxidation and resulted in an abnormal comet assay consistent with DNA damage; all these findings were reversed with the addition of iron chelator.45 In addition, iron chelation induces differentiation of several human myeloid cell lines (eg, HL60 and NB4) and MDS patients’ peripheral blood–derived CD34+ cells. Taken together, iron chelation in MDS patients ameliorates pathological effects of iron overload and consequent oxidative stress by decreasing iron-induced cytotoxicity, DNA damage, blocked differentiation in hematopoietic cells, and possibly transformation to leukemia.
Iron chelation improves ineffective erythropoiesis in MDS patients. The majority of deferoxamine (Desferal)-chelated MDS patients reduce RBC transfusion requirements by 50% and 46% became completely transfusion independent.99 Other studies in deferasirox-chelated MDS patients also observe reduced RBC transfusion requirements.100-102 In one study, 16% of patients achieved transfusion independence by 12 months with a median hemoglobin of 8 g. In this study, all patients reduced RBC transfusion frequency by 67% after 12 months of treatment.85 Most recently, chelation with deferasirox demonstrated a 1.5- to 1.8-g/dL increase in hemoglobin despite a decrease in RBC transfusion requirements in patients with low-risk MDS.103
Deferasirox also reduced the oxidative stress within RBCs, platelets, and neutrophils; reduced intracellular ROS and lipid peroxidation; and increased intracellular reduced glutathione stores.16 Similar results were independently presented by other groups.104
Conclusions and recommendations
Iron overload in low-risk MDS is an important clinical problem resulting from RBC transfusions to correct the anemia. Despite its potential impact, hepcidin appears to play a relatively minor role in iron overload compared with frequent RBC transfusions in transfusion-dependent MDS patients. However, in some transfusion-independent low-risk MDS patients (eg, RARS), increased erythropoietic activity results in hepcidin suppression and contributes to iron loading. It may therefore be prudent to monitor transfusion-independent RARS patients for iron overload.
The most appropriate direct method to diagnose iron overload is T2* MRI (ie, liver and heart). Furthermore, circulating NTBI and labile plasma iron concentration are becoming more available and have been used in several clinical studies. Currently, the recommendation to initiate iron chelation therapy in MDS patients is based on the total number of RBC transfusions and on elevated SF in transfusion-dependent patients. Because different patients undergo relatively different rates of parenchymal iron loading and different volume loss from blood sampling for diagnostic testing, liver T2* MRI is the most reasonable and objective measure to evaluate iron overload and would be reasonably assessed after 20 RBC units have been transfused. Although insufficiently sensitive or specific to diagnose iron overload, changes in SF and transferrin saturation are still useful measures of therapeutic efficacy of iron chelation in iron-overloaded patients.
NCCN guidelines mention hemoglobin of 10 g/dL as a reasonable and well-accepted goal of RBC transfusion in MDS (and in general),92 and transfusion dependence is reasonably defined in our view as requiring at least 2 RBC units per month for at least 1 year. This results in the delivery of at least 24 RBC units and 6 g of iron. In our view, once 20 RBC units have been transfused, even if <1 year has passed, evaluation for iron overload is warranted if the patient is expected to continue chronic RBC transfusion therapy.
Evidence from managing patients with β-thalassemia suggests that it is appropriate to start iron chelation when T2* MRI results are above the equivalent of LIC 3 to 4 mg/g dry weight105 and certainly if any evidence of cardiac iron overload is identified. As chronically transfused iron chelated patients with β-thalassemia are followed with annual T2* MRI to determine effectiveness of therapy, need for dose adjustments, and chelation holidays,105 low-risk MDS patients who receive chronic RBC transfusions can be managed similarly. Low-risk MDS patients with relatively lower RBC transfusion requirements may be considered for T2* MRI after every additional 10 to 20 RBC units to evaluate the need to initiate iron chelation, determine effectiveness of therapy, or the need for dose adjustments. Once iron chelation therapy is initiated, tests to monitor associated side effects should be performed. MDS patients on deferasirox should be assessed monthly for serum creatinine, bilirubin, aminotransferases, and complete blood count.105 Similar recommendations are appropriate for MDS patients treated with deferiprone, although insufficient data make practical recommendations premature.
The rationale for iron chelation therapy in MDS remains compelling but has not been tested in prospective randomized studies. Considerable evidence suggests that iron chelation decreases SF, LIC, and labile plasma iron. Data from multiple independent retrospective studies demonstrate that iron chelation therapy results in a marked survival benefit in low-risk MDS patients. Results of an ongoing double-blind, randomized, placebo-controlled trial are anticipated to provide additional survival and morbidity data. However, in view of the serious adverse effects of iron overload, it is reasonable to offer iron chelation therapy to low-risk MDS patients at high risk for developing iron overload.
Acknowledgments
The authors extend sincere appreciation to E. Nemeth (University of California, Los Angeles) for evaluation and contribution to the coherence of the figures.
Authorship
Contribution: N.S. wrote and edited the article; N.V. wrote the article; E.R. edited the article and figures; A.V. edited the article and figures; and Y.G. wrote and edited the article and figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yelena Ginzburg, Erythropoiesis Laboratory, Lindsley F. Kimball Research Institute, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: yginzburg@nybloodcenter.org.
References
- 1.Hellström-Lindberg E. Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42(2 suppl 1):S10–S13. doi: 10.1053/j.seminhematol.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. Hematology (Am Soc Hematol Educ Program) 2002:136–161. doi: 10.1182/asheducation-2002.1.136. [DOI] [PubMed] [Google Scholar]
- 3.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 4.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 5.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411(1):123–131. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 6.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 7.McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Liu DP, Wu L, et al. Proper developmental control of human globin genes reproduced by transgenic mice containing a 160-kb BAC carrying the human beta-globin locus. Blood Cells Mol Dis. 2000;26(6):598–610. doi: 10.1006/bcmd.2000.0339. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 11.von Bonsdorff L, Lindeberg E, Sahlstedt L, Lehto J, Parkkinen J. Bleomycin-detectable iron assay for non-transferrin-bound iron in hematologic malignancies. Clin Chem. 2002;48(2):307–314. [PubMed] [Google Scholar]
- 12.Piga A, Longo F, Duca L, et al. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol. 2009;84(1):29–33. doi: 10.1002/ajh.21317. [DOI] [PubMed] [Google Scholar]
- 13.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18(2):277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Pootrakul P, Breuer W, Sametband M, Sirankapracha P, Hershko C, Cabantchik ZI. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalassemia/HbE patients treated with an oral chelator. Blood. 2004;104(5):1504–1510. doi: 10.1182/blood-2004-02-0630. [DOI] [PubMed] [Google Scholar]
- 15.Ghoti H, Fibach E, Merkel D, Perez-Avraham G, Grisariu S, Rachmilewitz EA. Changes in parameters of oxidative stress and free iron biomarkers during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndromes. Haematologica. 2010;95(8):1433–1434. doi: 10.3324/haematol.2010.024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giardina PJ, Grady RW. Chelation therapy in beta-thalassemia: an optimistic update. Semin Hematol. 2001;38(4):360–366. doi: 10.1016/s0037-1963(01)90030-7. [DOI] [PubMed] [Google Scholar]
- 17.Hershko CM, Link GM, Konijn AM, Cabantchik ZI. Iron chelation therapy. Curr Hematol Rep. 2005;4(2):110–116. [PubMed] [Google Scholar]
- 18.Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353(11):1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 19.Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101(1):91–96. doi: 10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- 20.Chacko J, Pennell DJ, Tanner MA, et al. Myocardial iron loading by magnetic resonance imaging T2* in good prognostic myelodysplastic syndrome patients on long-term blood transfusions. Br J Haematol. 2007;138(5):587–593. doi: 10.1111/j.1365-2141.2007.06695.x. [DOI] [PubMed] [Google Scholar]
- 21.Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol. 2007;82(11):1013–1016. doi: 10.1002/ajh.20980. [DOI] [PubMed] [Google Scholar]
- 22.Di Tucci AA, Matta G, Deplano S, et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica. 2008;93(9):1385–1388. doi: 10.3324/haematol.12759. [DOI] [PubMed] [Google Scholar]
- 23.Roy NB, Myerson S, Schuh AH, et al. Cardiac iron overload in transfusion-dependent patients with myelodysplastic syndromes. Br J Haematol. 2011;154(4):521–524. doi: 10.1111/j.1365-2141.2011.08749.x. [DOI] [PubMed] [Google Scholar]
- 24.Leitch HA. Optimizing therapy for iron overload in the myelodysplastic syndromes: recent developments. Drugs. 2011;71(2):155–177. doi: 10.2165/11585280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122(12):4635–4644. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finch C. Regulators of iron balance in humans. Blood. 1994;84(6):1697–1702. [PubMed] [Google Scholar]
- 28.Tanno T, Noel P, Miller JL. Growth differentiation factor 15 in erythroid health and disease. Curr Opin Hematol. 2010;17(3):184–190. doi: 10.1097/MOH.0b013e328337b52f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casanovas G, Vujić Spasic M, Casu C, et al. The murine growth differentiation factor 15 is not essential for systemic iron homeostasis in phlebotomized mice. Haematologica. 2013;98(3):444–447. doi: 10.3324/haematol.2012.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santini V, Girelli D, Sanna A, et al. Hepcidin levels and their determinants in different types of myelodysplastic syndromes. PLoS ONE. 2011;6(8):e23109. doi: 10.1371/journal.pone.0023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism [published online ahead of print June 1, 2014]. Nat Genet. [DOI] [PMC free article] [PubMed]
- 32.Qin Y, Liu H, Ruan S, Cai YF, You XF, Song GQ. [Detection of Hepcidin in transfusion dependent myelodysplastic syndrome patients and its clinical significance]. Zhonghua Xue Ye Xue Za Zhi. 2011;32(11):758–761. [PubMed] [Google Scholar]
- 33.Gardenghi S, Marongiu MF, Ramos P, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearney SL, Nemeth E, Neufeld EJ, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48(1):57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi H, Arita K, Suzuki Y, et al. Iron chelation therapy for a case of transfusion-independent MDS-RARS with significant iron overload. Int J Hematol. 2013;97(1):151–153. doi: 10.1007/s12185-012-1230-7. [DOI] [PubMed] [Google Scholar]
- 36.Visconte V, Rogers HJ, Singh J, et al. SF3B1 haploinsufficiency leads to formation of ring sideroblasts in myelodysplastic syndromes. Blood. 2012;120(16):3173–3186. doi: 10.1182/blood-2012-05-430876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambaglio I, Malcovati L, Papaemmanuil E, et al. Inappropriately low hepcidin levels in patients with myelodysplastic syndrome carrying a somatic mutation of SF3B1. Haematologica. 2013;98(3):420–423. doi: 10.3324/haematol.2012.077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malcovati L, Papaemmanuil E, Bowen DT, et al. Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium and of the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood. 2011;118(24):6239–6246. doi: 10.1182/blood-2011-09-377275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattermann N. SF3B1 and the riddle of the ring sideroblast. Blood. 2012;120(16):3167–3168. doi: 10.1182/blood-2012-08-447094. [DOI] [PubMed] [Google Scholar]
- 40.Nearman ZP, Szpurka H, Serio B, et al. Hemochromatosis-associated gene mutations in patients with myelodysplastic syndromes with refractory anemia with ringed sideroblasts. Am J Hematol. 2007;82(12):1076–1079. doi: 10.1002/ajh.20995. [DOI] [PubMed] [Google Scholar]
- 41.Valent P, Krieger O, Stauder R, et al. Iron overload in myelodysplastic syndromes (MDS) - diagnosis, management, and response criteria: a proposal of the Austrian MDS platform. Eur J Clin Invest. 2008;38(3):143–149. doi: 10.1111/j.1365-2362.2007.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliva EN, Ronco F, Marino A, Alati C, Praticò G, Nobile F. Iron chelation therapy associated with improvement of hematopoiesis in transfusion-dependent patients. Transfusion. 2010;50(7):1568–1570. doi: 10.1111/j.1537-2995.2010.02617.x. [DOI] [PubMed] [Google Scholar]
- 43.Badawi MA, Vickars LM, Chase JM, Leitch HA. Red blood cell transfusion independence following the initiation of iron chelation therapy in myelodysplastic syndrome. Adv Hematol. 2010;2010:164045. doi: 10.1155/2010/164045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taoka K, Kumano K, Nakamura F, et al. The effect of iron overload and chelation on erythroid differentiation. Int J Hematol. 2012;95(2):149–159. doi: 10.1007/s12185-011-0988-3. [DOI] [PubMed] [Google Scholar]
- 45.Fibach E, Rachmilewitz EA. Selective toxicity towards myelodysplastic hematopoietic progenitors - another rationale for iron chelation in MDS. Leuk Res. 2012;36(8):962–963. doi: 10.1016/j.leukres.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Pan Z, Voehringer DW, Meyn RE. Analysis of redox regulation of cytochrome c-induced apoptosis in a cell-free system. Cell Death Differ. 1999;6(7):683–688. doi: 10.1038/sj.cdd.4400544. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann J, Braulke F, Sinzig U, et al. Iron overload impairs proliferation of erythroid progenitors cells (BFU-E) from patients with myelodysplastic syndromes. Leuk Res. 2013;37(3):327–332. doi: 10.1016/j.leukres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Camaschella C, Campanella A, De Falco L, et al. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110(4):1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Rybicki AC, Suzuka SM, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 50.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120(12):4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nai A, Pagani A, Mandelli G, et al. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of β-thalassemia. Blood. 2012;119(21):5021–5029. doi: 10.1182/blood-2012-01-401885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo S, Casu C, Gardenghi S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123(4):1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt PJ, Toudjarska I, Sendamarai AK, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood. 2013;121(7):1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dussiot MMT, Fricot A, Chartier C, et al. The activin receptor IIa ligand trap Sotatercept corrects ineffective erythropoiesis in beta-thalassemia. Nat Med. 2014;20(4):398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suragani RN, Cadena SM, Cawley SM, et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408–414. doi: 10.1038/nm.3512. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Nguyen AN, Sohal D, et al. Inhibition of the TGF-beta receptor I kinase promotes hematopoiesis in MDS. Blood. 2008;112(8):3434–3443. doi: 10.1182/blood-2008-02-139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, McMahon C, Bhagat T, et al. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71(3):955–963. doi: 10.1158/0008-5472.CAN-10-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kikuchi S, Kobune M, Iyama S, et al. Prognostic significance of serum ferritin level at diagnosis in myelodysplastic syndrome. Int J Hematol. 2012;95(5):527–534. doi: 10.1007/s12185-012-1048-3. [DOI] [PubMed] [Google Scholar]
- 59.Sanz G, Nomdedeu B, Such E, et al. Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome [abstract]. Blood (ASH Annual Meeting Abstracts). 2008;112: Abstract 640. [Google Scholar]
- 60.Fischer R, Harmatz PR. Non-invasive assessment of tissue iron overload. Hematology (Am Soc Hematol Educ Program) 2009:215–221. doi: 10.1182/asheducation-2009.1.215. [DOI] [PubMed] [Google Scholar]
- 61.Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment, and monitoring. Hemoglobin. 2009;33(suppl 1):S46–S57. doi: 10.3109/03630260903346676. [DOI] [PubMed] [Google Scholar]
- 62.Angelucci E, Baronciani D, Lucarelli G, et al. Needle liver biopsy in thalassaemia: analyses of diagnostic accuracy and safety in 1184 consecutive biopsies. Br J Haematol. 1995;89(4):757–761. doi: 10.1111/j.1365-2141.1995.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 63.Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110(4):971–977. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 64.Voskaridou E, Douskou M, Terpos E, et al. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol. 2004;126(5):736–742. doi: 10.1111/j.1365-2141.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 65.Cortelezzi A, Cattaneo C, Cristiani S, et al. Non-transferrin-bound iron in myelodysplastic syndromes: a marker of ineffective erythropoiesis? Hematol J. 2000;1(3):153–158. doi: 10.1038/sj.thj.6200028. [DOI] [PubMed] [Google Scholar]
- 66.Breuer W, Shvartsman M, Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol. 2008;40(3):350–354. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 67.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102(7):2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 68.Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355(9220):2051–2052. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- 69.Telfer P, Coen PG, Christou S, et al. Survival of medically treated thalassemia patients in Cyprus. Trends and risk factors over the period 1980-2004. Haematologica. 2006;91(9):1187–1192. [PubMed] [Google Scholar]
- 70.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 71.Pennell DJ. T2* magnetic resonance and myocardial iron in thalassemia. Ann N Y Acad Sci. 2005;1054:373–378. doi: 10.1196/annals.1345.045. [DOI] [PubMed] [Google Scholar]
- 72.Armand P, Sainvil MM, Kim HT, et al. Pre-transplantation iron chelation in patients with MDS or acute leukemia and iron overload undergoing myeloablative allo-SCT. Bone Marrow Transplant. 2013;48(1):146–147. doi: 10.1038/bmt.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takatoku M, Uchiyama T, Okamoto S, et al. Japanese National Research Group on Idiopathic Bone Marrow Failure Syndromes. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78(6):487–494. doi: 10.1111/j.1600-0609.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 74.Chee CE, Steensma DP, Wu W, Hanson CA, Tefferi A. Neither serum ferritin nor the number of red blood cell transfusions affect overall survival in refractory anemia with ringed sideroblasts. Am J Hematol. 2008;83(8):611–613. doi: 10.1002/ajh.21192. [DOI] [PubMed] [Google Scholar]
- 75.Leitch HA. Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res. 2007;31(suppl 3):S7–S9. doi: 10.1016/S0145-2126(07)70460-5. [DOI] [PubMed] [Google Scholar]
- 76.Rose C, Brechignac S, Vassilief D, et al. GFM (Groupe Francophone des Myélodysplasies) Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myélodysplasies). Leuk Res. 2010;34(7):864–870. doi: 10.1016/j.leukres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Metzgeroth G, Dinter D, Schultheis B, et al. Deferasirox in MDS patients with transfusion-caused iron overload—a phase-II study. Ann Hematol. 2009;88(4):301–310. doi: 10.1007/s00277-008-0588-3. [DOI] [PubMed] [Google Scholar]
- 78.Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80(2):168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gattermann N, Finelli C, Porta MD, et al. EPIC study investigators. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: Results from the large 1-year EPIC study. Leuk Res. 2010;34(9):1143–1150. doi: 10.1016/j.leukres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 80.List AF, Baer MR, Steensma DP, et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol. 2012;30(17):2134–2139. doi: 10.1200/JCO.2010.34.1222. [DOI] [PubMed] [Google Scholar]
- 81.Ghoti H, Fibach E, Westerman M, Gordana O, Ganz T, Rachmilewitz EA. Increased serum hepcidin levels during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndrome. Br J Haematol. 2011;153(1):118–120. doi: 10.1111/j.1365-2141.2011.08587.x. [DOI] [PubMed] [Google Scholar]
- 82.Al-Rousan RM, Paturi S, Laurino JP, et al. Deferasirox removes cardiac iron and attenuates oxidative stress in the iron-overloaded gerbil. Am J Hematol. 2009;84(9):565–570. doi: 10.1002/ajh.21487. [DOI] [PubMed] [Google Scholar]
- 83.Cappellini MD, Porter J, El-Beshlawy A, et al. EPIC Study Investigators. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010;95(4):557–566. doi: 10.3324/haematol.2009.014696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Angelucci E, Santini V, Di Tucci A, et al. Deferasirox chelation therapy in transfusion-dependent MDS patients: final report from the Gimema MDS0306 prospective trial [abstract]. Blood (ASH Annual Meeting Abstracts). 2012;120: Abstract 425. [Google Scholar]
- 85.Cancado R, Olivato MC, Bruniera P, et al. Two-year analysis of efficacy and safety of deferasirox treatment for transfusional iron overload in sickle cell anemia patients. Acta Haematol. 2012;128(2):113–118. doi: 10.1159/000338560. [DOI] [PubMed] [Google Scholar]
- 86.Steensma DP. The relevance of iron overload and the appropriateness of iron chelation therapy for patients with myelodysplastic syndromes: a dialogue and debate. Curr Hematol Malig Rep. 2011;6(2):136–144. doi: 10.1007/s11899-011-0084-z. [DOI] [PubMed] [Google Scholar]
- 87.Jaeger M, Aul C, Sohngen D, et al. Iron overload in polytransfused patients with MDS. Use of L1 for oral iron chelation. Drugs Today (Barc) 1992;28:143–147. [Google Scholar]
- 88.al-Refaie FN, Wonke B, Hoffbrand AV. Deferiprone-associated myelotoxicity. Eur J Haematol. 1994;53(5):298–301. doi: 10.1111/j.1600-0609.1994.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 89.Cermak J, Jonasova A, Vondrakova J, Cervinek L, Belohlavkova P, Neuwirtova R. A comparative study of deferasirox and deferiprone in the treatment of iron overload in patients with myelodysplastic syndromes. Leuk Res. 2013;37(12):1612–1615. doi: 10.1016/j.leukres.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 90.Cermak J, Jonasova A, Vondrakova J, et al. Efficacy and safety of administration of oral iron chelator deferiprone in patients with early myelodysplastic syndrome. Hemoglobin. 2011;35(3):217–227. doi: 10.3109/03630269.2011.578515. [DOI] [PubMed] [Google Scholar]
- 91.Bennett JM MDS Foundation’s Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83(11):858–861. doi: 10.1002/ajh.21269. [DOI] [PubMed] [Google Scholar]
- 92.Gattermann N. Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol. 2008;88(1):24–29. doi: 10.1007/s12185-008-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zipperer E, Pelz D, Nachtkamp K, et al. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica. 2009;94(5):729–732. doi: 10.3324/haematol.2008.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oliva EN, Dimitrov BD, Benedetto F, D’Angelo A, Nobile F. Hemoglobin level threshold for cardiac remodeling and quality of life in myelodysplastic syndrome. Leuk Res. 2005;29(10):1217–1219. doi: 10.1016/j.leukres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Hellström-Lindberg E, Gulbrandsen N, Lindberg G, et al. Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120(6):1037–1046. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 96.Gabrilove J, Paquette R, Lyons RM, et al. Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes. Br J Haematol. 2008;142(3):379–393. doi: 10.1111/j.1365-2141.2008.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novotna B, Bagryantseva Y, Siskova M, Neuwirtova R. Oxidative DNA damage in bone marrow cells of patients with low-risk myelodysplastic syndrome. Leuk Res. 2009;33(2):340–343. doi: 10.1016/j.leukres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Callens C, Coulon S, Naudin J, et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med. 2010;207(4):731–750. doi: 10.1084/jem.20091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jensen PD, Heickendorff L, Pedersen B, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996;94(2):288–299. doi: 10.1046/j.1365-2141.1996.d01-1795.x. [DOI] [PubMed] [Google Scholar]
- 100.Messa E, Cilloni D, Messa F, Arruga F, Roetto A, Saglio G. Deferasirox treatment improved the hemoglobin level and decreased transfusion requirements in four patients with the myelodysplastic syndrome and primary myelofibrosis. Acta Haematol. 2008;120(2):70–74. doi: 10.1159/000158631. [DOI] [PubMed] [Google Scholar]
- 101.Capalbo S, Spinosa G, Franzese MG, Palumbo G. Early deferasirox treatment in a patient with myelodysplastic syndrome results in a long-term reduction in transfusion requirements. Acta Haematol. 2009;121(1):19–20. doi: 10.1159/000209206. [DOI] [PubMed] [Google Scholar]
- 102.Gattermann N, Finelli C, Della Porta M, et al. Hematologic responses to deferasirox therapy in transfusion-dependent patients with myelodysplastic syndromes. Haematologica. 2012;97(9):1364–1371. doi: 10.3324/haematol.2011.048546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Improta S, Villa MR, Volpe A, et al. Transfusion-dependent low-risk myelodysplastic patients receiving deferasirox: Long-term follow-up. Oncol Lett. 2013;6(6):1774–1778. doi: 10.3892/ol.2013.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saigo K, Kono M, Takagi Y, et al. Deferasirox reduces oxidative stress in patients with transfusion dependency. J Clin Med Res. 2013;5(1):57–60. doi: 10.4021/jocmr1180w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364(2):146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]