Key Points
Clinical characteristics, causes of discontinuation, and outcome of patients who progress or fail ibrutinib are described.
Patients with CLL who progress early on ibrutinib therapy have poor outcomes.
Abstract
Ibrutinib is a Bruton tyrosine kinase inhibitor approved for the treatment of patients with relapsed refractory chronic lymphocytic leukemia (RR-CLL). We describe the characteristics, causes of discontinuation, and outcomes in patients who discontinued treatment with ibrutinib. One hundred twenty-seven patients were enrolled in various clinical trials of ibrutinib, with or without rituximab, at our center. Thirty-three (26%) patients have discontinued ibrutinib to date. The majority of those patients had high-risk features: 94% with unmutated immunoglobulin heavy chain variable gene rearrangement, 58% with del(17p) by fluorescence in situ hybridization, and 54% with a complex karyotype. Causes of discontinuation were disease transformation (7), progressive CLL (7), stem cell transplantation (3), adverse events (11), serious adverse events/deaths (3), and miscellaneous reasons (2). Twenty five patients (76%) died after discontinuing ibrutinib; the median overall survival was 3.1 months after discontinuation. Most patients with RR-CLL who discontinued ibrutinib early were difficult to treat and had poor outcomes.
Introduction
Bruton tyrosine kinase (BTK) is a critical enzyme in the B-cell receptor signaling pathway1 and is a novel therapeutic target in CLL.2,3 Ibrutinib (previously known as PCI-32765) is an irreversible BTK inhibitor that binds to cysteine 481 within the adenosine triphosphate binding site of the kinase domain. Ibrutinib given at a daily dose of 420 mg received an accelerated approval by the US Food and Drug Administration in February 2014 for patients with relapsed refractory chronic lymphocytic leukemia (RR-CLL) who had received 1 or more prior treatment and was recently approved as initial therapy in patients with deletion 17p. Ibrutinib alone3,4 or in combination with rituximab has demonstrated favorable results in patients with relapsed CLL and high-risk disease [eg, del(17p)].5
In a phase 3 trial,6 patients with RR-CLL were randomly assigned to either treatment with ibrutinib 420 mg orally daily (n = 195) or ofatumumab monotherapy (n = 196). As per the investigator’s assessment, the overall response rate (ORR) (complete response + partial response (PR) + PR with lymphocytosis) was 85% in the ibrutinib group vs 24% in the ofatumumab group. Ibrutinib was well tolerated. The most common adverse effects were diarrhea, fatigue, pyrexia, nausea, anemia, and neutropenia, seen in more than 20% of patients. Grade 3 to 4 hematological toxicity was comparable in the ibrutinib group vs the ofatumumab group (neutropenia, 16% vs 14%; anemia, 5% vs 8%; thrombocytopenia, 6% vs 4%). Bleeding such as petechiae or ecchymosis was more frequent in patients treated with ibrutinib (44% vs 12% in those treated with ofatumumab); however, major bleeding requiring red blood cell transfusion or hospitalization was 1% vs 2% with ibrutinib vs ofatumumab, respectively. Grade 3 nonhematologic adverse effects were diarrhea and atrial fibrillation (4% and 3% in the ibrutinib group). Of note, atrial fibrillation (any grade) occurred in 5% vs 1% of patients treated with ibrutinib vs ofatumumab. Fourteen percent (n = 28) of patients discontinued therapy after a median follow-up of 9.5 months. The most common causes of discontinuation of ibrutinib were disease progression, adverse events, deaths, patient decision, and stem cell transplant (SCT). Ibrutinib improved response rates, progression-free survival, and overall survival compared with ofatumumab in patients with RR-CLL.
In a phase 2 trial3,4 of 85 (117 in the 2013 update) patients with RR-CLL treated with single-agent ibrutinib, 36% (n = 31) of patients discontinued therapy after a median follow-up of 21 months. The most common causes of discontinuation of ibrutinib were disease progression, adverse events, patient decision, and SCT. Ibrutinib was also reported to be safe and efficacious in previously untreated patients with CLL aged 65 years or older with an ORR of 95% (8% complete response, 87% PR).7 In addition, the combination of ibrutinib with rituximab has produced positive early results with more rapid achievement of partial response compared with that seen with single-agent ibrutinib.5 In this report, we present the characteristics, causes of discontinuation, and outcome of those patients who discontinued ibrutinib.
Patients and methods
Patients with CLL (n = 127) enrolled in 4 different clinical trials of ibrutinib were included (Table 1). Patient characteristics at the time of starting ibrutinib are summarized in Table 2. Of note, 49 patients had missing karyotype data, 5 in those who discontinued (15%) and 44 in those who continued (47%); these patients were excluded from the comparison based on karyotype. Patients were treated at MD Anderson Cancer Center, Houston, Texas, from July 2010 until May 2014. Treatment protocols were approved by the institutional review board, and informed consent was obtained in accordance with the Declaration of Helsinki. These trials are registered at ClinicalTrials.gov. Patient charts were reviewed for the characteristics, causes of discontinuation, outcomes, and subsequent therapies. Kaplan-Meier survival postibrutinib was calculated from the date of discontinuing ibrutinib to the date of last follow-up. Duration of ibrutinib therapy was calculated from the date of starting ibrutinib to the date of discontinuing ibrutinib. Log-rank test was used to estimate the time to event outcomes.
Table 1.
Summary of clinical trials of ibrutinib in this analysis
| Protocol | Trial identification number | Regimen | Total number of patients enrolled | Number of patients who discontinued ibrutinib |
|---|---|---|---|---|
| 2010-0314 | NCT01105247 | Ibrutinib (RR-CLL) | 41 | 15 |
| 2011-0785 | NCT01520519 | Ibrutinib + rituximab (high-risk CLL) | 40 | 15 |
| 2012-0707 | NCT01578707 | Ibrutinib vs ofatumumab (RR-CLL) | 17 | 2 |
| 2012-0086 | NCT01752426 | Ibrutinib/heavy water | 29 | 1 |
| Total | 127 | 33 |
Table 2.
Characteristics of patients who discontinued ibrutinib
| Characteristic | Category | Overall, N = 33 |
|---|---|---|
| Age, years | Median (range) | 61 (36-83) |
| White blood cell, K/μL | Median (range) | 24 (2-323) |
| Rai stage, 3-4 | Advanced | 76% |
| CD38, >30% | High | 52% |
| Zap-70 positive | By immunohistochemistry | 70% |
| β2 M, mg/L | ≥4 mg/L | 61% |
| IGHV mutation | Unmutated | 94% |
| Fluorescence in situ hybridization category | del17p/del11q/others | 58%/18%/24% |
| Karyotype | Complex | 54% |
| Number of prior therapies | ≥3 | 45% |
| Median number of prior therapies | Median (range) | 2 (0-7) |
| Median time from diagnosis to ibrutinib, months | Median (range) | 59 (8-150) |
Results
Twenty-six percent (33/127) of patients have discontinued ibrutinib. The median age of the patients was 61 years. The majority of patients exhibited high-risk features at the time of starting ibrutinib: 25 (76%) patients had advanced Rai stage, 17 (52%) patients had overexpression of CD38, 21/30 (70%) overexpressed Zap-70, 20/33 (61%) had a Beta2 microglobin higher than 4 mg/L, 29/31 (94%) had unmutated immunoglobulin heavy chain variable gene rearrangement (IGHV), 19 patients (58%) had del(17p) by fluorescence in situ hybridization, and 15/28 (54%) had complex karyotype. The median number of prior therapies was 2 (range, 0-7), and 45% patients received 3 or more therapies before ibrutinib. The median time from diagnosis to the start of ibrutinib was 59 months (range, 8-150 months), and the median duration of ibrutinib treatment was 13 months (range, 2-39 months). Twenty-five patients died, and 8 are alive. Six were on-study deaths (Table 3). Of note, the rates of unmutated IGHV, 17p deletion, and complex karyotype for the 94 patients who did not discontinue ibrutinib therapy were 74%, 25%, and 46%, respectively. The rates of 17p deletion and unmutated IGHV were significantly higher in patients who discontinued ibrutinib therapy (P = .0005 and .02, respectively).
Table 3.
Summary of patient characteristics, causes of discontinuation, and outcomes of patients after discontinuing ibrutinib (n = 33)
| Patients | Patient characteristics | Cause of discontinuation | Outcome of patients after ibrutinib | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | Zap-70 Status | β2 M (mg/L) | IGHV mutation status | Fluorescence in situ hybridization | Number of prior therapies | Duration of ibrutinib, months | Survival status | Survival post-ibrutinib, months | |||
| Patients who transformed (n = 7) | |||||||||||
| 1‡ | 57 | + | 6.8 | UM | del11q + del13q | 5 | 13.1 | Dead | 0.0 | Histiocytic sarcoma transformation | Died on study |
| 2 | 52 | ND | 6 | UM | del13q | 4 | 4.5 | Dead | 0.9 | Richter transformation | Died |
| 3‡ | 72 | + | 4 | UM | del17p | 3 | 21.9 | Alive | 2+ | Richter transformation | On small molecule clinical trial |
| 4 | 73 | + | 12 | UM | del17p | 1 | 2 | Dead | 2.1 | Richter transformation | Chemoimmunotherapy ×1* |
| 5 | 60 | + | 10 | UM | del11q | 2 | 13.1 | Dead | 2.6 | Richter transformation | R-MP (1 course) |
| 6 | 65 | + | 2.1 | UM | del17p | 0 | 10 | Dead | 3.1 | Richter transformation | Chemoimmunotherapy* |
| 7 | 63 | + | 2 | UM | del17p + 11q + 13q | 4 | 9.4 | Dead | 15.0 | Richter transformation | Chemoimmunotherapy×1* |
| Patients who progressed not transformed (n = 7) | |||||||||||
| 1‡ | 73 | + | 4 | UM | del17p | 1 | 14.2 | Dead | 1.6 | Progressive disease | Chronic obstructive pulmonary disease, infections, no further treatment of CLL |
| 2‡ | 78 | — | 5.2 | UM | del17p | 2 | 22.5 | Alive | 1.8+ | Progressive disease | Ofatumumab |
| 3‡ | 52 | + | 3 | UM | del13q | 2 | 21.4 | Alive | 1.9+ | Progressive disease | On small molecule clinical trial |
| 4‡ | 36 | — | 5.9 | UM | del11q | 2 | 6.9 | Dead | 2.1 | Progressive disease | Infections, pneumonia, no further treatment of CLL |
| 5 | 63 | + | 5.1 | UM | del11q + 13q | 5 | 38.7 | Dead | 2.8 | Progressive disease | Pneumonia |
| 6 | 56 | + | 4.1 | ND | del17p + 13q | 2 | 33.1 | Alive | 11.5+ | Progressive disease | R-MP (4 infusions, PR), on small molecule clinical trial |
| 7 | 83 | — | 10.8 | UM | del17p, +12, 13q | 4 | 18.5 | Alive | 14.4+ | Progressive disease | Ofatumumab |
| Patients who discontinued because of stem cell transplantation (n = 3) | |||||||||||
| 1 | 61 | + | 2.3 | UM | del17p + del 11q | 3 | 11.7 | Dead | 0.1 | Stem cell transplantation | Died of graft vs host disease |
| 2‡ | 64 | — | 2.4 | UM | Negative | 3 | 12.9 | Dead | 4.2 | Stem cell transplantation | Complications of transplant |
| 3‡ | 63 | + | 5.1 | UM | Del17p | 1 | 11.9 | Alive | 11+ | Stem cell transplantation | Remission post-SCT |
| Patients who discontinued because of adverse events or sudden death (n = 14) | |||||||||||
| 1‡ | 61 | + | 5.1 | UM | Del17p | 1 | 11 | Dead | 0 | Pseudomonas sepsis and bronchiectasis | No further treatment |
| 2 | 64 | + | 10.8 | ND | Del13q | 3 | 20 | Dead | 0 | Recurrent fungal pneumonia, altered mental status | No further treatment, died on study |
| 3 | 50 | ND | 3.7 | UM | Negative | 1 | 6 | Dead | 0 | Sudden death† | Died on study |
| 4 | 64 | + | 3 | UM | Trisomy 12 | 0 | 6.2 | Dead | 0 | Suicide | Died on study |
| 5‡ | 67 | — | 2.6 | UM | Del11q | 1 | 16.4 | Dead | 0 | Sudden death† | Died on study |
| 6‡ | 76 | + | 6 | UM | Del17p | 2 | 2.1 | Dead | 0.2 | Extensive aspergillosis | Multifocal aspergillosis, no further treatment of CLL |
| 7‡ | 68 | — | 3.9 | UM | del11q | 2 | 21.5 | Alive | 0.3+ | Diarrhea, subdural hematoma, | Plan to restart ibrutinib |
| 8 | 68 | — | 6 | M | del17p + del11q + del13q | 5 | 31.9 | Dead | 1 | Diarrhea and pneumonia with DIC | No treatment |
| 9‡ | 62 | + | 5.4 | UM | Del17p | 0 | 17 | Dead | 1.8 | Recurrent ear bleeding, past history of cancer oropharynx | Died on study, but off ibrutinib |
| 10‡ | 82 | + | 4.1 | UM | Del17p | 1 | 8.4 | Dead | 4 | Comorbidities and recurrent infections, chronic obstructive pulmonary disease | No further treatment |
| 11 | 66 | ND | 4.3 | UM | del17p +12 del13q | 7 | 3.9 | Dead | 5 | Gastrointestinal bleeding, acquired von Willebrand disease, accelerated CLL | Hyper-CVAD with ofatumumab |
| 12‡ | 57 | + | 3.2 | M | Del17p | 3 | 6 | Dead | 12.8 | Recurrent oral ulcers, intolerance | OFAR1 cycle, ibrutinib restarted |
| 13 | 50 | ND | 2.2 | UM | del13q | 3 | 16.1 | Alive | 24.1+ | Diarrhea, colitis | Ofatumumab and MP, progressed with ascites and C1 OFAR-2 again on ibrutinib |
| 14 | 68 | + | 3.4 | UM | del13q | 4 | 1.9 | Dead | 41.8 | Subdural hematoma, atrial fibrillation | Metastatic cancer of jejunum |
| Patients who discontinued because of miscellaneous causes (n = 2) | |||||||||||
| 1 | 55 | ND | 5.1 | UM | del17p + del11q + del13q | 6 | 27.4 | Dead | 1.1 | Therapy-related myelodysplasia, cytopenias | Died because of therapy-related myelodysplasia |
| 2 | 54 | + | 4.2 | UM | Del17p | 2 | 17 | Dead | 1.4 | Patient choice | No further treatment |
ara-C, rituximab; CVAD, cyclophosphamide, vincristine, adriamycin (doxorubicin) and dexamethasone; DIC, disseminated intravascular coagulation; M, mutated; MP, methylprednisolone; OFAR, oxaliplatin, fludarabine; R-MP, rituximab with methylprednisolone; UM, unmutated.
Chemoimmunotherapy - OFAR- Oxaliplatin, fludarabine, ara-C, Rituximab.
Patients who are alive.
Cause of sudden death in these 2 patients was unknown (autopsies not performed), 1 patient had no prior cardiac history and another had prior hypertension but no arrhythmias.
Patients treated with ibrutinib and rituximab.
Seven patients transformed to an aggressive lymphoma: 6 had diffuse large B-cell lymphoma (Richter transformation) and 1 had a histiocytic sarcoma discovered only at autopsy. The cause of death in the patient with histiocytic sarcoma transformation was multiorgan dysfunction secondary to histiocytic sarcoma transformation. Four (58%) patients transformed within the first 12 months of starting ibrutinib. Among these 7 patients, 6 (86%) have died, and 1 patient died on study (transformation found at autopsy). One patient with Richter transformation is alive and being treated with a small molecule in a clinical trial. Among the 6 patients who died, 2 died within a month because of sepsis and disease progression without further therapy. Three died within 3 months after treatment with intensive chemoimmunotherapy (n = 2) or an investigational agent (n = 1). One patient failed chemoimmunotherapy and died in hospice after 15 months.
Seven patients progressed without transformation. Four patients are alive, and 3 patients died. Among the 3 patients who died, 2 had pneumonia and progressive disease and 1 failed to respond to further therapy. Four patients are alive: 2 are receiving investigational agents, 1 responded to ofatumumab, and 1 is not receiving therapy.
Three patients responding to ibrutinib proceeded to SCT; 2 patients have died and another is alive and in remission. Two patients died because of complications of SCT and severe graft vs host disease. Fourteen patients came off therapy because of adverse events (n = 11) or death (n = 3). Adverse events included extensive aspergillosis, diarrhea and subdural hematoma, recurrent ear bleeding, recurrent oral ulcers, subdural hematoma and atrial fibrillation, Pseudomonas sepsis, extensive fungal pneumonia, diarrhea and pneumonia, recurrent infections, gastrointestinal bleeding, and colitis (summarized in Table 3).
Two patients discontinued ibrutinib for other reasons, including therapy-related myelodysplasia (prior therapy with fludarabine and bendamustine) and financial issues.
Overall, 6 patients died on study. They received ibrutinib for a median of 12 months (range, 6-20 months). In addition to the patient dying of transformation to histiocytic sarcoma, there were 5 other deaths on study. Two patients died suddenly at home (cause of death is unknown in these 2 patients), and 1 patient committed suicide because of depression. Two patients developed severe infections.
We assessed whether there was any difference in the causes of discontinuation between patients enrolled on studies with rituximab (n = 15) vs without (n = 18). Of 15 patients treated with rituximab and ibrutinib, 2 patients discontinued because of transformation, 4 because of disease progression, 2 because of SCT, and 7 secondary to adverse events (including 1 because of suicide). There does not appear to be any significant difference in reasons for discontinuation between patients enrolled on studies with rituximab vs those without.
Of the 33 patients in this analysis, only 3 patients were previously untreated, and they discontinued ibrutinib secondary to Richter transformation, suicide, and recurrent ear bleeding.
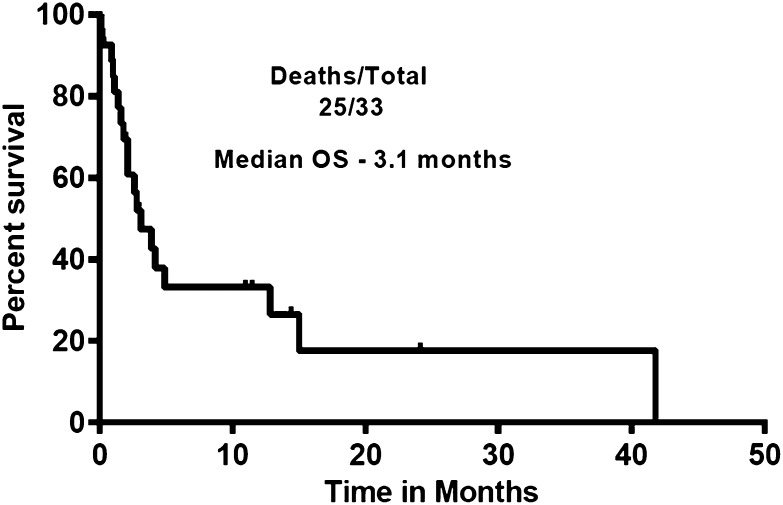
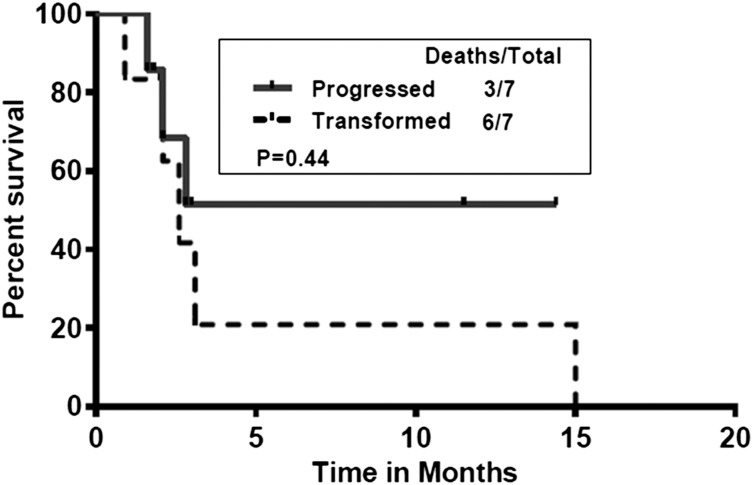
Figure 1 shows the survival of patients after discontinuing ibrutinib. The median survival for all the patients was 3.1 months and did not differ significantly between transformed and untransformed (those who progressed and those who discontinued for other reasons) patients with CLL. We also compared the postibrutinib survival of patients who transformed with those patients who progressed with CLL (Figure 2). Median survival in patients who progressed (untransformed) was not reached vs 2.6 months in those who transformed. There was no significant difference in survival (P = .44).
Figure 1.
Survival of patients after discontinuation of ibrutinib. Patients survived for a median of 3.1 months after discontinuation of ibrutinib (n = 33).
Figure 2.
Postibrutinib survival of patients who progressed (without transformation; n = 7) vs those who transformed (n = 7). Median survival in patients who progressed (untransformed) was not reached vs 2.6 months in those who transformed (P = .44).
Discussion
Our data suggest that survival of most previously treated patients who discontinued ibrutinib “early” is poor, and few salvage treatment options are available for these patients. The median duration of ibrutinib therapy before discontinuation in our study population was 13 months. In the most recent follow-up of the phase 2 study of ibrutinib, the median progression-free survival was not reached at 30 months. In our analysis, all but 3 patients relapsed before the median of 30 months, and therefore are considered “early” progressors, partly accounting for such poor outcomes.8 Another possible reason for poor salvageability of these patients after discontinuing ibrutinib could be the higher proportion of these patients having del(17p) (58% vs 25%) and unmutated IGHV (94% vs 74%) compared with the 94 patients who continued receiving ibrutinib therapy. Response rates with ibrutinib are high; however, some patients develop progressive CLL or transform when receiving ibrutinib. Many of the patients enrolled on clinical trials with ibrutinib had relapsed CLL with poor prognostic features [eg, unmutated IGHV, del(17p), multiple prior therapies, and complex karyotype]. The presence of del(17p)/TP53 mutation and complex karyotype may also contribute to the genomic instability in CLL cells, and prior therapies can promote subclonal mutations and refractory disease in patients with CLL. BTK mutations and clonal evolution may occur and produce ibrutinib resistance. Two groups have reported on acquired ibrutinib resistance and BTK mutation (C481S and phospholipase Cγ2) in patients with CLL who progressed on ibrutinib therapy.9,10 It is unclear whether BTK mutations contribute to clonal evolution and promote transformations of CLL on ibrutinib therapy. Further studies are required to characterize the BTK mutations in our cohort. Patients with relapsed refractory CLL who progress early on ibrutinib are difficult to treat and have poor outcomes.
Footnotes
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.J. and S.O. designed the study and analyzed results; P.J., M.K., W.W., B.G., D.J., J.B., and S.O. wrote and reviewed the article; M.K., J.B., W.W., N.J., A.F., Z.E., H.K., and S.O. contributed patient samples; and all authors reviewed and gave the final approval for the article.
Conflict-of-interest disclosure: S.O. and D.J. received research support from Pharmacyclics; D.J. is an employee of Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Susan O’Brien, Department of Leukemia, Unit 428, 1515 Holcombe Blvd, Houston, TX; e-mail: sobrien@mdanderson.org.
References
- 1.Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol. 2013;23(6):410–421. doi: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14(4):219–232. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien S, Furman RR, Fowler N, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Monotherapy Demonstrates Long-Term Safety and Durability Of Response In Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) Patients In An Open-Label Extension Study. Blood. 2013;122(21):4163. [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd JC, Brown JR, O'Brien S, et al. RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien S, Coutre S, Flinn I, et al. Independent evaluation of ibrutinib efficacy 3 years post-initiation of monotherapy in patients with chronic lymphocytic leukemia/small lymphocytic leukemia including deletion 17p disease. J Clin Oncol. 2014;32(5s) Abstract 7014. [Google Scholar]
- 9.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landau D, Hoellenriegel J, Sougnez C, et al. Clonal Evolution In Patients With Chronic Lymphocytic Leukemia (CLL) Developing Resistance To BTK Inhibition. Blood. 2013;122(21):866. [Google Scholar]