Heart failure is a costly and deadly disease affecting over 23 million patients worldwide1. At the core of the pathophysiology of heart failure is the inability of the adult mammalian heart to regenerate following myocardial loss, which is in marked contrast to terelost fish2–4, urodele amphibians5–8, and mammalian neonates9, 10. In mammals most of the cardiomyocytes are permanently withdrawn from cell cycle soon after birth, and despite extensive efforts to identify regulators of cardiomyocyte cell cycle in mammals11–15, the signaling cascades that activate/repress cell cycle in mammalian cardiomyocytes remain unclear.
Hippo signaling, which is an evolutionary conserved pathway that regulates cell proliferation, survival differentiation, and organ size16, has received significant attention in the field of regenerative medicine recently. Upon activation of the Hippo pathway in mice, Ste20 family kinases MST1/2 interact with scaffolding protein WW45 to phosphorylate and activate LATS1/2 kinases which in turn forms a complex with its cofactor MOB1 to phosphorylate and inactivate YAP and TAZ, the downstream transcriptional regulators of the signaling pathway. In contrast, when the Hippo pathway is inactivated, YAP and TAZ accumulate in the nucleus to interact with TEA domain (TEAD) family of proteins, along with other proteins such as SMADs, OCT4 or AMOT, to promote gene expression for cellular proliferation and organ growth17.
Recently, several studies have uncovered the importance of Hippo signaling pathway in heart development and regeneration. Knock-down of an upstream effector of Hippo cascade, Salv (WW45)18, and also forced expression of a constitutively active form of YAP (S127A in human and S112A in mouse) in the fetal heart, promoted cadiomyocyte proliferation and thickening of myocardial wall19, 20. In contrast, specific deletion of Yap in cardiomyocytes resulted in cardiac hypoplasia and lethality19, 20, demonstrating the necessity of Hippo signaling pathway in cardiomyocyte proliferation during embryonic development. The significance of Hippo pathway in postnatal cardiac homeostasis and repair has also been demonstrated where cardiomyocyte specific knockout of Yap and/or Taz results in lethal cardiomyopathy13, whereas expression of YAPS112A in mouse heart stimulated postnatal re-activation of cardiomyocyte proliferation and enhanced cardiac function in mice after myocardial infarction (MI) injury 13, 21. In addition, deletion of Salv or Lats1/2 in postnatal mice with postnatal day 7 apex resection or adult with MI promotes heart regeneration22. These results indicate that Hippo signaling is a potentially important target for promoting myocardial regeneration.
Despite these results, downstream mediators of Hippo signaling pathway that regulate cardiomyocyte proliferation are not fully understood. Previous studies using constitutively active YAP revealed that the Hippo-YAP pathway augments insulin-like growth factor (IGF) signaling, which in turn induces activation of the PI3K–AKT pathway19. Phosphorylated AKT inactivates GSK-3β by increasing its phosphorylation, leading to the stabilization of β-catenin, which in turn is required for Yap-mediated proliferation. This pathway has been well studied in a variety of disease models such cancer and diabetes23–25 26, 27.
In this issue, Lin et al28 reported the identification of one of the direct transcriptional targets of YAP, an isoform of PI3K catalytic subunit PIK3CB, that regulates cardiomyocyte proliferation as a downstream mediator of Hippo-YAP signaling. ChIP-seq analysis combined with three different systems – (1) overexpression of YAP in cardiomyocyte-like HL cells, (2) overexpression of YAP in rat neonatal ventricular cardiomyocytes (NRVMs), and (3) cardiomyocyte specific Yap homozygous knockout, identified YAP-binding sites which are significantly enriched in genes related to heart development. Among candidate YAP target genes, the authors focused on Pik3cb, the function of which has not been understood thus far. A YAP-bound region containing conserved TEAD motif, was in the first-intron of Pik3cb, through which YAP-TEAD activates transcription of Pik3cb. Importantly, the authors showed that forced-expression of PIK3CB is sufficient to activate PI3K-AKT pathway, and hence to regulate cardiomyocyte proliferation. Adenoviral transfection of Pik3cb to NRVMs, as well as in vivo overexpression of YAP driven by cTNT promoter in neonatal cardiomyocyte using AAV9, both significantly activated AKT by triggering the phosphorylation of AKT, and induced cardiomyocyte proliferation assessed by BrdU uptake and immune-staining with phosphorylated histone H3 (pH3) antibody. Mice with cardiomyocyte-specific Yap deletion showed reduced phosphorylated AKT but not total AKT, which is consistent with the findings in cancer cell lines29 and neonatal cardiomyocytes19.
Moreover, Lin et al showed that Pik3cb is necessary for Yap-mediated activation of AKT and cardiomyocyte proliferation. AAV9-mediated overexpression of YAP together with scrambled control or specific shRNA targeting Pik3cb showed that while YAP overexpression (with scrambled Pik3cb shRNA) promoted cardiomyocyte proliferation as previously described13, 19, 20, addition of Pik3cb shRNA resulted in a diminished effect of YAP overexpression on AKT phosphorylation and cardiomyocyte proliferation. Although these are convincing results, utilizing a Pik3cb knockout model would eliminate the potential off-target effects of shRNA. Finally, the authors show that AAV9-mediated overexpression of PIK3CB in the cardiomyocyte-specific Yap knockout mice induced cardiomyocyte proliferation, improved contractile function, and attenuated cardiomyocyte hypertrophy to an extent, demonstrating that Pik3cb can partially rescue the Yap knockout phenotype in cardiomyocytes. In summary, Hippo-YAP mediated activation of PI3K/AKT pathway, along with cardiomyocyte growth, is at least in part mediated by direct transcriptional activation of Pik3cb by Hippo-signaling mediator YAP/TEAD complex.
Despite these important findings, some questions remain. For example, much of the terminal effect on cell cycle is attributed to p27, however the mechanism of regulation of p27 or the effect on other CDK Inhibitors has not been fully examined. In addition, in the loss of function studies both decreased proliferation and survival are noted. However, the isolated effects on cell survival and proliferation are not clearly dissected, although admittedly this may be quite a difficult task. Finally, it would be important for future studies to examine upstream regulators of Hippo-YAP pathway, and how this pathway is regulated in the postnatal heart. Nevertheless, this report demonstrates that Pik3cb is an important link between Hippo-YAP and PI3K-AKT pathways, and brings us one step closer to an understanding of molecular mechanism regulating cardiomyocyte growth and proliferation.
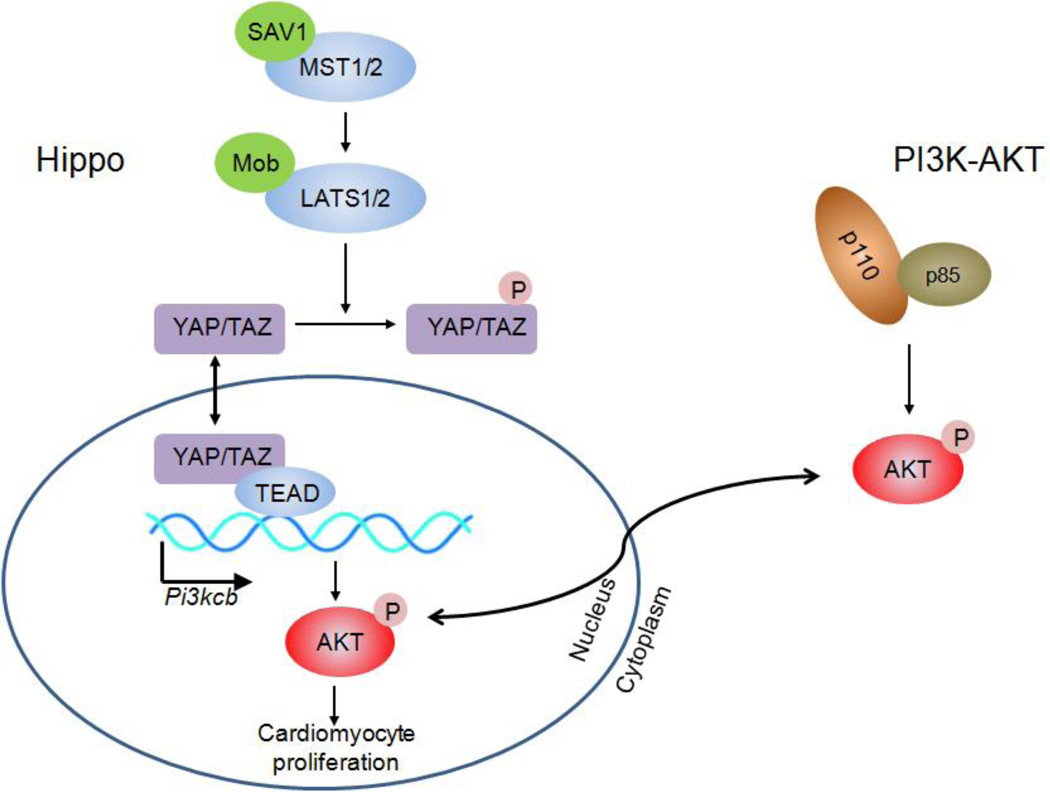
Figure 1. Hippo and PI3K-Akt signaling pathways stimulate the cardiomyocyte proliferation.
Pik3cb is a gene encoding for the catalytic subunit p110 of the Class IA PI3K. Lin et al found that Pik3cb is a direct target of YAP which links Hippo and PI3K-Akt signaling pathways to stimulate cardiomyocyte proliferation.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nature reviews. Cardiology. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science (New York, N.Y. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development (Cambridge, England) 2011;138:3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development (Cambridge, England) 2011;138:1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 5.Becker RO, Chapin S, Sherry R. Regeneration of the ventricular myocardium in amphibians. Nature. 1974;248:145–147. doi: 10.1038/248145a0. [DOI] [PubMed] [Google Scholar]
- 6.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. The Journal of experimental zoology. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 7.Neff AW, Dent AE, Armstrong JB. Heart development and regeneration in urodeles. The International journal of developmental biology. 1996;40:719–725. [PubMed] [Google Scholar]
- 8.Flink IL. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, amblystoma mexicanum: Confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anatomy and embryology. 2002;205:235–244. doi: 10.1007/s00429-002-0249-6. [DOI] [PubMed] [Google Scholar]
- 9.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, 2nd, van Rooij E, Olson EN. Mir-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circulation research. 2013;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science (New York, N.Y.) 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sdek P, Zhao P, Wang Y, Huang CJ, Ko CY, Butler PC, Weiss JN, Maclellan WR. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. The Journal of cell biology. 2011;194:407–423. doi: 10.1083/jcb.201012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R, Wang DZ. Mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circulation research. 2013;112:1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development (Cambridge, England) 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong W, Guan KL. The yap and taz transcription co-activators: Key downstream effectors of the mammalian hippo pathway. Seminars in cell & developmental biology. 2012;23:785–793. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits wnt signaling to restrain cardiomyocyte proliferation and heart size. Science (New York, N.Y.) 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size. Science signaling. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. Yap1, the nuclear target of hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific yap activation improves cardiac function and survival in an experimental murine mi model. Circulation research. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development (Cambridge, England) 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Molecular cell. 2005;20:33–44. doi: 10.1016/j.molcel.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Sabatini DM. Mtor and cancer: Insights into a complex relationship. Nature reviews. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 25.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin d1 proteolysis and subcellular localization. Genes & development. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science (New York, N.Y.) 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 27.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase b kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase b. Science (New York, N.Y.) 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 28.Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, Guo H, van Gorp PR, Wang DZ, Pu WT. Pi3kcb links hippo-yap and pi3k-akt signaling pathways to promote cardiomyocyte proliferation and survival. Circulation research. 2014;116:xxx–xxx. doi: 10.1161/CIRCRESAHA.115.304457. [in this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W. Cell-cycle-regulated activation of akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]