Abstract
Staphylococcus aureus is the most prevalent etiologic agent of sepsis. Statins, primarily prescribed for their cholesterol-lowering capabilities, may be beneficial for treating sepsis due to their anti-inflammatory properties. This study examined the effect of low dose, short term simvastatin pretreatment in conjunction with antibiotic treatment on host survival and demonstrated that pretreatment with simvastatin increased survival of C57BL/6 mice in response to S. aureus infection. In vitro studies revealed that short term simvastatin pretreatment did not reduce S. aureus-stimulated expression of surface proteins necessary for macrophage presentation of antigen to T cells, such as MHC Class II and co-stimulatory molecules CD80 and CD86, but did reduce both basal and S. aureus-stimulated levels of C5aR. Additionally, this work demonstrated the ability of simvastatin to dampen macrophage responses initiated not only by bacteria directly but by membrane vesicles shed in response to infection, revealing a new mechanism of immune modulation by statins. These data demonstrate the ability of short term simvastatin pretreatment to modulate immune responses and identify new insights into the underlying mechanisms of the anti-inflammatory properties of simvastatin that may decrease the pathophysiological effects leading to sepsis.
Keywords: COMPLEMENT, INFLAMMATION, MACROPHAGE, MEMBRANE VESICLE, SEPSIS, SIMVASTATIN, STAPHYLOCOCCUS AUREUS
Introduction
Invasion of host tissue by a pathogen results in the activation of a variety of innate immune responses. Complement is a collection of serum proteins that are activated in response to pathogens and serve to contain and destroy the pathogen as well as initiate and aid innate immune responses [1]. Upon pathogen encounter, tissue macrophages are activated, increasing the expression of proteins on their cell surface, production of cytokines, phagocytic ability, and killing mechanisms. Furthermore, activated macrophages initiate and coordinate an inflammatory response that induces local vascular changes, allowing fluid, proteins, and cells to accumulate at the site of infection to contain and eliminate the pathogen [2]. In addition to the activities of complement and macrophages, there is increasing evidence of membrane vesicles (microparticles, microvesicles, ectosomes, exosomes) in regulating immune processes. Membrane vesicles are released from a variety of cell types, which can be enhanced during infection. These vesicles have immunostimulatory and immunosuppressive abilities, regulating the activation and function of immune cells and subsequently influencing inflammatory immunity [3–5]. Despite the multitude of innate immune responses, not all pathogens are contained locally. The spread of a pathogen results in a systemic inflammatory response, referred to as sepsis [2].
S. aureus is a Gram-positive, opportunistic pathogen that can cause a range of health conditions, from minor skin infections to more serious conditions including pneumonia, toxic shock syndrome, and meningitis. In the United States, S. aureus is the most prevalent bacterial pathogen causing infections in hospital inpatients and is the second leading cause of bacterial infections in outpatients [6]. Specifically, S. aureus is the most prominent pathogen causing bloodstream infections that result in sepsis [7, 8], and bloodstream infections caused by S. aureus are associated with the highest mortality [9].
The increasing frequency of S. aureus infections is primarily attributed to increases in the use of invasive procedures, and numbers of immunocompromised patients [10–12]. Current antibiotic therapies are increasingly ineffective for treating S. aureus infections, prompting a need to develop alternative and adjunctive therapies. Recently, statins have been shown to have beneficial effects on cardiovascular processes, independent of lowering cholesterol, and these benefits extend to effects on inflammatory diseases, highlighting the potential anti-inflammatory properties of statin drugs [13]. There is mounting evidence for the prospective usefulness of statin drugs in a variety of inflammation-driven diseases. Recent studies have demonstrated a role for statin therapy in the treatment of sepsis. LPS-induced sepsis in a murine model demonstrated that pretreatment with cerivastatin increased survival [14]. Similarly, mice treated with cerivastatin 24 hours pre- and up to 72 hours post- LPS or live bacterial challenge were protected from sepsis-related death [15]. Furthermore, mice pretreated with simvastatin had improved survival in response to sepsis induced via cecal ligation and perforation (CLP) [16], and simvastatin treatment prior to CLP induction of sepsis in a mouse burn model increased survival [17]. Low-dose, long- term simvastatin treatment also has been found to increase survival in a murine model of S. aureus-induced sepsis [18]. Several clinical studies provide further evidence of statins as possible effective treatments for sepsis and support the findings from studies using mouse models [13]. Taken together, the results from these studies demonstrate the potential for statins as an effective treatment for sepsis. However, the data are not fully conclusive and a better understanding of how statins achieve these beneficial effects through their modulation of immune responses is necessary. Toward this end, simvastatin was examined in a murine model of systemic infection to assess effects on immune responses and survivability and macrophage-mediated innate immune responses elicited by S. aureus infection were assessed in vitro.
Materials and methods
Cell culture
HUVEC (human umbilical vein endothelial cells, Life Technologies, Carlsbad, CA) were grown in filter-sterilized M200 (Life Technologies ) antibiotic-free media (M200 supplemented with low serum growth supplement (Life Technologies ) at 37°C in 5% CO2. RAW 264.7 macrophages (American Type Culture Collection, Manassas, VA) were grown in filter-sterilized DMEM (Lonza, Allendale, NJ ) antibiotic-free media supplemented with 1% v/v L-glutamine (BioWhittaker) and 10% v/v FBS (Atlanta Biologicals, Lawrenceville, GA) or RPMI-1640 (BioWhittaker) supplemented with 1% v/v L-glutamine (BioWhittaker) and 10% v/v FBS (Atlanta Biologicals) at 37°C in 5% CO2.
S. aureus preparation
Methicillin-sensitive Staphylococcus aureus (MSSA) (#29213, ATCC) were cultured in tryptic soy broth (TSB) (Sigma-Aldrich, St. Louis, MO) at 37°C shaking at 200 rpm. Bacteria were washed and diluted to 3×108 bacteria/mL. This bacterial strain was selected based upon extensive characterization of the inhibition of invasiveness by simvastatin [20].
Mice and infection model
All protocols were approved by the Ball State University Animal Care and Use Committee prior to initiation of each study. C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were housed individually in filtered cages. Mice were separated into groups based on treatments with no fewer than three mice per group. Based on average body weight of males and females separately for each study, a stock of 10 mg/ml of simvastatin (Calbiochem [EMD Chemicals], Gibbstown, NJ), dissolved in ethanol, was diluted with saline for a final dosage of 1000 ng simvastatin per gram of mouse body weight and a total injection volume of 100 µL. For each study, mice in experimental groups received simvastatin 18 and 3 hours prior to S. aureus infection via intraperitoneal (i.p.) injection. Control groups were given 1% ethanol in saline as a vehicle control. Mice were inoculated with S. aureus at 1× 107 colony forming units (cfu) by i.p. in 1 mL of 5% mucin (BD, Franklin Lakes, NJ) diluted in 0.85% sterile saline. Based on average weight, 10 mg of gentamicin (Sigma-Aldrich) per kg of mouse body weight was administered in saline in a final volume of 100 µL. The dosage for gentamicin was at the ED30 (effective dose for 30% survival). This dosage was used in order to detect augmented protection by simvastatin, if it existed. Gentamicin was used because it is poorly permeable to host cell membranes and was previously used in the in vitro studies to determine inhibition of invasiveness with this strain of S. aureus [20]. All mice received gentamicin via i.p. injection at 3, 6, 12, 24, and 48 hours post-infection.
Analysis of S. aureus in the blood
Mice were euthanized at 24 or 48 hours post-infection via pentobarbital injection (5 mg in saline) and blood was collected via cardiac puncture. Whole blood (100 µL) was plated on pre-warmed tryptic soy agar (TSA) (Sigma-Aldrich) supplemented with 7.5% sodium chloride for selective growth of S. aureus. Following overnight incubation of plates at 37°C, cfu were counted.
S. aureus invasion assay
S. aureus was inoculated from glycerol stock in tryptic soy broth (Sigma-Aldrich) and subcultured daily for 2 days prior to running the assay (200 rpm, 37°C, 24 h). On the day of the assay, bacteria were pelleted (10000 rpm, 37°C, 3 min), washed in saline, pelleted as above, resuspended in saline, then fluorescently labeled by incubation with rabbit anti-mouse IgG Alexa Fluor 488 (final concentration 8 µg/ml, RT, 20 minutes, Invitrogen). Protein A, a S. aureus cell surface protein, avidly binds IgG thereby labeling the bacteria. Labeled bacteria were washed twice as above and resuspended to 2.3×108 cfu/ml in saline. Cell cultures of THP-1 (ATCC, TIB-202) were collected and resuspended to a cell density of 3 × 105 cells/ml in 35 mm dishes in RPMI-1640 (ATCC, 30-2001) supplemented with 10% fetal bovine serum (Atlanta Biologicals)/0.1% 2-mercaptoethanol (Life Technologies). Following incubation with simvastatin (Calbiochem [EMD Chemicals]) or DMSO (Thermo Fisher Scientific) as the negative control (37°C, 5% CO2, 20 h), host invasion was initiated by addition of 488-labeled S. aureus at a multiplicity of infection of 30 (37°C, 5% CO2, 1 h). Invasion was stopped by placing plates at 4°C (10 minutes). Extracellular bacteria were labeled by incubation with anti-Staph polyclonal antibody, raised against soluble and structural antigens to S. aureus, (final concentration 16 µg/ml, 4°C, 40 min, Meridian Life Science, Saco, ME) followed by incubation with goat anti-rabbit 555 (final concentration 8 µg/ml, 4°C, 40 min, Life Technologies). Cells were fixed in a final concentration of 1.5% formaldehyde and samples read using the Accuri C6 cytometer (BD, San Jose, CA).
Membrane vesicle preparation and cellular stimulation
Tissue culture plates (100 mm and 35 mm) were treated with Attachment Factor (Life Technologies). HUVEC (1.2×106) were seeded on 100 mm plates, and RAW cells were seeded at 2.0×105 cells on 35 mm plates. Cells were allowed to grow overnight at 37°C at 5% CO2. HUVEC and RAW cells were then either treated with 1 μM simvastatin (Calbiochem [EMD Chemicals]) or 1 μM DMSO (Thermo Fisher Scientific) overnight. All HUVEC plates were infected (post-simvastatin or DMSO treatment) for 2 hours with 6×108 bacteria (MOI of 300) in saline to induce microvesicle formation due to MSSA infection. The HUVEC supernatant was removed from the plates and centrifuged at 1500g in 15 ml conical tubes for 10 minutes at 10°C to remove HUVEC fraction. Supernatant was incubated with 80 µg/mL gentamicin (Sigma-Aldrich), and 32 µg/mL lysostaphin (Sigma-Aldrich) for 40 minutes at 37°C in 5% CO2, mixing every 10 minutes (previously determined to rid microvesicle preparation of MSSA). Tubes were centrifuged at 17000g for 30 minutes at 4°C, and the supernatant was completely removed. One mL of cold RAW media was used to resuspend the microvesicle pellet, and 200 µL of the microvesicle preparation was added to the corresponding simvastatin or DMSO treated RAW plates and incubated at 37°C in 5% CO2. Following overnight incubation, supernatant and RAW cells were harvested for analysis. An additional 200 µL of the microvesicle preparation from simvastatin and DMSO groups was diluted in 2 ml cold TSB and spread on a TSA plate to confirm no MSSA remained in the supernatant.
S. aureus infection of RAW macrophages
RAW cells were plated onto 35mm plates at 2.5 X 105 cells/ml in supplemented RPMI (BioWhittaker). Cells were pre-treated with 1 µM simvastatin (Calbiochem [EMD Chemicals]) or DMSO (Thermo Fisher Scientific) for 18–24 hours prior to infection. Each plate received either 1 X 108 bacteria (MOI 400) or an equal volume of 0.85% saline and incubated at 37°C and 5% CO2. After one hour, media from each plate was replaced with media containing 50 μg/ml Gentamicin (Sigma-Aldrich) and 20 μg/ml Lysostaphin (Sigma-Aldrich) and incubated for 12–24 hours before analysis of cell surface marker expression by flow cytometry (described below).
Flow cytometry
RAW macrophages (1×106 cells per sample), stimulated by microvesicle treatment or infected with S. aureus as described above, were washed and stained in FACS buffer (PBS containing 2% BSA and 0.1% NaN3). Cells were first incubated with diluted normal rat serum (Jackson ImmunoResearch, West Grove, PA) for 5 minutes at 4 °C then washed once in FACS buffer. Samples were stained with antibodies (MHC Class II, CD80, CD86, CD40, and C5aR) conjugated to FITC, PE, CyChrome, or allophycocyanin (eBioscience, San Diego, CA). Staining was performed at 4°C for 15 minutes. Cells were washed and then fixed in FACS buffer containing 0.5% formaldehyde. Samples were analyzed using an Accuri C6 flow cytometer. Mean fluorescence intensity was recorded and normalized to the background fluorescence intensity of the negative control.
Enzyme-linked immunosorbant assay (ELISA)
Levels of TNF-alpha present in the supernatants of microvesicle-treated RAW cells were detected using a TNF-alpha ELISA kit (eBioscience). The procedure was performed according to the manufacturer’s guidelines. Samples were analyzed in duplicate using a microplate reader (Model 680, Bio-Rad).
Statistical analyses
Results are represented as mean +/− standard error of the mean (SEM). For the survival studies, statistical differences were assessed by the Kaplan-Meier Log-Rank test. For flow cytometric analysis of cell surface marker expression on macrophages, one way ANOVA was performed followed by Student Neuman-Keuls post-hoc analysis. For the bacterial clearance assays, the Mann-Whitney Rank Sum test was performed. Student's t-test was used for assessing all other assays. Differences between groups were considered statistically significant at p ≤ 0.05.
Results
Simvastatin pretreatment increases survival of mice infected with S. aureus
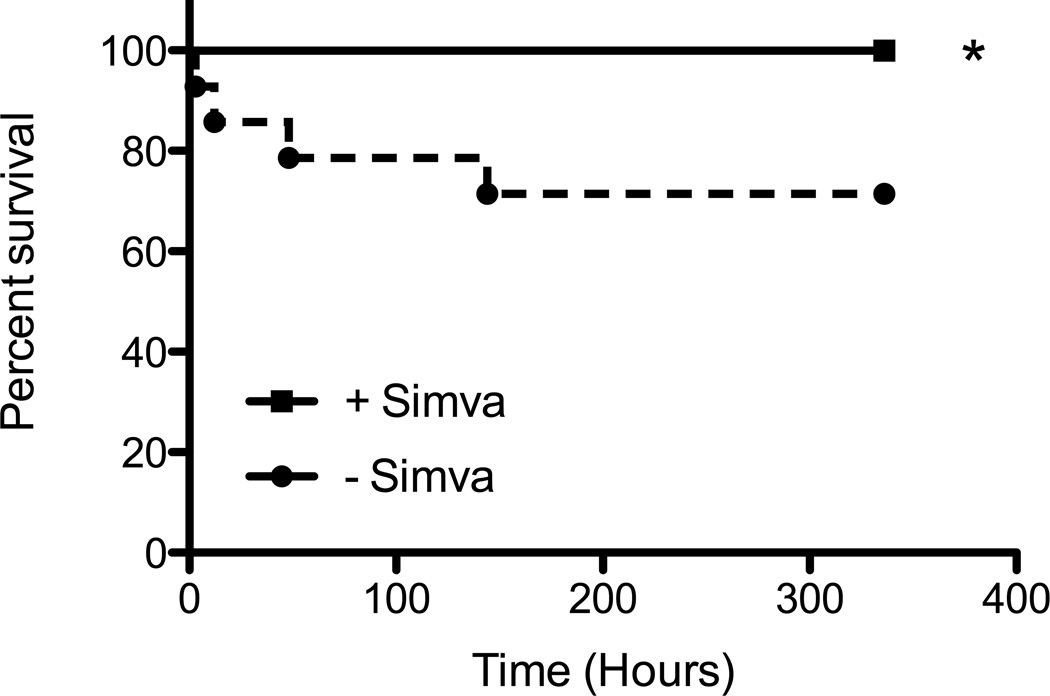
To investigate the effect of low dose (below the predicted range of therapeutic dosage [21]) and short term (less than 24 hours) simvastatin pretreatment on the induction and progression of sepsis due to S. aureus infection, C57BL/6 mice were pretreated with simvastatin or vehicle control prior to infection with S. aureus. It has been recently reported that the resistance of C57BL/6 mice to S. aureus infection is dependent upon innate immune mechanisms, due in part to their ability to efficiently recruit neutrophils to the site of infection [19]. Furthermore, strains of mice that are highly susceptible to S. aureus infection, such as Balb/c, demonstrate impaired neutrophil recruitment that prevents them from quickly mobilizing an effective innate response to control the infection [19]. Thus, C57BL/6 mice were used for this survival study to examine the ability of simvastatin to affect immune responses under normal innate immune conditions. Antibiotic treatment at the ED30 was given post-infection to examine whether simvastatin augmented the antibiotic therapy. Survival was assessed for 14 days and demonstrated that mice pretreated with simvastatin prior to infection with S. aureus had increased survival compared to control mice (Figure 1).
Figure 1. Simvastatin pretreatment increases survival of mice infected with S. aureus.
Male and female mice were pretreated with simvastatin (+Simva) or vehicle control (−Simva) 18 and 3 hours prior to infection with S. aureus, treated with gentamicin 3, 6, 12, 24, and 48 hours post-infection and observed for 14 days. The graph represents individual data points combined from 3 replicate studies (n=13–14/group). * p ≤ 0.05 by Kaplan-Meier Log Rank analysis.
Clearance of S. aureus from the blood is not enhanced by simvastatin pretreatment
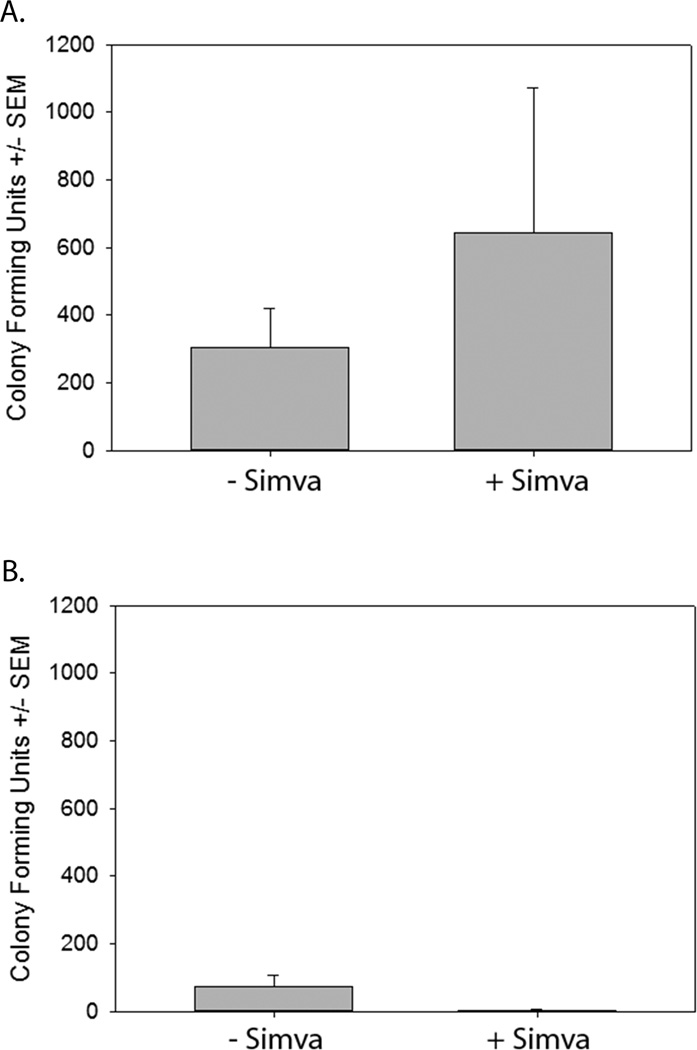
At higher doses, simvastatin has been reported to have anti-microbial effects on S. aureus in vitro [22] and in vivo higher dosage [23] or longer duration [18] improved clearance. To investigate the possibility that short term, low-dose simvastatin treatment may enhance the clearance of S. aureus, levels of S. aureus in the blood were examined at 24 and 48 hours post-infection in mice pretreated with simvastatin or vehicle control. Twenty-four hours post-infection, no significant difference in numbers of bacteria in the blood between simvastatin pretreated and vehicle pretreated mice was observed (Figure 2a), and by 48 hours, almost all of the bacteria were cleared from the blood in both treatment groups (Figure 2b). These data demonstrate that the increased survival to severe S. aureus infection of simvastatin-treated mice is independent of bacterial clearance.
Figure 2. Clearance of S. aureus from the blood is not enhanced by simvastatin pretreatment.
Mice were pretreated with simvastatin (+Simva) or vehicle control (−Simva) 18 and 3 hours prior to infection with S. aureus and treated with gentamicin 3, 6, 12, 24, and 48 hours post-infection. Whole blood was isolated 24 hours (a) and 48 hours (b) post infection and plated on tryptic soy agar. Data were pooled from 2 replicate experiments (n=5–10/group) and analyzed using the Mann-Whitney rank sum test.
Macrophage functions are altered in response to short term simvastatin pretreatment
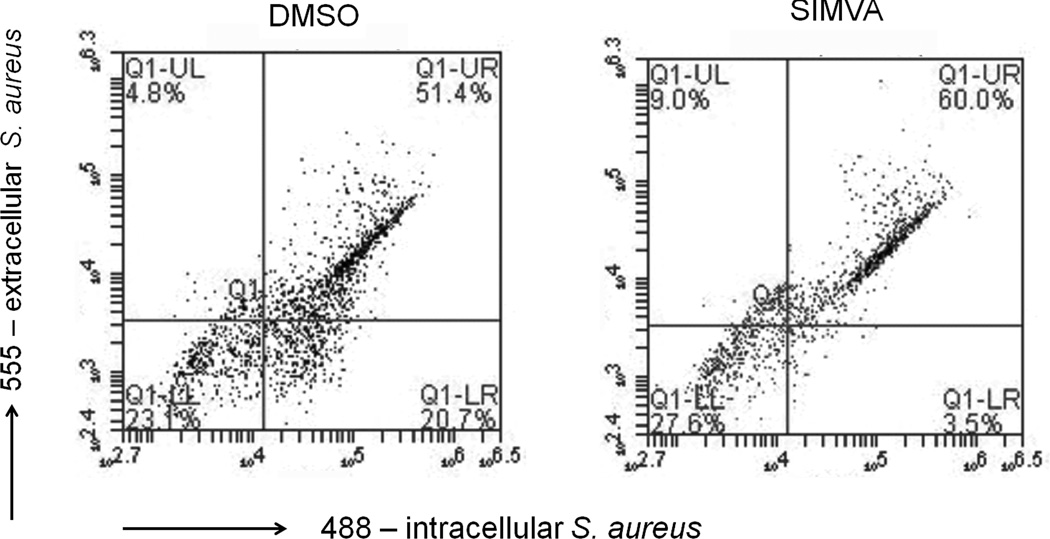
Macrophages are important orchestrators of inflammatory reactions and thus modulation of macrophage activation and function may affect the initiation and progression of sepsis and subsequent survival of the host to infection [2]. To investigate the ability of short term simvastatin pretreatment to modulate macrophage activities in response to S. aureus infection, macrophage activities were investigated in vitro. Following pathogen invasion of host tissue, macrophages are stimulated to ingest invading pathogens. Previous reports examining the effects of statin treatments on the phagocytic abilities of monocytes and macrophages have demonstrated the ability of statins to both enhance [24, 25] and inhibit [26, 27] phagocytosis. To examine the effects of short term simvastatin pretreatment on the phagocytic activity of macrophages in response to S. aureus infection, macrophages were pretreated with simvastatin or vehicle control and infected with Alexa Fluor 488-labeled S. aureus. Following invasion, extracellular bacteria were made distinguishable from intracellular bacteria by post-labeling of extracellular bacteria with an antibody to soluble and structural antigens to S. aureus raised in rabbit -followed by anti-rabbit Alexa Fluor 555. Macrophages were then analyzed by flow cytometry to assess bacterial internalization (Figure 3). The results demonstrated that macrophages pretreated with simvastatin had reduced S. aureus invasion compared to vehicle control treated cells.
Figure 3. Simvastatin inhibits S. aureus host cell invasion.
THP-1 were pretreated with 10 µM simvastatin (SIMVA) or dimethyl sulfoxide (DMSO) for 20 hours followed by invasion by Alexa Fluor 488-labeled S. aureus (1 hour). Following invasion, extracellular bacteria are made distinguishable from intracellular bacteria by post-labeling extracellular bacteria with anti-S. aureus followed by anti-rabbit Alexa Fluor 555. Lower right quadrant indicates intracellular, 488-labeled bacteria. Data is representative of 3 experiments.
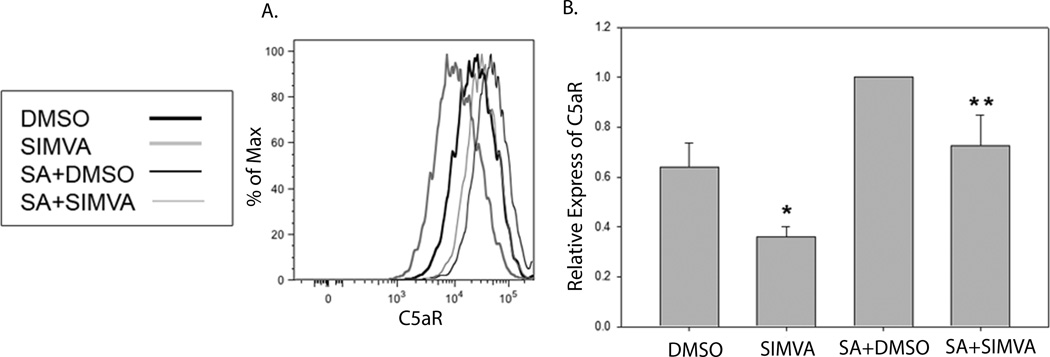
Pathogen invasion not only enhances phagocytosis by macrophages, but also induces the production of a large amount of pro-inflammatory cytokines, which can be further enhanced by the presence of the complement protein C5a. C5aR expression is enhanced on macrophages in response to pathogen invasion and its binding of C5a augments the production of pro-inflammatory cytokines by macrophages, contributing to a septic pathology [28]. To investigate the effect of short term simvastatin pretreatment on C5aR expression, simvastatin or control treated RAW macrophages, mock-infected or infected with S. aureus, were examined by flow cytometry (Figure 4). Simvastatin pretreatment resulted in lower basal expression of C5aR on mock-infected macrophages and decreased C5aR expression stimulated by S. aureus infection compared to controls. These data suggest that simvastatin may modulate complement activity stimulated by S. aureus infection by down-regulating the expression of complement receptors, decreasing the binding of complement proteins and subsequently decreasing the activation of cells such as macrophages.
Figure 4. Simvastatin pretreatment reduces C5aR expression on macrophages.
RAW cells were treated with 1 µM simvastatin (SIMVA) or dimethyl sulfoxide (DMSO) 18–20 hours prior to infection with S. aureus for 1 hour (SA+DMSO and SA+ SIMVA). The infection was stopped and cells were analyzed 18 hours post-infection for expression of C5aR. Histograms (a) are from one experiment that is representative of 3 total experiments. Normalized mean fluorescence intensity (b) was pooled from all experiments and analyzed by one-way ANOVA followed by Student Neuman-Keuls post-hoc analysis. *p ≤ 0.05 compared to DMSO. **p ≤ 0.05 compared to SA+DMSO.
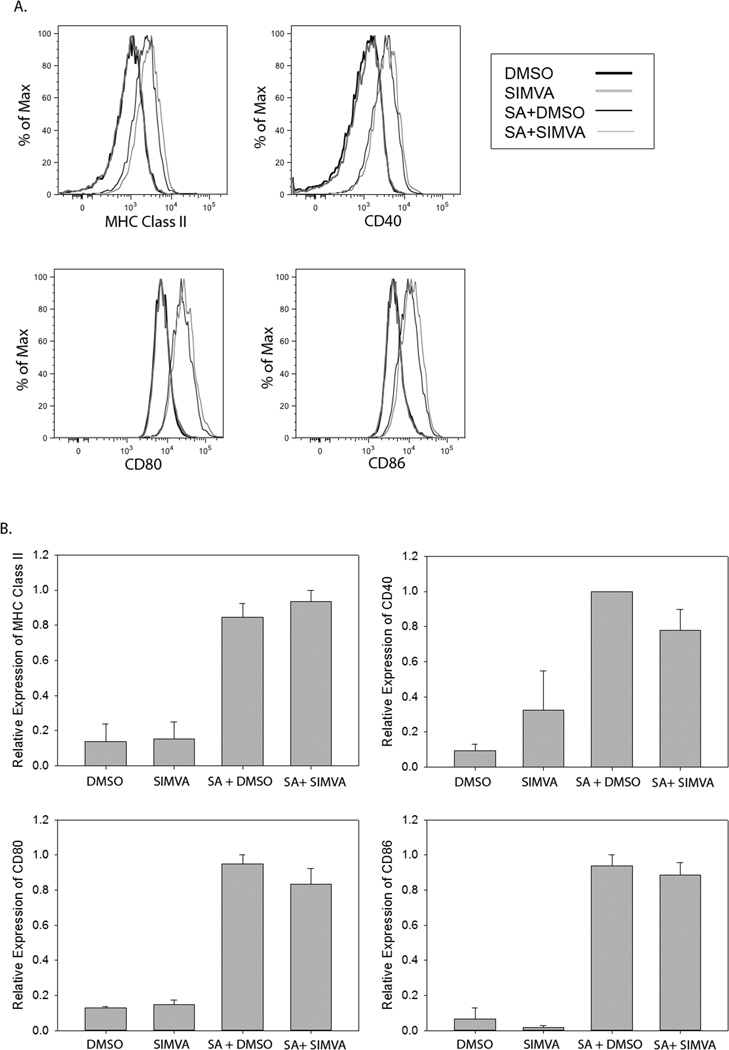
In human macrophage cell lines, simultaneous treatment with simvastatin and IFN-gamma results in decreased expression of activation markers such as MHC Class II, CD40, CD80, and CD86 compared to IFN-gamma-stimulated controls [29]. To investigate the effect of short term simvastatin pretreatment on gene expression in macrophages, the expression of cell surface activation markers on simvastatin or control treated RAW macrophages mock-infected or infected with S. aureus was examined by flow cytometry (Figure 5). Short term simvastatin pretreatment did not reduce the expression of S. aureus-stimulated activation marker expression on RAW macrophages nor alter the basal expression level of the cell surface proteins. Taken together these data suggest that short term simvastatin pretreatment is sufficient to decrease phagocytic activity and subsequent uptake of bacterial pathogens as well as complement receptor expression but does not affect the expression of cell surface proteins indicative of macrophage activation.
Figure 5. Simvastatin does not alter the expression of S. aureus-induced surface proteins on macrophages.
RAW cells were treated with 1 µM simvastatin (SIMVA) or dimethyl sulfoxide (DMSO) 18–20 hours prior to infection with S. aureus for 1 hour (SA+DMSO and SA+ SIMVA). The infection was stopped and cells were analyzed 18 hours post-infection for expression of MHC Class II, CD40, CD80, and CD86. Histograms (a) are from one experiment that is representative of 3 total experiments. Normalized mean fluorescence intensity (b) was pooled from all experiments and analyzed by one-way ANOVA followed by Student Neuman-Keuls post-hoc analysis.
Macrophage activation stimulated by membrane vesicles from S. aureus-infected cells is dampened by short term simvastatin pretreatment
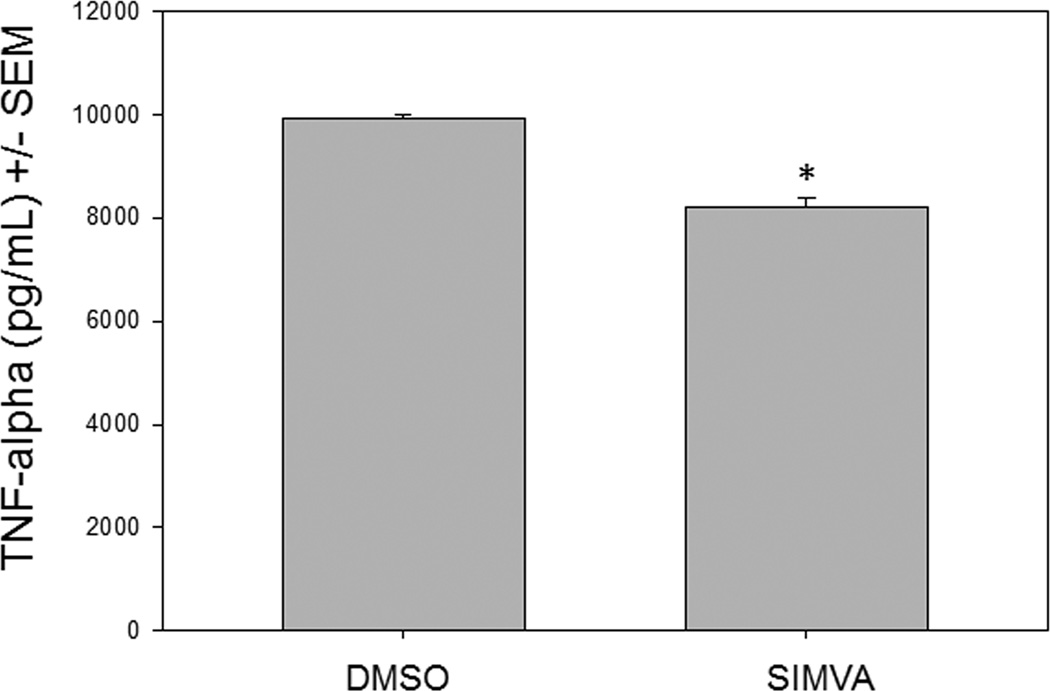
Recent studies have demonstrated that the membrane vesicles, released from a variety of different cells, interact with immune cells and can modulate immune responses [4]. Specifically, membrane vesicles released from infected macrophages can interact with uninfected macrophages and induce a pro-inflammatory response [30]. S. aureus invasion of host tissue results in its uptake by immune and non-immune cells, and recently pretreatment with simvastatin has been shown to inhibit S. aureus internalization by endothelial cells [20]. To investigate the influence of short term simvastatin pretreatment on membrane vesicle release by infected non-immune cells and subsequent activation of macrophages, simvastatin or control treated HUVEC were infected with S. aureus and membrane particles were harvested from the supernatant. Simvastatin or vehicle control treated RAW macrophages were then incubated with the isolated membrane particles for 24 hours and levels of TNF-alpha were analyzed by ELISA (Figure 6). Simvastatin pretreatment of HUVEC and RAW macrophages resulted in decreased TNF-alpha production compared to controls, demonstrating the ability of short term simvastatin pretreatment to modulate the stimulating activity of membrane vesicle transfer to immune cells, resulting in dampened macrophage activation.
Figure 6. Macrophage activation stimulated by membrane vesicles (MV) from S. aureus-infected cells is dampened by simvastatin pretreatment.
RAW cells were pretreated with vehicle control (DMSO) or with1 µM simvastatin (SIMVA) for 20 hours, and then incubated for 2 hours with MV harvested from pretreated, infected human umbilical vein endothelial cells. TNF-alpha was measured at 24 hours by ELISA. *p ≤ 0.05 by Student’s t-test.
Discussion
This study demonstrated the ability of low dose, short term simvastatin pretreatment to enhance survival to S. aureus infection and highlighted novel mechanisms by which short term simvastatin pretreatment modulates macrophage-mediated innate immune responses to S. aureus. The beneficial effect of statin treatment on the ability of mice to survive sepsis-related death due to S. aureus infection has been investigated previously [15]. The findings from our work compliment the previous study by demonstrating the ability of simvastatin as only a short term pretreatment to induce systemic changes prior to S. aureus exposure that result in enhanced survivability following infection. In the previous study, cerivastatin was administered to Balb/c mice 24 hours prior to challenge with S. aureus and treatments continued for 72 hours post-infection. In contrast, our findings demonstrate that two doses of simvastatin (below the range of therapeutic dosage) administered to C57BL/6 mice within 24 hours prior to S. aureus challenge with no continuing treatments is sufficient to increase survival. Additionally, this previous study did not incorporate antibiotics into the study design. This study administered gentamicin at the ED30 for 48 hours post-infection to examine if antibiotic efficacy could be enhanced by simvastatin treatment. Possibly, the failure to observe a difference in bacterial clearance in our study (Figure 2) is due to the presence of antibiotics, lending further support that our design revealed a role for immunomodulation over and above clearance.
Our in vitro investigations examining macrophage-mediated innate immune mechanisms demonstrated novel mechanisms by which simvastatin may down-regulate hyper-inflammatory reactions that contribute to a septic pathology; through regulation of membrane vesicle activity and complement receptor surface expression. Our finding that simvastatin pretreatment did not reduce S. aureus-stimulated expression of surface proteins necessary for macrophage presentation of antigen to T cells (Figure 5), but did reduce both basal and S. aureus-stimulated levels of C5aR (Figure 4), which is a stimulator of macrophage inflammatory activities when engaged, demonstrates specificity and selectiveness by simvastatin to modulate these immune responses. This finding supports a previous report examining the effects of simvastatin on macrophages stimulated with opsonized S. aureus. Simvastatin impaired the bactericidal response (phagocytosis and oxidative burst) of macrophages to IgG-coated S. aureus and enhanced the production of inflammatory mediators such as TNF-alpha through a mechanism dependent upon FcgammaR since non-opsonized S.aureus did not elicit enhanced production of inflammatory mediators [31]. Our findings, together with Benati et al., highlight the ability of simvastatin to differentially regulate gene expression and cellular functions when encountering opsonized and non-opsonized S. aureus and demonstrate an important selective property of simvastatin that may be a necessary aspect of its anti-inflammatory properties and ability to increase survival of mice infected with S. aureus.
Interestingly, in our mouse model, C5a serum levels were not different between control and simvastatin-pretreated mice (data not shown), which would suggest that this aspect of complement activity is unaffected by simvastatin. Indeed, reports examining the effects of statin treatments on complement activity and complement-mediated inflammation in a variety of in vivo models have been conflicting [32–39]. However, our finding that simvastatin treatment reduced basal and S. aureus-stimulated levels of C5aR on macrophages in vitro suggests modulation of complement activity by simvastatin is a result of alterations in receptor expression, and potentially provides an explanation for the conflicting reports found in the literature While it has been previously shown that simvastatin treatment impairs surface protein recycling [40], the demonstration of this ability applied to surface receptors intimately involved in the pathogenesis of sepsis is novel and highlights a potential mechanism through which simvastatin mediates its anti-inflammatory effects.
Importantly, our work has revealed a unique mechanism through which simvastatin regulates macrophage activation. Recent work indicated that membrane vesicles released in response to bacterial infection stimulate an inflammatory response in uninfected, recipient macrophages [30]. Therefore, we investigated whether this inflammatory response would be dampened by simvastatin treatment of the infected host cells and of the recipient, non-infected macrophage and found that simvastatin attenuated the robust pro-inflammatory response of recipient macrophages. This finding is in contrast to previous work demonstrating simvastatin’s ability to enhance the production of pro-inflammatory mediators [31], while seemingly contrasting, the two studies actually provide more evidence for the very specific affects that simvastatin exerts on cellular functions such that the previous work was examining FcgammaR-stimulated functions, while our study examined membrane vesicle-stimulated functions. Potentially, simvastatin’s specific dampening of the early macrophage response to not only bacterial invasion but also to membrane vesicles is a modulatory mechanism regulating the hyper-inflammatory status that is the hallmark of sepsis.
Conclusion
This study identified the ability of short term simvastatin pretreatment to differentially regulate macrophage functions: dampening activities that increase macrophage activation, such as phagocytosis, bacterial uptake, C5aR expression, and sensitivity to membrane vesicles, while leaving functions necessary for the stimulation of adaptive responses unaffected. Additionally, our finding that pro-inflammatory, macrophage-mediated responses were initiated not only by bacteria directly but by membrane vesicles shed in response to infection reveal that the immunomodulatory role of statins during infection extends beyond direct interaction with bacterial pathogens, potentially dampening the overall hyper-responsiveness through multiple mechanisms at the level of the host and potentially decreasing the pathophysiological effects leading to sepsis. Taken together these data identify new insights into the underlying mechanisms of the anti-inflammatory properties of simvastatin and highlight its ability to be a very specific immunomodulatory agent.
Acknowledgments
We thank Mark Kaplan and Jennifer Metzler for thorough review of the manuscript. This work was supported by funding from the National Institutes of Health, 1R15HL092504-01 (SAM), the Indiana Academy of Science (HAB), and the Ball State University Department of Biology and Sponsored Programs Office (HAB and SAM).
References
- 1.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41(11):1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101(3):439–451. [PubMed] [Google Scholar]
- 4.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 5.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13(1):1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 6.Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diekema D, Pfaller M, Schmitz F, Smayevsky J, Bell J, Jones R, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical Infectious Diseases. 2001;32(Suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 8.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 9.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34(10):2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 10.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293(24):3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 11.Shorr AF. Epidemiology of staphylococcal resistance. Clin Infect Dis. 2007;45(Suppl 3):S171–S176. doi: 10.1086/519473. [DOI] [PubMed] [Google Scholar]
- 12.Lowy F. Staphylococcus aureus infections. New England Journal of Medicine. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 13.Smaldone C, Brugaletta S, Pazzano V, Liuzzo G. Immunomodulator activity of 3-hydroxy-3-methilglutaryl-CoA inhibitors. Cardiovasc Hematol Agents Med Chem. 2009;7(4):279–294. doi: 10.2174/187152509789541864. [DOI] [PubMed] [Google Scholar]
- 14.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. The Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):1043–1046. [PubMed] [Google Scholar]
- 15.Chaudhry MZ, Wang JH, Blankson S, Redmond HP. Statin (cerivastatin) protects mice against sepsis-related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surgical Infections. 2008;9(2):183–194. doi: 10.1089/sur.2006.077. [DOI] [PubMed] [Google Scholar]
- 16.Merx M, Liehn E, Janssens U, Lutticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 17.Beffa DC, Fischman AJ, Fagan SP, Hamrahi VF, Paul KW, Kaneki M, Yu YM, Tompkins RG, Carter EA. Simvastatin treatment improves survival in a murine model of burn sepsis: Role of interleukin 6. Burns. 2011;37(2):222–226. doi: 10.1016/j.burns.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDowell SA, Ma Y, Kusano R, Akinbi HT. Simvastatin is Protective During Staphylococcus aureus Pneumonia. Curr Pharm Biotechnol. 2011;12(9):1455–1462. doi: 10.2174/138920111798281027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Kockritz-Blickwede M, Rohde M, Oehmcke S, Miller LS, Cheung AL, Herwald H, Foster S, Medina E. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173(6):1657–1668. doi: 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn M, Knecht S, Rushing F, Birdsong J, Siddall C, Johnson C, Abraham T, Brown A, Volk C, Gammon K, Bishop D, McKillip J, McDowell S. Simvastatin inhibits Staphylococcus aureus host cell invasion through modulation of isoprenoid intermediates. Journal of Pharmacology and Experimental Therapeutics. 2008;326:135–143. doi: 10.1124/jpet.108.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275(1):55–60. [PubMed] [Google Scholar]
- 22.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. Journal of Antimicrobial Chemotherapy. 2008;61:362–364. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 23.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 8(5):445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djaldetti M, Salman H, Bergman M, Bessler H. Effect of pravastatin, simvastatin and atorvastatin on the phagocytic activity of mouse peritoneal macrophages. Exp Mol Pathol. 2006;80(2):160–164. doi: 10.1016/j.yexmp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Salman H, Bergman M, Djaldetti M, Bessler H. Hydrophobic but not hydrophilic statins enhance phagocytosis and decrease apoptosis of human peripheral blood cells in vitro. Biomed Pharmacother. 2008;62(1):41–45. doi: 10.1016/j.biopha.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Loike J, Shabtai D, Neuhut R, Malitzky S, Lu E, Husemann J, Goldberg I, Silverstein S. Statin inhibition of Fc receptor-mediated phagocytosis by macrophages is modulated by cell activation and cholesterol. Arterioscler Thromb Vasc Biol. 2004;24:2051–2056. doi: 10.1161/01.ATV.0000143858.15909.29. [DOI] [PubMed] [Google Scholar]
- 27.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. The Journal of Biological Chemistry. 2005;280(40):34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 28.Ward PA. The harmful role of c5a on innate immunity in sepsis. J Innate Immun. 2010;2(5):439–445. doi: 10.1159/000317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum Immunol. 2005;66(6):653–665. doi: 10.1016/j.humimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110(9):3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benati D, Ferro M, Savino MT, Ulivieri C, Schiavo E, Nuccitelli A, Pasini FL, Baldari CT. Opposite effects of simvastatin on the bactericidal and inflammatory response of macrophages to opsonized S. aureus. J Leukoc Biol. 2010;87(3):433–442. doi: 10.1189/jlb.0409273. [DOI] [PubMed] [Google Scholar]
- 32.Halkes CJ, van Dijk H, de Jaegere PP, Plokker HW, van Der Helm Y, Erkelens DW, Castro Cabezas M. Postprandial increase of complement component 3 in normolipidemic patients with coronary artery disease: effects of expanded-dose simvastatin. Arterioscler Thromb Vasc Biol. 2001;21(9):1526–1530. doi: 10.1161/hq0901.095276. [DOI] [PubMed] [Google Scholar]
- 33.Muscari A, Bastagi L, Poggiopollini G, Tomassetti V, Massarelli G, Boni P, Puddu P. Short term effect of atorvastatin and vitamin E on serum levels of C3, a sensitive marker of the risk of myocardial infarction in men. Cardiovasc Drugs Ther. 2001;15(5):453–458. doi: 10.1023/a:1013314227857. [DOI] [PubMed] [Google Scholar]
- 34.Verseyden C, Meijssen S, van Dijk H, Jansen H, Castro Cabezas M. Effects of atorvastatin on fasting and postprandial complement component 3 response in familial combined hyperlipidemia. J Lipid Res. 2003;44(11):2100–2108. doi: 10.1194/jlr.M300201-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Viedt C, Shen W, Fei J, Kamimura M, Hansch GM, Katus HA, Kreuzer J. HMG-CoA reductase inhibition reduces the proinflammatory activation of human vascular smooth muscle cells by the terminal complement factor C5b-9. Basic Res Cardiol. 2003;98(6):353–361. doi: 10.1007/s00395-003-0437-4. [DOI] [PubMed] [Google Scholar]
- 36.Fischetti F, Carretta R, Borotto G, Durigutto P, Bulla R, Meroni PL, Tedesco F. Fluvastatin treatment inhibits leucocyte adhesion and extravasation in models of complement-mediated acute inflammation. Clin Exp Immunol. 2004;135(2):186–193. doi: 10.1111/j.1365-2249.2003.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura A, Inui K, Nemoto T, Uda S, Sugenoya Y, Watanabe S, Yokota N, Taira T, Iwasaki S, Ideura T. Simvastatin suppresses glomerular cell proliferation and macrophage infiltration in rats with mesangial proliferative nephritis. J Am Soc Nephrol. 1998;9(11):2027–2039. doi: 10.1681/ASN.V9112027. [DOI] [PubMed] [Google Scholar]
- 38.Neale TJ, Ojha PP, Exner M, Poczewski H, Ruger B, Witztum JL, Davis P, Kerjaschki D. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest. 1994;94(4):1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappegard KT, Hvassing T, Mollnes TE. Statin drugs do not affect serum complement activation in vitro. Scand J Immunol. 2004;60(1–2):178–183. doi: 10.1111/j.0300-9475.2004.01439.x. [DOI] [PubMed] [Google Scholar]
- 40.Stankiewicz TE, Haaning KL, Owens JM, Jordan AS, Gammon K, Bruns HA, McDowell SA. GTPase activating protein function of p85 facilitates uptake and recycling of the beta1 integrin. Biochem Biophys Res Commun. 2010;391(1):443–448. doi: 10.1016/j.bbrc.2009.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]