Summary
β-arrestins regulate G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors (GPCRs) through receptor desensitization while also acting as signaling scaffolds to facilitate numerous effector pathways. Recent studies have provided evidence that β-arrestins play a key role in inflammatory responses. We here summarize these advances on the roles of β-arrestins in immune regulation and inflammatory responses under physiological and pathological conditions, with an emphasis on translational implications of β-arrestins on human diseases.
I. Introduction
β-arrestins are classically known to regulate GPCR signaling through receptor desensitization and internalization 1. The structure and molecular characterization of β-arrestins have been established 2; 3; 4. Collaborating with GPCR kinases (GRKs) 5, β-arrestins are known for their role in desensitizing the β2-adrenergic receptor 6. Much of the investigation on the role of β-arrestins has been associated with the deactivation and desensitization of the GPCRs. GPCR internalization is believed to occur through clathrin-mediated endocytosis, which consists of three processes: receptor desensitization, sequestration of receptors to clathrin-coated pits, and receptor internalization. These steps are each regulated by distinct signaling events and β-arrestins have a role in these processes. In addition, β-arrestins have been shown to mediate non-GPCR signaling pathways, such as through TGF-β receptor III 7.
Reports have also shown that β-arrestins facilitate the activation of numerous effector pathways, such as the mitogen-activated protein kinases (MAPKs) and Akt 8. β-arrestin 2 serves as s scaffold to enhance receptor tyrosine kinase (cRaf-1) and MAP kinase kinase (MEK)-dependent activation of extracellular signal-regulated kinase 2 (ERK2) 9 (also reviews 10; 11). Furthermore, evidence suggests that β-arrestins mediate signaling pathways through transcriptional regulation (review 12). For example, β-arrestin 1 is able to translocate from the cytosol to the nucleus and recruit histone acetyltransferase p300, leading to enhanced local histone H4 acetylation and transcription of p27 and c-fos 13.
Extensive research demonstrates that β-arrestins have functions in development 14; 15, cancer 16; 17, and chemotaxis 18; 19. During the last several years, roles of β-arrestins in innate and adaptive immunity, as well as in inflammatory responses have been reported. Studies in mice with targeted deletion β-arrestins have identified important roles for these adaptor molecules in immune responses and regulation of the pathogenesis of several disease processes have begun to been explored.
The immune system consists of innate and adaptive responses. Innate immune cells, receptor systems, and related signaling pathways defend the host from infections such as bacteria and virus, as well as from non-infectious tissue injuries. Defense by the innate immune system includes chemotaxis of inflammatory cells, release of cytokines, pathogen clearance, as well as the activation of the adaptive immune system through antigen presentation. The adaptive immune system, on the other hand, consists of highly specialized cells (such as T lymphocytes and B lymphocytes), proteins, and processes that aim to further eliminate pathogens. In addition to their role in pathogen clearance, both systems have a role in tissue injury, inflammation, and repair processes. Expression of β-arrestins has been found in innate immune cells such as macrophages, polymorphonuclear neutrophiles (PMNs), as well as in adaptive immune cells including T lymphocytes and B cells. β-arrestins have been suggested to have a role in both systems. In addition, β-arrestins may have a role in mammalian hematopoiesis, since β-arrestin 1 deficient mice demonstrate basal hematologic defects 20. Similarly in zebrafish, β-arrestin 1 relieved polycomb group-mediated repression of the cdx4-hox pathway, regulating hematopoietic lineage specification during primitive hematopoiesis 20.
II. β-arrestins in innate immunity
A. β-arrestins and macrophages
β-arrestins are expressed in macrophages. Both β-arrestin 1 and β-arrestin 2 mRNA and protein expression are found in both human and mouse mononuclear cells and macrophages 21; 22. Lipopolysaccharides down regulates β-arrestin 2 expression in RAW264 macrophages 23. Furthermore, β-arrestin 1 expression can be down-regulated by activation of TLR2 and TLR4 in primary mouse macrophages, and the regulation is both transcriptional and post-translational 24. Expression of β-arrestin 1 and β-arrestin 2 by macrophages can be regulated by cytokines. Cytokine granulocyte-macrophage colony-stimulating factor-1 increased β-arrestin 1 expression, associated with down-regulation of surface chemokine receptor CXCR4 expression in human primary monocytes-macrophages 25. Lectin phitoemagglutinin reduces β-arrestin 1 expression in mononuclear leukocytes, and interferon β-1a can quench the effect of phitoemagglutinin on the expression of β-arrestin 1 26. β-arrestin 2 mRNA and protein levels were significantly elevated in peripheral blood mononuclear cells of cryptococcal meningitis patients 27. β-arrestin 1 and 2 expressions are reduced in blood leukocytes during acute kidney transplantation 28. The modulation of β-arrestin expression by cytokines is involved in regulating related effector pathways.
β-arrestins regulate macrophage chemotaxis induced by chemokines and serine proteases. Chemokine CCL4 (MIP-1β) binding to CCR5 induces the translocation of β-arrestins, PI3K and Pyk2 to the membrane and forms a multi-kinase signaling complex mediating macrophage migration 29. Knockdown of β-arrestin 1 and 2 by siRNA impairs complex formation and inhibits macrophage chemotaxis toward CCL4 29. Chemokine CCL2 (MCP-1) binds with the CCR2 receptor, leading to formation of a CCR2/β-arrestin/GRK2 complex in human monocytes/macrophages. This process promotes the rapid desensitization of CCL2-induced calcium flux responses, which are essential for chemotaxis in macrophages 30. Protease-activated receptor-2 (PAR-2) activation has been shown to enhance β-arrestin and ERK1/2-regulated assembly of the actin cytoskeleton in pseudopodia, promoting polarized pseudopodia extension and facilitating macrophage chemotaxis 31. Collectively, these studies demonstrate that, β-arrestins are an integrated regulator of macrophage-mediated innate immune responses.
B. β-arrestins and polymorphonuclear leukocytes
Polymorphonuclear leukocytes (PMNs) are essential for host defense against infection and the activation of PMNs is pivotal in the process of innate immunity 32; 33. β-arrestin 1 is highly expressed in peripheral blood leukocytes including PMNs 22. β-arrestin 1 and 2 are critical modulators of inflammatory responses in PMNs, including PMN cytokine production, recruitment 34, and granule release 35. β-arrestins and GRK2 associated with the CCR2 receptor play an important role in CCL2 (MCP-1)-regulated leukocyte activation and migration following CCL2 binding 30. PMNs from β-arrestin 2 deficient mice show enhanced release of inflammatory cytokines, including TNF-α and IL-6, compared to WT PMNs 34. β-arrestin 2 deficiency, results in augmented PMN recruitment with increased expression of CD18 and CD62L, and enhanced PMN infiltration in the lung after cecal ligation and puncture (CLP) 34. Furthermore, β-arrestins regulate interleukin (IL)-8-induced granule exocytosis in PMNs, which is critical for the innate immune response against infectious bacteria 35. β-arrestin 2 plays a negative role in CXCR2 signaling in neutrophils. Neutrophils isolated from peritoneal cavity of β-arrestins 2-deficient mice show enhanced calcium mobilization, superoxide anion production, and GTPase activity, but decreased receptor internalization in CXCR2 signaling 36. β-arrestin 2-deficient mice also demonstrate increased recruitment of PMNs in response to CXCL1 in the air pouch model and in excisional wound beds, leading to faster wound re-epithelialization 36.
C. β-arrestins and natural killer (NK) cells
NK cells constitute a major component of the innate immune system that play a significant role in tumor rejection and viral clearance. NK cell receptors include activating NK receptors such as NKG2D and inhibitory receptors such as killer-cell immunoglobulin-like receptors. β-arrestin 2 has been reported to interact with inhibitory receptor KIR2DL1 and mediate the binding of tyrosine phosphatases to KIR2DL1 to facilitate the inhibitory signaling of NK cells 37. KIR2DL1 with an arginine residue at position 245 in its transmembrane domain recruited more β-arrestin 2 and Src-homology-2 domain-containing protein tyrosine phosphatase 2 38. NK cells isolated from β-arrestin 2 deficient mice demonstrated higher cytotoxicity than WT mice, while NK cells from β-arrestin 2 transgenic mice showed reduced cytotoxicity which could be restored by β-arrestin 2 knockdown 37. β-arrestin 2 is negative regulator NK cell cytotoxicity.
D. β-arrestins and mast cells
Mast cells contain granules rich in histamine and heparin, and play a role in allergy and anaphylaxis, as well as in tissue injury 7. Mast cells can be activated to degranulate by injury, cross-linking of Immunoglobulin E (IgE) receptors, and complement proteins 7. In the intestine, mast cells release tryptase to activate PAR2 in colonocytes. PAR2 then associates with β-arrestins to activate ERK1/2, which increases epithelial permeability by regulating the assembly of perijunctional F-actin 39. β-arrestins may play a role in the increased epithelial permeability of the intestine during stress and inflammation through the mediation of mast cells activation.
E. β-arrestins and complement
The complement system contains more than 30 proteins, which regulate tissue injury and inflammation 40. The complement system can be activated as part of the innate immune as well as adaptive immune responses. Complement fragments bind to specific receptors to induce cellular responses that trigger inflammatory and immune responses. β-arrestin 1 and β-arrestin 2 are involved in complement receptor C3aR desensitization, internalization, degranulation, NF-κB activation and chemokine generation in mast cells 41. Stable knockdown of β-arrrestin 2 expression attenuated C3aR desensitization, internalization and NF-κB activity as well as chemokine generation in human mast cell lines that endogenously express C3aR 41. However, silencing β-arrestin 1 decreased C3a–induced mast cell degranulation, not C3aR desensitization. Knockdown of β-arrestin 1, β-arrestin 2 or both enhanced the early response to C3a but inhibited G protein-dependent ERK1/2 phosphorylation 41. Others studies suggested that although C3aR interacts with β-arrestins, they do not appear to be involved in C3a–induced ERK activation in a leukemia cell line 42. Complement C5a binds to the C5a receptor (C5aR, a GPCR) and C5L2 receptor (a non-GPCR) to elicit its proinflammatory functions. A recent study suggested that C5a binding to C5a receptor recruits C5L2 and β-arrestin 2 43. Activation of C5L2 results in inhibition of C5aR-β-arrestin-mediated ERK1/2 activation, with no apparent alteration of G protein-mediated functions such as calcium influx and receptor endocytosis 43. Bone marrow-derived macrophages devoid of β-arrestin 2 showed decreased complement C1q gene expression and enhanced factor-independent survival of CSF-1 through JNK/ERK signaling 44. Therefore, β-arrestins appear to regulate complement functions through receptor desensitization as well as ERK signaling.
III. β-arrestins in adaptive immunity
A. β-arrestins and lymphocytes
Both β-arrestin 1 and β-arrestin 2 are expressed in lymphocytes 45. In thymocytes, β-arrestin 1 expression was very low compared with that of splenocytes or from lymph nodes 46. Compared with B cells, T cells contain substantially more β-arrestin 2 45. For both naive and activated cells, CD4+ T cells had much more nuclear β-arrestin 1 than CD8+ T cells 46. CD4+ T lymphocytes of mice with allergic asthma expressed higher levels of β-arrestin 2 at both mRNA and protein levels compared to normal mice 47. β-arrestin expression levels are dependent upon cell type, activation status, and disease states.
B. T cell activation
T lymphocytes are activated by the binding of the T cell receptor (TCR) to antigens bound by major histocompatibility complex (MHC) molecules on antigen presenting cells s(APC). The binding of co-stimulatory molecules to the CD28 receptor is also required for full T cell activation. In addition, the activation is mediated by kinases, phosphatases, and transcription factors. Engagement of the TCR is followed by rapid cAMP production in lipid rafts and activation of the cAMP-protein kinase A (PKA)-Csk pathway inhibiting proximal T-cell signaling 48; 49. It appears that β-arrestins do not regulate the cAMP-PKA-Csk pathway 48; 49. However, β-arrestins participate in CD28 receptor co-ligation signaling 49. Upon TCR and CD28 co-ligation, β-arrestin was recruited to lipid rafts, and augmented TCR/CD28-stimulated cytokine production 48; 50. β-arrestins form complexes with cAMP phosphodiesterase 4 and mediate cross-talk between phosphatidylinositol 3-kinase and cyclic AMP-protein kinase A signaling pathways 51. A PDE4/β-arrestin complex attenuates PKA phosphorylation of Csk 48; 52, allowing full T-cell activation to proceed.
C. Migration and chemotaxis
The migration of T cells is important in adaptive immune responses. T cells must migrate to sites where antigen is found, because T cells respond to pathogens only on direct contact with pathogen-derived antigen 53. β-arrestins function as fundamental adaptors connecting receptors to cell trafficking machinery 48. β-arrestin-2 activation of the p38 MAPK cascade is required for CXCR4-mediated migration of human embryonic kidney 293 cells to CXCL12 (SDF-1α) 54. It has been reported that lymphocytes devoid of β-arrestin-2 demonstrate impaired migration toward the chemotactic factor CXCL12 in vitro 45. A subsequent study showed that β-arrestin 2 deficient mice have defective macrophage-derived chemokine-mediated CD4+ T cell migration to the lung 55, and T cells in β-arrestin 2 deficient mice exhibit migration defects in a model of metastatic tumor growth 17.
D. Lymphocyte survival and apoptosis
Several studies indicate that β-arrestins play a critical role in preventing apoptosis 56; 57. For example, neuropeptide substance P inhibits apoptosis via a β-arrestin scaffolding complex 58, and β-arrestins play a critical role in the suppression of apoptosis mediated by stimulated GPCRs using β-arrestin 1/2-knockout cells 57. IGF1-mediated anti-apoptosis was diminished by knockdown of β-arrestin 1/2 and was restored by transfection of β-arrestin 1 56. β-arrestin 1 epigenetically regulates Bcl2 expression, regulating the survival and homeostasis of CD4+ T cells 46. β-arrestin 1 promotes acetylation of histone H4 59 at the Bcl2 locus and Bcl2 expression in both naïve and activated CD4+ T cells 46. Furthermore, β-arrestin 1 is highly expressed in CD4+ T cells from multiple sclerosis patients, and RNAi knockdown of β-arrestin 1 in those cells have increased apoptosis after induction by cytokine withdrawal 46. β-arrestin 1 did not affect the homeostasis of CD8+ T cells 46, possibly due to the difference in the subcellular distribution of β-arrestin 1 in CD4+ and CD8+ T cells. Moreover, β-arrestin 2 over-expression and interference can decrease and increase, respectively, the level of gp120/morphine-induced lymphocyte apoptosis 60. Collectively, these studies support a role for β-arrestins in regulating lymphocyte survival.
E. Th1, Th2, and Th17 cells
Classically, helper T cells include Th1 and Th2 T cells. T cell antigen receptor-mediated activation of the Ras/MAP kinase pathway controls IL-4 receptor function and Th2 cell differentiation 61. Although the direct demonstration of β-arrestins in Th1 and Th2 skewing is lacking, it seems that β-arrestins play a role in both conditions. For example, β-arrestin 2 deficient mice showed a reduced CD4+ T cell accumulation in airway in an asthma model 55. This may be a Th2 dependent event, since β-arrestin 2 deficient mice showed a normal inflammatory response in a LPS model 55. Knocking down β-arrestin 2 with RNAi decreased expression of the Th2 cytokine IL-4 as well as the T cell specific transcription factor GATA3 in CD4+ T lymphocytes after terbutaline stimulation 47. On the other hand, overexpression of β-arrestin 1 increased Th1 cytokine interferon-γ production 59. The stress-induced suppression of Th1 cytokines and the increased production of Th2 cytokines were greatly enhanced in β-arrestin 2-deficient mice compared with wild-type mice 62. These studies suggest that β-arrestins may not have a role in T cell differentiation, but has a role in Th1 and Th2 responses during inflammation.
The role of β-arrestins in Th17 cell function has not been reported. Blocking CCL2-CCR2 interactions with a fusion peptide failed to recruit β−arrestin 2, leading to blocking IL-17 production in vitro 63. Silencing β-arrestin 2 with specific siRNA reduced IL-17 expression 64. Overexpression of β-arrestin 1 increased IL-17 expression, possibly by acetylation of histone H4 in the promoter region of IL-17 59. It would be interesting to determine if β-arrestins have any role in activation of Th17 cells.
IV. β-arrestins and structural cells
β-arrestins are expressed in epithelial cells 65; 66, endothelial cells 22; 67; 68, and smooth muscle 22. β-arrestin 1 has been found to be expressed in human endothelial cells and smooth muscle cells 22. In these cells, β-arrestin 1 expression can be upregulated by cAMP-inducing agents such as cholera toxin, forskolin, iloprost, and isoproterenol 22. β-arrestins mediate the infections of Streptococcus pneumonia 67 and Neisseria meningitides 69 through interactions wth endothelial cells. β-arrestin 2 regulates thrombin-PAR1 signaling in endothelial cell adhesion 68.
β-arrestin influences CXCL12-mediated epithelial cell migration. CXCL12-stimulated cell migration is enhanced by CXCR7/CXCR4 co-expression in a β-arrestin dependent manner 65. In a CXCR4/CXCR7-expressing MDA-MB-231 breast cancer epithelial cell line, inhibition of β-arrestin using either siRNA knockdown or a dominant negative mutant suppressed CXCL12-induced cell migration 65.
V. β-arrestins regulate immune signaling pathways
A. β-arrestins and chemokine receptors
Chemokines are a family of small cytokines secreted by immune cells and inflammatory cells. Receptors for chemokines are typically seven-transmembrane GPCRs. Binding of chemokines and their cognate receptors regulate chemotaxis of leukocytes into inflammatory sites 70 and cytokine release. β-arrestins are recruited to the receptors and mediate receptor desensitization and internalization (reviews 11; 18; 21; 71).
CXC chemokines are one of the main subfamily of chemokines. β-arrestins have been shown to mediate the internalization and desensitization of IL-8 induced CXCR1 72; 73, CXCR2 36; 73; 74, and CXCL9- and CXCL10-induced CXCR3 75. β-arrestins regulate CXCR4 internalization in response to the specific ligand CXCL12/SDF-1 76. CXCR4 can also form a heteromeric complex with the CXCR7 chemokine receptor upon in response to CXCL12 65. Defects chemokine receptor-β-arrestin interactions may promote human disease states. WHIM (warts, hypogammaglobulinemia, infections, and myelokatexis) syndrome is an immunodeficiency syndrome linked to heterozygous mutations of the chemokine receptor CXCR4 resulting in truncations of its cytoplasmic tail. WHIM-associated mutant CXCR4 was unable to recruit β-arrestin 2, leading to the impaired CXCR4 internalization and enhanced chemotaxis in response to CXCL12 77.
CC chemokines are a second group of chemokines. Reports showed that β-arrestins regulate CC chemokine-induced CC chemokine receptor activation by desensitization and internalization. CCL2 (MCP-1) binds to CCR2 inducing the activation of CCR2 and, shortly after stimulation, recruits a protein complex including GRK2 and β-arrestin 30. In addition, the actin binding protein filamin A binds CCR2B 78 and together with β-arrestin regulates the internalization of the receptor complex. Chemokine CCL5 (RANTES) binding to the CCR5 induces the phosphorylation of CCR5. β-arrestin 1 is able to desensitize and internalize homo- and hetero-oligomers of CCR5 79; 80. β-arrestins thus regulate immune and inflammatory responses in part by desensitization and internalization of activated chemokine receptors.
β-arrestins also regulate chemokine signaling non-canonical G protein pathways. β-arrestin 2 and GRK6 play positive regulatory roles, mediating the chemotactic responses of T and B lymphocytes to CXCL12 45. β-arrestin 2 strengthened CXCR4 activation through the ASK1-p38MAPK pathway 54. In addition to the formation of a CXCR4 homodimer, CXCL12 also binds to CXCR7 81; 82, and induces CXCR4-CXCR7 heterodimers 65. The CXCR4-CXCR7 heterodimer complex upon CXCL12 binding recruits β-arrestin, resulting in preferential activation of β-arrestin-linked signaling pathways over canonical G protein pathways 65. This activation was dependent on β-arrestin-mediated cell signaling pathways, such as ERK1/2, p38 MAPK, and SAPK, leading to enhanced cell migration in response to CXCL12 stimulation 65. The CC chemokine CCL4 (MIP-1β) binds to CCR5 regulating macrophage migration. Chemotaxis upon CCR5 stimulation by CCL4 requires activation of Pyk2, PI3K p85, and Lyn, and ERK. β-arrestins regulate the receptor complex formation and enhance macrophage chemotaxis toward the ligand 29.
B. β-arrestins and other GPCRs in inflammation
Angiotensin II receptor Type 1A
Angiotensin II is a potent vasopressor peptide hormone, binds its receptor, the angiotensin II type 1A receptor (AGTR1, AT1R, or AT1AR), a GPCR, to mediate the formation of reactive oxygen species and inflammatory responses 83, in addition to its role as an important effector controlling blood pressure. For example in liver, angiotensin II-dependent NF-κB activation leads to proinflammatory cytokine release from hepatocytes and stellate cells that is implicated in the development of hepatic fibrosis 84. Studies suggested that β-arrestins promote desensitization and sequestration of AGTR1 85. Both β-arrestin 1 and β-arrestin 2 showed similar roles in agonist-stimulated receptor desensitization, while the β-arrestin 2 may have more profound effects on angiotensin II-stimulated internalization of the AGTR1 85. Furthermore, β-arrestin 2 also mediates chemotaxis through an AGTR1-dependent and G-protein-independent mechanism 86.
However, β-arrestins also facilitate GPCR-mediated ERK activation but inhibit ERK-dependent transcription to target activated ERK1/2 to non-nuclear substrates following angiotensin AT1a receptor stimulation 87. Furthermore, β-arrestin 2 binds JNK and mediates ASK1 and MKK4 activity 88.
The angiotensin II receptor has been shown to play a role in immune-mediated renal injury 89; 90. Deletion of AGTR1 in mice markedly attenuated glomerular expression of CCL2 (MCP-1), proteinuria, and tissue damage in an anti-glomerular basement membrane nephritis model 89. Angiotensin II binds its receptors on immune cells, triggering the proliferation of splenic lymphocytes and leading to the potency of cellular alloimmune responses 90. The absence of AGTR1 signaling accentuates the immunosuppressive effects of the calcineurin inhibitor cyclosporine 90. The manipulation of β-arrestin-mediated renin-angiotensin signaling can be used as a way to regulate immune responses.
β2-adrenergic receptor (β2AR)
β-arrestin attenuates agonist-induced β2-adrenergic receptor signaling by GRK-mediated desensitization 91 and by clathrin-mediated receptor internalization 92. It is reported that both isoforms of β-arrestins promote desensitization of β2-adrenergic receptors 85, however, deficiency in β-arrestin 2, not in β-arrestin 1, significantly compromised the sequestration of β2-adrenergic receptors 85. A cAMP phosphodiesterase PDE4 is recruited to the receptor-β-arrestin complex and PDE4 plays a role in the regulation of G protein switching by the β2-adrenergic receptor in cardiac myocytes 93.
Lipid signaling receptors
Prostaglandins are lipid compounds derived from fatty acids. Prostaglandins bind to their respective receptors to elicit functions including inflammatory regulation. Prostaglandin E2 receptor (EP4) is a typical GPCR. Studies suggested that β-arrestins have both positive and negative effects on the EP4 receptor. β-arrestin 1 is necessary for maximal agonist-induced internalization through the interaction with a Ser/Thr cluster in the C-terminal domain of EP4 receptor 94.
Leukotrienes are arachidonic acid metabolites, binding to their respective receptors to elicit their role in inflammatory responses. β-arrestin 1 is required for internalization of the leukotriene B4-activated leukotriene B4 receptor 95.
Lysophosphatidylcholines are phospholipids that bind to G2A receptors on PMNs recruiting clathrin, β-arrestin-1 and GRK6 for receptor de-sensitization. Phospholipids cause acute lung injury 96 and are involved in transfusion-related acute lung injury 97. β-arrestin 1 both propagates and terminates inflammatory signals 97.
Lysophosphatidic acid binds to lysophosphatidic acid receptors, including LPAR1 and mediates diverse biologic roles such as proliferation, platelet aggregation, smooth muscle contraction, chemotaxis, fibroblast migration, and tumor cell invasion. β-arrestin 1 and GRK2 mediate lysophosphatidic acid receptor LPAR1 desensitization 98. Furthermore, β-arrestin 2 promotes a distinct subset of ERK1/2-mediated responses to LPA receptor activation 99 and lysophosphatidic acid-induced NF-κB activation 100. Suppression of β-arrestin 2 expression using small interfering RNA abolished chemotaxis induced by lysophosphatidic acid 86. β-arrestin signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells 101
Phospholipid platelet-activating factor (PAF) is an effective chemoattractant that primes PMN oxidases. The binding of PAF with the PAF receptor (PAFR, or PTAFT) recruits β-arrestin 1 and desensitizes the receptor 102. β-arrestin recruitment blocks both NF-κB activation and Ca2+/calcineurin-dependent signaling pathways, leading to reduced cytokine production 103. Platelet-activating factor-induced clathrin-mediated endocytosis requires β-arrestin 1-MKK3-p38 MAPK complex and p38 MAPK activation 104. Finally, the same scaffolding complex regulates actin rearrangement in human neutrophils 104; 105.
Protease-activated receptors
Both protease-activated receptor-1 (PAR-1) and PAR-2 mediate leukocyte recruitment to sites of injury and infection. β-arrestins may play a dual role in both in PAR-1 and PAR-2 desensitization and scaffolding. A report suggested that only β-arrestin 1 is capable of PAR-1 receptor desensitization, but receptor internalization is independent of either β-arrestin 106. In contrast, β-arrestins function as scaffolds for PAR-2 activation. A recent study reported that upon PAR-2 activation, β-arrestins scaffolding aggregates the intracellular actin-modulating protein cofilin and activator chronophin, to facilitate the localized generation of free actin barbed ends, leading to the reorganization of the actin cytoskeleton and chemotaxis 107. The formation of the signaling complex of β-arrestins and activated ERK1/2 is required to mediate PAR-2 signaling 108.
C. β-arrestins and TLR signaling
Toll-like receptors (TLRs) are pattern recognition receptors that recognize pathogen-associated molecular patterns such as bacterial coat components 109 as well as endogenous ligands 110. TLRs belong to the interleukin-1 receptor/Toll-like receptor superfamily that play a key role in the innate immune system 109. β-arrestins are implicated in TLR4 signaling in several studies111; 112; 113; 114. β-arrestin 2 attenuates TLR4-initiated apoptosis by controlling the activation and inactivation of GSK-3β 115. β-arrestin 2 modulates immune responses induced by stress through TLR2-mediated signaling 116.
Studies suggest that endosomal trafficking of the LPS-TLR receptor complex is essential for signal termination and LPS-associated antigen presentation 117. Reports have shown that β-arrestins may have a direct role in regulating TLR sensitization and internalization. β-arrestin 2 directly interacts with TRAF6 in response to LPS or IL-1β stimulation, preventing TRAF6 autoubiquitination and oligomerization 111. β-arrestin 2 also inhibits AP-1 and NF-κB activation and inhibits LPS- and IL-1-induced inflammatory cytokine production 111. Moreover, β-arrestin 2 and GRK5 negatively regulate TLR4 signaling through NF-κB and ERK phosphorylation 118. On the other hand, a study suggested that β-arrestins regulate cytokine production following LPS-induced chromatin modification and not MAPK and IκBα-NFκB pathways 112. In contrast, in drosophila, β-arrestin Kurtz inhibits MAPK and Toll signaling in Drosophila development 119.
β-arrestins may have a role in regulating cross-talk between TLRs and other receptor families (Figure 1). A recent study showed that there was cross-talk between the β2-adrenergic receptor and TLR4, and the suppression of β-arrestin 2 eliminated the anti-inflammatory effects of β2-adrenergic receptor activation in monocytes 120. β-arrestin-2 mediated redistribution of CD14 and TLR4/CD14 complex 120. β2-adrenergic receptor regulates TLR4-induced late-phase NF-κB activation 121. Furthermore, recent studies also suggest there is cross-talk between TLRs and chemokine receptors. TLR2 activation leads to β-arrestin-mediated endocytosis of CCR1, CCR2, and CCR5 on human blood monocytes, providing a molecular mechanism for inhibiting monocyte migration after pathogen recognition 122. Moreover, it seems that TLRs also directly regulate β-arrestins. For instance, activation of TLR2 and TLR4 significantly decrease β-arrestin 2 protein in macrophages 24.
Figure 1.
β-arrestins regulate GPCR and TLR signaling during inflammation. Upon binding of chemokines and their respective receptors, β-arrestins are recruited to the receptors and regulate chemotaxis of leukocytes into inflammatory sites and cytokine release in two ways: mediating receptor desensitization and internalization, and acting as signaling scaffolds to facilitate numerous effector pathways. Classically, LPS-TLR interaction induces signaling cascade including TRAF6 and NF-κB activation, leading inflammatory response. The LPS-TLR binding also recruits β-arrestins and the latter mediates TLR signaling through regulating TRAF6, NF-κB, as well as chromatin modification. Furthermore, β-arrestins are able to mediate the crosstalk to chemokine receptors.
D. Other non-GPCR signaling pathways
Peroxisome proliferator-activated receptors are nuclear receptor proteins, regulating metabolism and inflammation. β-arrestin 1 binds peroxisome proliferator-activated receptor-γ (PPARγ) to elicit the repression of PPARγ/9-cis-retinoic acid receptor-α function and to promote PPARγ/nuclear receptor corepressor function in PPARγ-mediated adipogenesis and inflammatory cytokine expression 123.
TNFα binds to its TNF receptor, a type I transmembrane protein, recruiting death domain containing proteins and leading to apoptosis pathways. As TNFα is such a pleiotropic cytokine, the interaction of TNF and its cognate receptor regulates an array of biological functions including apoptosis, immune regulation, and inflammatory response. β-arrestin 1 can function as a signaling molecule in the TNFα action cascade that stimulates ERK activation and lipolysis, mediates phosphatidylinositol 3-kinase activation and inflammatory gene expression 60. β-Arrestin 1 and 2 also bind and prevent degradation of IκBα-inhibiting NF-κB activation 124; 125, leading to the suppression of TNFα-induced proinflammatory cytokines. A subsequent report confirmed that although TNF receptor-1 is a non-GPCR, it functions through a Gα (q/11) signaling complex and through ERK activation, mediating TNFα-induced phosphatidylinositol 3-kinase activation and inflammatory gene expression 126. β-arrestin 1 was found to interact with STAT1 upon interferon-γ stimulation 127. β-arrestin 1 accelerates STAT1 tyrosine dephosphorylation by recruiting T cell protein tyrosine phosphatase (TC45) and negatively regulates interferon-γ induced gene transcription 127. Interestingly, β-arrestin 2 did not have a similar effect in regulating interferon-γ signaling, although β-arrestin 2 also binds to STAT1.
TGF-β binds to TGF-β receptors, regulating cell growth, differentiation, and immune responses. β-arrestin 2, but not β-arrestin 1, binds to the phosphorylated type III TGF-β receptor and mediates endocytosis of the type II TGF-β receptor/type III TGF-β receptor complex 128. Further study suggested that β-arrestin 2 modulates the association of type III TGF-β receptor with ALK6 and ALK3 and enhances ALK6-mediated bone morphogenetic protein 2 signaling 129. TGF-β signaling mediates immune regulation, inflammatory responses, and tissue fibrosis. However, functional studies on β-arrestins-mediating TGF-β signaling are largely lacking. Interaction of β-arrestin 2 and type III TGF-β receptors regulate epithelial cell migration through activation of Cdc42 130. In a tissue fibrosis model, our initial finding suggests that deficiency in β-arrestins may not affect TGF-β-induced matrix production 131. Detailed analysis such as prolonged treatment with TGF-β may shed some light on the role of TGF-β and β-arrestins in inflammatory and fibrotic diseases. In addition, β-arrestins have been shown to regulate the orexin-1 receptor 132 and T cell receptor and co-receptor 133. Through these interactions with these signaling pathways, β-arrestins regulate inflammatory responses.
VI. Role of β-arrestins in human diseases
A. Pathogen recognition and clearance
β-arrestins play a role in bacterial-induced inflammation and clearance, such as in sepsis 34; 134; 135, and Cryptococcus infections 27. For example, deficiency in β-arrestin-2 in mice showed decreased mortality and reduced infections. Therefore, inhibition of β-arrestins may be used to limit bacterial and fungal infections in some instances 27; 69.
Neisseria meningitides
Neisseria meningitides, a commensal bacterium causing cerebrospinal meningitis, is an intracellular human-specific pathogen that functions through Type IV pilus-mediated adhesion to human brain endothelial cells, leading to the opening of intercellular junctions and allowing meningeal colonization 136. The signaling receptor activated by the pathogen may be the β2-adrenoceptor 69. Studies have suggested that the β2-adrenergic receptor/β-arrestin signaling pathway may mediate bacterial colonization 136; 137. Type IV pili of N. meningitidis are able to activate the β2-adrenergic receptor/β-arrestin signaling pathway, thus recruiting the polarity complex and the cell junction proteins and allowing the opening of a paracellular route in endothelial cells 138. By this process, Neisseria meningitidis is able to cross of the blood-brain barrier and affect the central nervous system 138. Activation of β-adrenoceptor endocytosis with specific agonists prevents signaling events downstream of N. meningitidis adhesion and inhibits bacterial crossing of the endothelial barrier 69. Therefore, targeting the β2-adrenergic receptor/β-arrestin signaling pathway may be used for treatment and prevention of meningococcal infection 69.
Streptococcus pneumoniae
β-arrestin 1 has been reported to be involved in pneumococci infection. A report showed that upon infection with Streptococcus pneumoniae, β-arrestin 1 moved to the plasma membrane of endothelial cells 67. By transfection of β-arrestin 1, pneumococcal invasion was enhanced in a cell line. β-arrestin 1 was shown to interact with the platelet-activating factor receptor and contribute to successful infection by Streptococcus pneumoniae 67.
Polymicrobial sepsis
β-arrestin 2 protein expression was reduced in a murine model of CLP-induced polymicrobial sepsis 134. β-arrestin 2 deficient mice exhibit decreased mortality, increased IL-6 and elevated caecal myeloperoxidase in a mouse model of polymicrobial sepsis by caecal ligation and puncture (CLP). Splenocytes devoid of β-arrestin 2 produced higher levels of TNF-α, IL-6 and IL-10 135. This is reminiscent of another study showing that CLP-induced myeloperoxidase was increased in β-arrestin 2 deficient mouse lung 34. β-arrestin 1 and β-arrestin 2 deficient mice were protected from TLR4-mediated endotoxic shock and lethality 112. These studies suggest that β-arrestin 2 is a negative regulator in polymicrobial sepsis. Therefore, treatment with flavocoxid preserved β-arrestin 2 expression and reduced cytokine production, leading to protection against tissue damage induced by CLP presumably by decreasing myeloperoxidase activity in the lung and liver 134.
Since chemokine receptors play key roles in pathogen recognition and β-arrestins regulate chemokine receptor internalization, it is not surprising that β-arrestins regulate chemokine receptor-mediated pathogen clearance. For example, during Staphylococcus aureus associated lipoteichoic acid induced pathogen recognition and monocyte migration, β-arrestins mediate endocytosis of CCR5 into recycling endosomes. Thus facilitating the TLR2 negative regulation of CCR1, CCR2 and CCR5 on monocytes 122.
Cryptococcal meningitis
β-arrestin 2 mRNA and protein levels were significantly elevated in peripheral blood mononuclear cells of patients with cryptococcal meningitis. The increased β-arrestin 2 levels were concurrent with reduced cytotoxic activity against Cryptococcus, increased IL-10 levels and reduced IFN-γ expression in patient serum 27. This study suggests that increasing INF-γ by inhibition of β-arrestin 2 could be a therapeutic strategy in Cryptococcus infections27.
Viral responses
Reports suggest that β-arrestins mediate antiviral responses. β-arrestin 1 accelerates STAT1 tyrosine dephosphorylation by recruiting T cell protein tyrosine phosphatase (TC45) and negatively regulates IFN-γ induced gene transcription in IFN-γ-induced cellular antiviral responses 127. β-arrestin 1 knockdown promotes the antiviral immune response in vesicular stomatitis virus-infected cells 127. β-arrestin 2 plays a negative role in HIV-1 gp120 and opioids-induced lymphocyte cell death 60. Increased or decreased β-arrestin 2 expression inhibited or enhanced gp120/morphine-induced apoptosis, respectively 60. β-arrestin 1 and 2 can function as both positive and negative regulators of adenovirus-induced innate immune responses both in vivo and in vitro 114.
B. Fibrosis
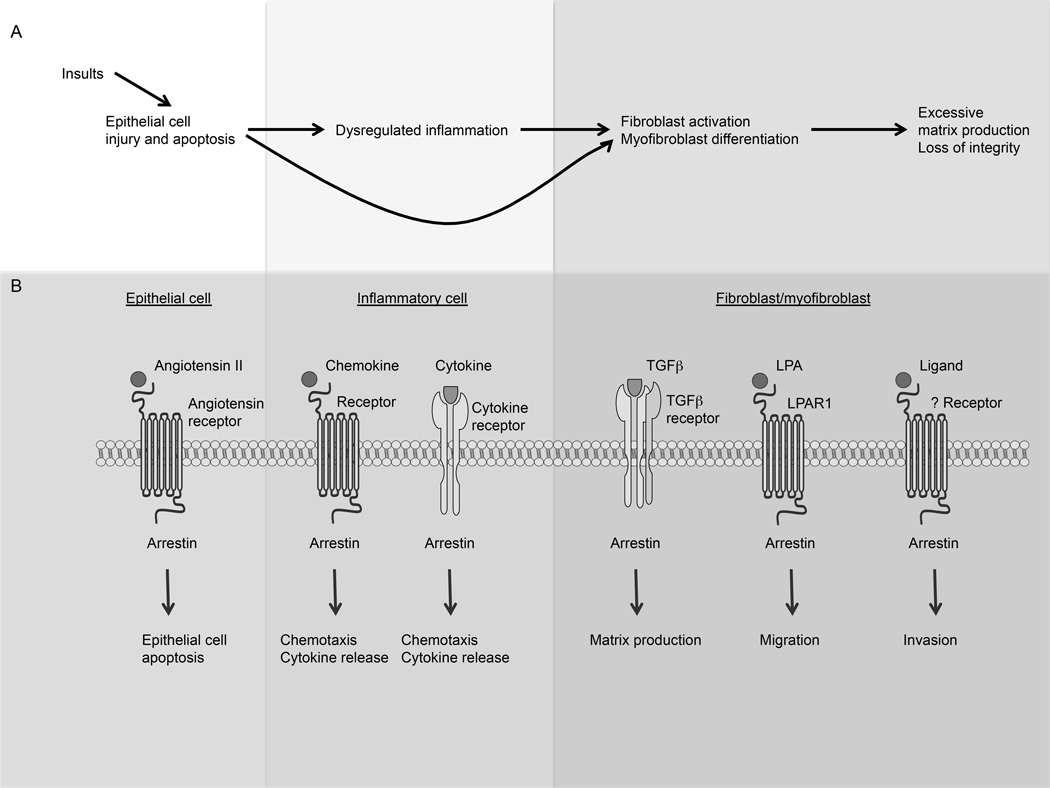
The molecular mechanisms that control progressive tissue fibrosis remain poorly understood. Idiopathic pulmonary fibrosis (IPF) is a progressive disease and a major cause of morbidity and mortality. Progressive pulmonary fibrosis results from numerous micro-injuries to the alveolar epithelia that lead to detonation of excessive fibroblast activity. Lung epithelial cell apoptosis is believed to be responsible for initiation of fibrogenesis. β-arrestins are known to regulate cell apoptosis (reviews 19; 57; 139). Some GPCRs are reported to have a role in lung epithelial apoptosis. For instance, angiotensin II receptors are expressed by lung alveolar epithelial cells, and the interaction of angiotensin II and its receptors mediate alveolar epithelial cell apoptosis 140; 141. However, it is unclear whether β-arrestins mediate type II epithelial cell apoptosis during lung fibrosis. During tissue injury, TGF-β is released by fibroblasts and the epithelium. There is abundant evidence that TGF-β is critical for the progression of pulmonary fibrosis due to its role in regulating collagen synthesis, fibroblast proliferation, apoptosis, and myofibroblast differentiation 142; 143; 144. β-arrestin 2, but not β-arrestin 1, mediates endocytosis of type II TGF-β receptor/type III TGF-β receptor complex and down-regulates TGF-β signaling 128 and bone morphogenetic protein 2 signaling 129. In a lung fibrosis model, our initial findings suggest that deficiency in β-arrestins may not affect TGF-β-induced matrix production 131. Detailed analysis such as prolonged treatment with TGF-β may shed some light on the role of β-arrestins on TGF-β signaling in inflammatory and fibrotic diseases (Figure 2).
Figure 2.
β-arrestins mediate signaling pathways during fibrogenesis. A simplified model of tissue fibrosis depicts that epithelial cell injury and apoptosis leads to dysregulated inflammatory responses and fibroblast/myofibroblast activation resulting to excessive matrix production and eventually fibrosis. β-arrestins regulate signaling pathways mediating cell injury and apoptosis in epithelial cells, inflammatory responses in immune cells, and fibroblast migration and invasiveness, matrix production in effector cells.
Fibroblast and myofibroblast migration is an important process during fibrogenesis. Chemokines and chemokine receptors regulate fibroblast migration 145; 146. Furthermore, lysophosphatidic acid in bronchoalveolar lavage fluid interacts with its receptor LPAR1 regulating fibroblast migration during fibrogenesis 147. β-arrestins regulate chemokine signaling as well as LPAR1 signaling. The role of β-arrestins in fibroblast migration has not been explored. We have shown that deletion of β-arrestins did not significantly affect fibroblast migration 131.
The destruction of alveolar basement membrane was observed in experimental lung fibrosis and the biopsies of patients with lung fibrosis 148. Moreover, fibroblasts and myofibroblasts from patients with progressive lung fibrosis have been shown to have the ability to invade extracellular matrix in the manner of metastatic cancer cells 148. β-arrestins have been shown to play a role in tumor metastasis 16; 17. For example, β-arrestin signaling mediates lysophosphatidic acid-induced cancer cell migration and invasion 101. We found that fibroblasts from bleomycin-treated β-arrestin deficient mice and human IPF fibroblasts with β-arrestin 2 knockdown appear failed to invade extracellular matrix 131. Furthermore, we showed that loss of β-arrestin 1 or β-arrestin 2 in primary lung fibroblasts results in altered expression of genes involved in matrix production, basement membrane degradation, and cell adhesion 131. Therefore, this study suggests that inhibiting β-arrestin expression in lung fibroblasts would be a potential therapy for IPF and other types of pulmonary fibrosis.
β-arrestin 1 expression is increased in T cells from primary biliary cirrhosis patients. There is also a positive correlation between the levels of β-arrestin 1 mRNA in peripheral blood mononuclear cells and progression of cirrhosis 59. β-arrestin 1 was overexpressed in autoreactive T cells from cirrhosis patients augmented cell proliferation, increased interferon release, and regulated autoimmune-related gene expression 59. The study suggests that β-arrestin 1 overexpression contributes to the pathogenesis of primary biliary cirrhosis. Angiotensin II binds its receptor angiotensin II type 1A receptor to mediate inflammatory and fibrotic responses 83. β-arrestins regulate desensitization and sequestration of AGTR1 85, 149. In cirrhosis, impaired vascular reactivity leads to vasodilation and contributes to portal hypertension. Vascular hyporesponsiveness to angiotensin II in CCl4-treated rats is due to enhanced interaction of the AGTR1 with β-arrestin 2 and alterations in receptor function 150. Silencing β-arrestins might be a therapeutic strategy for cirrhosis.
C. Arthritis
Rheumatoid arthritis is an autoimmune disease characterized by joint inflammation and destruction. GPCRs such as chemokine receptors, G-protein-coupled receptor kinases, and β-arrestin1 and 2 are involved in the pathogenesis of rheumatoid arthritis. Reduction in GRK activity with a decreased level of GRK-2 and GRK-6 protein expression was found in patients with rheumatoid arthritis, while β-arrestin-1 expression was unchanged between rheumatoid arthritis patients and controls 151. In a rat adjuvant arthritis model, the expression of β-arrestin 1 in splenocytes was increased at the most severe period of the disease 152. Using collagen-induced arthritis and human TNFα transgenic mouse models, another group demonstrated that expression of β-arrestin 1 and 2 was elevated in joint tissue 153. Furthermore, β-arrestins differentially regulated inflammatory cytokine production by fibroblast-like synoviocytes, in which β-arrestin 1 increased TNFα and IL-6 production while β-arrestin decreased their production by synoviocytes 153. Finally, mice deficient in β-arrestin 2 exhibited more severe arthritis in the collagen-induced arthritis model 153.
The role of chemokine receptor CCR2 and β-arrestins has been investigated in inflammatory arthritis, with some studies suggesting that blocking CCR2 signaling may ameliorate arthritis in animal models. A fusion protein using GM-CSF and an N-terminal truncated version of CCL2 binding to CCR2 led to altered conformational changes in the CCR2 homodimer and failed to induce the recruitment of β-arrestin 2 to the receptor 63. The fusion peptide was capable of blocking IL-17 production in vitro and attenuated inflammatory arthritis in vivo in mice 63. This studysuggests that suppressing expression of β-arrestins may protect joints from rheumatoid arthritis.
Thus, strategies that regulate β-arrestin expression or activity may be developed for patients with rheumatoid arthritis. For example, Paeoniflorin, a monoterpene glucoside, decreased expression of β-arrestin 1 and β-arrestin 2, and increased β2-adrenergic receptor and GRK2 in the mesenteric lymph node lymphocytes, significantly attenuated arthritis scores, and reversed the changes of cytokines 154.
D. Multiple sclerosis
Multiple sclerosis is characterized by the presence of multiple plaques of demyelination throughout the central nervous system. The destruction of myelin is believed to involve autoimmune mechanisms. β-arrestin 1 was autoantibodies have been found in sera from multiple sclerosis patients 155. Multiple sclerosis patients had a greater prevalence of positive T-cell proliferative responses to β-arrestin than healthy controls 156. These studies suggest a role for β-arrestin 1 in the pathogenesis of multiple sclerosis. β-arrestin 1 expression was increased in brains of multiple sclerosis patients relative to non-multiple sclerosis brains 157. This suggests β-arrestin 1 may be a negative regulator in multiple sclerosis. In contrast, another study showed β-arrestin 1 mRNA levels were reduced by phitoemagglutinin (PHA), but, increased in interferon β-1a-treated mononuclear leukocytes? 26. Since IFNβ-1a is known to ameliorate the course of multiple sclerosis, β-arrestin 1 may be a downstream mediator in multiple sclerosis. Additional studies suggest that β-arrestin 1 is critical for CD4+ T cell survival and may be a factor in susceptibility to autoimmunity 46.
E. Encephalomyelitis
β-arrestin protein expression was significantly increased in splenocytes following myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE), while G-protein-coupled receptor kinases (GRK) 2, GRK 6, and A1 adenosine receptor were decreased during the disease 157; 158. Mice deficient in β-arrestin 1 were much more resistant to experimental autoimmune encephalomyelitis, whereas overexpression of β-arrestin 1 increased susceptibility to the disease 46. Glucocorticoid treatment eliminated EAE-induced neuroinflammation and neurobehavioral deficits, and also reduced β-arrestin 1 expression and enhanced A1 adenosine receptor expression 157. These suggest that β-arrestins may play a pathogenic role in EAE.
F. Acute rejection in organ transplant
β-arrestin 1 and 2 are expressed by intravascular renal graft monocytes and T cells in a rat model of kidney transplantation, and β-arrestin 1 and 2 expression is decreased in monocytes during rejection. Decreased β-arrestin 2 expression was concurrent with depression of I-κB and augmentation of NF-κB 28. These data suggest that the up-regulation of β-arrestin 2 in renal isografts may suppress the activation of blood monocytes via NF-κB activation. Ischemic injury following heart transplantation reduced β-arrestin 1 expression 159. Immunomodulatory peptide α-melanocyte stimulating hormone was able to preserve β-arrestin 1 protein levels 159 and reduced damage in transplanted heart grafts.
G. Asthma
Asthma is characterized by chronic airway inflammation and airway hyperresponsiveness. Studies have showed that Th2 cytokines such as IL-4 and IL-13 play a key role in the pathogenesis. β-arrestin 2 regulated T cell function in human asthma and animal models of allergic asthma. β-arrestin 2 knockout mice are protected from OVA induced allergic-asthmatic inflammation but not LPS mediated non-allergic inflammation suggesting that neutrophil recruitment is not dependent on. β-arrestin 2 β-arrestin 2 deficient mice are competent in their ability to present antigen, to generate antigen-specific T cell responses, and to undergo Ig isotype switching, but they have defective macrophage-derived chemokine-mediated CD4+ T lymphocytes migration to sites of inflammation 55. A subsequent study determined the cell types required for β-arrestin 2-dependent allergic pathology and inflammation. By using bone marrow chimera mice, the same group was able to show expression of β-arrestin 2 in eosinophilic and lymphocytic cells contributes to the airway inflammation, while expression of β-arrestin 2 in structural cells contributes to the airway hyperresponsiveness 66. Another study investigated the role of β-arrestin 2 in CD4+ T lymphocytes, and found that β-arrestin 2 regulated IL-4 production and GATA3 expression 47.
β-arrestins can regulate beta-agonist-induced desensitization of airway smooth muscle beta-2-adrenergic receptors, which is the cause of loss of bronchoprotective effect and deterioration of asthma control by beta-agonists. β-arrestin 2 deficient mice show augmented beta-agonist-mediated airway smooth muscle relaxation, and increased beta-agonist-stimulated cyclic adenosine monophosphate production. Knockdown of β-arrestins in cultured human airway smooth muscle cells demonstrated increased beta-agonist-stimulated cyclic adenosine monophosphate production. Collectively, these studies suggest that β-arrestins regulate beta-2-adrenergic receptor signaling and function in airway smooth muscle 160; 161; 162.
Interleukin-17 is a pro-inflammatory cytokine that plays a critical role in the pathogenesis of allergic asthma. A recent study found that β-arrestin2 and IL-17 expression in CD4+ T cells from a murine asthma model were increased compared with those from wild-type mice 64. Moreover, β-arrestin2 stimulated IL-17 production and expression of CD4+ T lymphocytes in a murine asthma model 64.
H. Cystic fibrosis
β-arrestin 2 protein level is increased in cystic fibrosis cell models, Cftr−/− mouse nasal epithelia, and nasal scrapes obtained from cystic fibrosis patients 163. Elevated β-arrestin 2 expression leads to increased cAMP response element binding protein (CREB) activation and ERK activation in βarr2-GFP expressing tracheal epithelial cells. Inhibition of ERK resulted in diminished cAMP response element (CRE) activation in both cells with and without βarr2-GFP expressiion. Nasal epithelium excised from Cftr and β-arrestin 2 double knockout mice exhibit reduced pCREB and pERK levels compared to Cftr−/− mice, but similar to WT mice. Thus, β-arrestin 2 directly regulates cystic fibrosis induced CREB activation through the ERK signaling pathway 164.
I. Cardioprotection
In addition to regulating the PKA-apoptosis pathway, β1AR activation also signals through the β-arrestin-Src-MMP pathway, leading to transactivation of the EGF receptor and promoting cardiomyocyte survival 165. Both β-arrestin 1 and β-arrestin 2 are required for β1AR transactivation of the EGF receptor 165. Mechanical stretch in the heart triggers activation of AT1aR and β-arrestin recruitment to the receptor in the absence of ligand 166. β-arrestin deficient hearts failed to induce a cardioprotective response to mechanical stretch and demonstrated enhanced myocyte apoptosis 166, suggesting that the heart responds to mechanical stress by activating β-arrestin-mediated cell survival signals 167; 168.
VII. β-arrestins in therapeutic development for inflammatory diseases
Understanding β-arrestin biology offers the potential to accelerate drug development in targeting GPCR functions (reviews 169; 170). Several assay systems have been proposed and tested to target β-arrestins. For example, β-arrestin conformational changes were used to develop a biased agonist to the GPR109A receptor, leading to a therapeutic targeting lipolytic effect, but devoid of potential cutaneous side effects171.
RNA interference targeting β-arrestins has been used to demonstrate the role of arrestins in desensitization, internalization, and signaling functions 125; 172. Furthermore, these specific siRNAs have been used in vitro as well as in vivo settings. Silencing β-arrestin 2 with RNA interference in allergic asthmatic mice reduced Th2 cytokines such as IL-4 47. Silencing β-arrestin 2 reduced fibroblast invasiveness 131. Suppression of beta-arrestin 2 expression using small interfering RNA eliminated AT(1A)R- and LPAR1-mediated chemotaxis 86.
There are few reports exploring the interplay between microRNAs and β-arrestins. For example, β-arrestin 2-mediated ERK phosphorylation is required for the down-regulation of miR-190 173. Expression of miR-326 is decreased in human glioblastomas and correllated with decreased expression of host gene β-arrestin 1 174. Although there are no reports identifying β-arrestins as targets of any microRNAs thus far, microRNAs regulating β-arrestin expression may prove to be a powerful strategy for therapeutic development.
Therapeutic targeting of β-arrestins has been challenging thus far. β-arrestins have been shown to interact with an array of receptors as well as downstream proteins. For example, there are over a hundred proteins interacting with either β-arrestin when angiotensin II type 1a receptor is activated, by proteomic analysis 175. Furthermore, there are 171 proteins with increased phosphorylation, and 53 with decreased phosphorylation, which represent a β-arrestin-mediated kinase-substrate network involved in a number of cellular pathways 176. However, the protean impact of β-arrestins in the pathobiology of a variety of diseases processes suggest future efforts at developing agonists or antagonists will continue.
Table 1.
Disease models with β-arrestin 1 and β-arrestin 2 deficient mice
| Disease | Disease model | Mouse model | Key phenotype | References |
|---|---|---|---|---|
| Lung fibrosis | Bleomycin-induced lung fibrosis | βarr1−/− or βarr2−/− | Reduced susceptibility and lung fibrosis | 131 |
| Asthma | Ovalbumin sensitization model of asthma | βarr2−/− | Reduced airyway hyperresponsiveness | 55,66 |
| Infection | Cytomegalovirus infection | βarr2−/− | Reduced cytomegalovirus infection | 37 |
| Sepsis | Galactosamine-D sensitized model of endotoxic shock | βarr2−/− | More susceptible to endotoxic shock | 111 |
| Sepsis | LPS injection or Caecal ligation and puncture | βarr1−/− or βarr2−/− | Protected from endotoxic shock and lethality. | 112,135 |
| Wound healing | Skin air pouch model of inflammation | βarr2−/− | Increased re-epithelialization | 36 |
| Encephalomyelitis | Experimental autoimmune encephalomyelitis | βarr1−/− | Resistant to experimental autoimmune encephalomyelitis | 46 |
| Stress | Chronic physical restraint | βarr2−/− | Augments stress-induced immune suppression | 62; 116 |
| Cutaneous flushing | Nicotinic acid induced-cutaneous flushing | βarr1−/− | Reduced cutaneous flushing in response to nicotinic acid | 171 |
| Lung cancer | Teterotopic murine Lewis lung cancer and tail vein metastasis tumor model | βarr2−/− | Increase in Lewis lung cancer tumor growth and metastasis | 17 |
| Rheumatoid arthritis | Collagen-induced arthritis | βarr2−/− | More severe arthritis in collagen-induced arthritis | 153 |
Contributor Information
Dianhua Jiang, Email: dianhua.jiang@duke.edu.
Paul W. Noble, Email: paul.noble@duke.edu.
References
- 1.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane Translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 3.Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. Activation-dependent conformational changes in {beta}-arrestin 2. J Biol Chem. 2004;279:55744–55753. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
- 4.Vishnivetskiy SA, Schubert C, Climaco GC, Gurevich YV, Velez MG, Gurevich VV. An additional phosphate-binding element in arrestin molecule. Implications for the mechanism of arrestin activation. J Biol Chem. 2000;275:41049–41057. doi: 10.1074/jbc.M007159200. [DOI] [PubMed] [Google Scholar]
- 5.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 6.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 7.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 8.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 9.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 11.Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 13.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A–stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci U S A. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghuwanshi SK, Nasser MW, Chen X, Strieter RM, Richardson RM. Depletion of beta-arrestin-2 promotes tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2008;180:5699–5706. doi: 10.4049/jimmunol.180.8.5699. [DOI] [PubMed] [Google Scholar]
- 18.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 19.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 20.Yue R, Kang J, Zhao C, Hu W, Tang Y, Liu X, Pei G. Beta-arrestin1 regulates zebrafish hematopoiesis through binding to YY1 and relieving polycomb group repression. Cell. 2009;139:535–546. doi: 10.1016/j.cell.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 21.Lattin J, Zidar DA, Schroder K, Kellie S, Hume DA, Sweet MJ. G-protein-coupled receptor expression, function, and signaling in macrophages. J Leukoc Biol. 2007;82:16–32. doi: 10.1189/jlb.0107051. [DOI] [PubMed] [Google Scholar]
- 22.Parruti G, Peracchia F, Sallese M, Ambrosini G, Masini M, Rotilio D, De Blasi A. Molecular analysis of human beta-arrestin-1: cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J Biol Chem. 1993;268:9753–9761. [PubMed] [Google Scholar]
- 23.Kizaki T, Izawa T, Sakurai T, Haga S, Taniguchi N, Tajiri H, Watanabe K, Day NK, Toba K, Ohno H. Beta2-adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin 2. Immunology. 2008;124:348–356. doi: 10.1111/j.1365-2567.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loniewski K, Shi Y, Pestka J, Parameswaran N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol Immunol. 2008;45:2312–2322. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem. 2001;276:49236–49243. doi: 10.1074/jbc.M108523200. [DOI] [PubMed] [Google Scholar]
- 26.Giorelli M, Livrea P, Defazio G, Iacovelli L, Capobianco L, Picascia A, Sallese M, Martino D, Aniello MS, Trojano M, De Blasi A. Interferon beta-1a counteracts effects of activation on the expression of G-protein-coupled receptor kinases 2 and 3, beta-arrestin-1, and regulators of G-protein signalling 2 and 16 in human mononuclear leukocytes. Cell Signal. 2002;14:673–678. doi: 10.1016/s0898-6568(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 27.Xia R, Hu Z, Sun Y, Chen S, Gu M, Zhou Y, Han Z, Zhong R, Deng A, Wen H. Overexpression of beta-arrestin 2 in peripheral blood mononuclear cells of patients with cryptococcal meningitis. J Interferon Cytokine Res. 2010;30:155–162. doi: 10.1089/jir.2009.0017. [DOI] [PubMed] [Google Scholar]
- 28.Zakrzewicz A, Krasteva G, Wilhelm J, Dietrich H, Wilker S, Padberg W, Wygrecka M, Grau V. Reduced expression of arrestin beta 2 by graft monocytes during acute rejection of rat kidneys. Immunobiology. 2011;216:854–861. doi: 10.1016/j.imbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Cheung R, Malik M, Ravyn V, Tomkowicz B, Ptasznik A, Collman RG. An arrestin-dependent multi-kinase signaling complex mediates MIP-1beta/CCL4 signaling and chemotaxis of primary human macrophages. Journal of leukocyte biology. 2009;86:833–845. doi: 10.1189/jlb.0908551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez AC, Mayor F., Jr Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc Natl Acad Sci U S A. 1998;95:2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 32.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 34.Basher F, Fan H, Zingarelli B, Borg KT, Luttrell LM, Tempel GE, Halushka PV, Cook JA. beta-Arrestin 2: a Negative Regulator of Inflammatory Responses in Polymorphonuclear Leukocytes. Int J Clin Exp Med. 2008;1:32–41. [PMC free article] [PubMed] [Google Scholar]
- 35.Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ. Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol. 2000;1:227–233. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- 36.Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. Journal of immunology. 2005;175:5396–5402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu MC, Su LL, Zou L, Liu Y, Wu N, Kong L, Zhuang ZH, Sun L, Liu HP, Hu JH, Li D, Strominger JL, Zang JW, Pei G, Ge BX. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol. 2008;9:898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]
- 38.Bari R, Bell T, Leung WH, Vong QP, Chan WK, Das Gupta N, Holladay M, Rooney B, Leung W. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood. 2009;114:5182–5190. doi: 10.1182/blood-2009-07-231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M, Bunnett NW. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. The Journal of biological chemistry. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 40.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 41.Vibhuti A, Gupta K, Subramanian H, Guo Q, Ali H. Distinct and shared roles of beta-arrestin-1 and beta-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One. 2011;6:e19585. doi: 10.1371/journal.pone.0019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahamed J, Haribabu B, Ali H. Cutting edge: Differential regulation of chemoattractant receptor-induced degranulation and chemokine production by receptor phosphorylation. J Immunol. 2001;167:3559–3563. doi: 10.4049/jimmunol.167.7.3559. [DOI] [PubMed] [Google Scholar]
- 43.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lattin JE, Greenwood KP, Daly NL, Kelly G, Zidar DA, Clark RJ, Thomas WG, Kellie S, Craik DJ, Hume DA, Sweet MJ. Beta-arrestin 2 is required for complement C1q expression in macrophages and constrains factor-independent survival. Molecular immunology. 2009;47:340–347. doi: 10.1016/j.molimm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y, Feng Y, Kang J, Liu C, Li Z, Li D, Cao W, Qiu J, Guo Z, Bi E, Zang L, Lu C, Zhang JZ, Pei G. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Liu Y, Yang M, Liu S, Ma L, Gong S, Li K, Zhang L, Xiang X. Effects of beta-arrestin 2 on cytokine production of CD4+ T lymphocytes of mice with allergic asthma. Indian J Exp Biol. 2011;49:585–593. [PubMed] [Google Scholar]
- 48.Bjorgo E, Solheim SA, Abrahamsen H, Baillie GS, Brown KM, Berge T, Okkenhaug K, Houslay MD, Tasken K. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Mol Cell Biol. 2010;30:1660–1672. doi: 10.1128/MCB.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjorgo E, Moltu K, Tasken K. Phosphodiesterases as targets for modulating T-cell responses. Handb Exp Pharmacol. 2011:345–363. doi: 10.1007/978-3-642-17969-3_15. [DOI] [PubMed] [Google Scholar]
- 50.Abrahamsen H, Baillie G, Ngai J, Vang T, Nika K, Ruppelt A, Mustelin T, Zaccolo M, Houslay M, Tasken K. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR signaling. J Immunol. 2004;173:4847–4858. doi: 10.4049/jimmunol.173.8.4847. [DOI] [PubMed] [Google Scholar]
- 51.Bjorgo E, Tasken K. Role of cAMP phosphodiesterase 4 in regulation of T-cell function. Crit Rev Immunol. 2006;26:443–451. doi: 10.1615/critrevimmunol.v26.i5.40. [DOI] [PubMed] [Google Scholar]
- 52.Baillie GS, Houslay MD. Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes. Curr Opin Cell Biol. 2005;17:129–134. doi: 10.1016/j.ceb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 53.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 55.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 57.Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 58.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu Z, Huang Y, Liu Y, Sun Y, Zhou Y, Gu M, Chen Y, Xia R, Chen S, Deng A, Zhong R. beta-Arrestin 1 modulates functions of autoimmune T cells from primary biliary cirrhosis patients. J Clin Immunol. 2011;31:346–355. doi: 10.1007/s10875-010-9492-4. [DOI] [PubMed] [Google Scholar]
- 60.Moorman J, Zhang Y, Liu B, LeSage G, Chen Y, Stuart C, Prayther D, Yin D. HIV-1 gp120 primes lymphocytes for opioid-induced, beta-arrestin 2-dependent apoptosis. Biochim Biophys Acta. 2009;1793:1366–1371. doi: 10.1016/j.bbamcr.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashita M, Kimura M, Kubo M, Shimizu C, Tada T, Perlmutter RM, Nakayama T. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci U S A. 1999;96:1024–1029. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Smalligan DA, Xie N, Javer A, Zhang Y, Hanley G, Yin D. beta-Arrestin 2-mediated immune suppression induced by chronic stress. Neuroimmunomodulation. 2011;18:142–149. doi: 10.1159/000322868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafei M, Berchiche YA, Birman E, Boivin MN, Young YK, Wu JH, Heveker N, Galipeau J. An engineered GM-CSF-CCL2 fusokine is a potent inhibitor of CCR2-driven inflammation as demonstrated in a murine model of inflammatory arthritis. J Immunol. 2009;183:1759–1766. doi: 10.4049/jimmunol.0900523. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Wang GY, Liu SK, Yang MY, Ma LB, Li K, Gong SB, Zhang L, Chen P, Xiang XD. beta-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model. Iran J Allergy Asthma Immunol. 2011;10:171–182. [PubMed] [Google Scholar]
- 65.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. The Journal of biological chemistry. 2011;286:32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollingsworth JW, Theriot BS, Li Z, Lawson BL, Sunday M, Schwartz DA, Walker JK. Both hematopoietic-derived and non-hematopoietic-derived {beta}-arrestin-2 regulates murine allergic airway disease. Am J Respir Cell Mol Biol. 2010;43:269–275. doi: 10.1165/rcmb.2009-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen EI. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delekta PC, Apel IJ, Gu S, Siu K, Hattori Y, McAllister-Lucas LM, Lucas PC. Thrombin-dependent NF-{kappa}B activation and monocyte/endothelial adhesion are mediated by the CARMA3.Bcl10.MALT1 signalosome. J Biol Chem. 2010;285:41432–41442. doi: 10.1074/jbc.M110.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coureuil M, Lecuyer H, Scott MG, Boularan C, Enslen H, Soyer M, Mikaty G, Bourdoulous S, Nassif X, Marullo S. Meningococcus Hijacks a beta2-adrenoceptor/beta-Arrestin pathway to cross brain microvasculature endothelium. Cell. 2010;143:1149–1160. doi: 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 70.Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci U S A. 1997;94:14489–14894. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Barlic J, Khandaker MH, Mahon E, Andrews J, DeVries ME, Mitchell GB, Rahimpour R, Tan CM, Ferguson SS, Kelvin DJ. beta-arrestins regulate interleukin-8-induced CXCR1 internalization. J Biol Chem. 1999;274:16287–16294. doi: 10.1074/jbc.274.23.16287. [DOI] [PubMed] [Google Scholar]
- 73.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 74.Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 76.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 77.McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS One. 2009;4:e8102. doi: 10.1371/journal.pone.0008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minsaas L, Planaguma J, Madziva M, Krakstad BF, Masia-Balague M, Katz AA, Aragay AM. Filamin a binds to CCR2B and regulates its internalization. PLoS One. 2010;5:e12212. doi: 10.1371/journal.pone.0012212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huttenrauch F, Nitzki A, Lin FT, Honing S, Oppermann M. Beta-arrestin binding to CC chemokine receptor 5 requires multiple C-terminal receptor phosphorylation sites and involves a conserved Asp-Arg-Tyr sequence motif. J Biol Chem. 2002;277:30769–30777. doi: 10.1074/jbc.M204033200. [DOI] [PubMed] [Google Scholar]
- 80.Huttenrauch F, Pollok-Kopp B, Oppermann M. G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem. 2005;280:37503–37515. doi: 10.1074/jbc.M500535200. [DOI] [PubMed] [Google Scholar]
- 81.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 83.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 84.McAllister-Lucas LM, Ruland J, Siu K, Jin X, Gu S, Kim DS, Kuffa P, Kohrt D, Mak TW, Nunez G, Lucas PC. CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci U S A. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, Milligan G, Patel DD, Lefkowitz RJ. Beta-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol Pharmacol. 2005;67:1229–1236. doi: 10.1124/mol.104.006270. [DOI] [PubMed] [Google Scholar]
- 87.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 88.Willoughby EA, Collins MK. Dynamic interaction between the dual specificity phosphatase MKP7 and the JNK3 scaffold protein beta-arrestin 2. J Biol Chem. 2005;280:25651–25658. doi: 10.1074/jbc.M501926200. [DOI] [PubMed] [Google Scholar]
- 89.Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest. 1999;104:1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 92.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 93.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Neuschafer-Rube F, Hermosilla R, Rehwald M, Ronnstrand L, Schulein R, Wernstedt C, Puschel GP. Identification of a Ser/Thr cluster in the C-terminal domain of the human prostaglandin receptor EP4 that is essential for agonist-induced beta-arrestin1 recruitment but differs from the apparent principal phosphorylation site. Biochem J. 2004;379:573–585. doi: 10.1042/BJ20031820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jala VR, Shao WH, Haribabu B. Phosphorylation-independent beta-arrestin translocation and internalization of leukotriene B4 receptors. J Biol Chem. 2005;280:4880–4887. doi: 10.1074/jbc.M409821200. [DOI] [PubMed] [Google Scholar]
- 96.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khan SY, McLaughlin NJ, Kelher MR, Eckels P, Gamboni-Robertson F, Banerjee A, Silliman CC. Lysophosphatidylcholines activate G2A inducing G(alphai)-/G(alphaq/)- Ca(2)(+) flux, G(betagamma)-Hck activation and clathrin/beta-arrestin-1/GRK6 recruitment in PMNs. Biochem J. 2010;432:35–45. doi: 10.1042/BJ20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iacovelli L, Capobianco L, D'Ancona GM, Picascia A, De Blasi A. Regulation of lysophosphatidic acid receptor-stimulated response by G-protein-coupled receptor kinase-2 and beta-arrestin1 in FRTL-5 rat thyroid cells. J Endocrinol. 2002;174:103–110. doi: 10.1677/joe.0.1740103. [DOI] [PubMed] [Google Scholar]
- 99.Gesty-Palmer D, El Shewy H, Kohout TA, Luttrell LM. beta-Arrestin 2 expression determines the transcriptional response to lysophosphatidic acid stimulation in murine embryo fibroblasts. J Biol Chem. 2005;280:32157–32167. doi: 10.1074/jbc.M507460200. [DOI] [PubMed] [Google Scholar]
- 100.Sun J, Lin X. Beta-arrestin 2 is required for lysophosphatidic acid-induced NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:17085–17090. doi: 10.1073/pnas.0802701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res. 2009;7:1064–1077. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- 102.Chen Z, Dupre DJ, Le Gouill C, Rola-Pleszczynski M, Stankova J. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C terminus and the (D/N)PXXY motif. J Biol Chem. 2002;277:7356–7362. doi: 10.1074/jbc.M110058200. [DOI] [PubMed] [Google Scholar]
- 103.Venkatesha RT, Ahamed J, Nuesch C, Zaidi AK, Ali H. Platelet-activating factor-induced chemokine gene expression requires NF-kappaB activation and Ca2+/calcineurin signaling pathways. Inhibition by receptor phosphorylation and beta-arrestin recruitment. J Biol Chem. 2004;279:44606–44612. doi: 10.1074/jbc.M408035200. [DOI] [PubMed] [Google Scholar]
- 104.McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol. 2006;176:7039–7050. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- 105.McLaughlin NJ, Banerjee A, Khan SY, Lieber JL, Kelher MR, Gamboni-Robertson F, Sheppard FR, Moore EE, Mierau GW, Elzi DJ, Silliman CC. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J Immunol. 2008;180:8192–8203. doi: 10.4049/jimmunol.180.12.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. beta -Arrestins regulate protease-activated receptor-1 desensitization but not internalization or Down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 107.Zoudilova M, Min J, Richards HL, Carter D, Huang T, DeFea KA. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem. 2010;285:14318–14329. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 110.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 112.Porter KJ, Gonipeta B, Parvataneni S, Appledorn DM, Patial S, Sharma D, Gangur V, Amalfitano A, Parameswaran N. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by beta-arrestins. J Cell Physiol. 2010;225:406–416. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. Beta-arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol. 2007;44:3092–3099. doi: 10.1016/j.molimm.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seregin SS, Appledorn DM, Patial S, Bujold M, Nance W, Godbehere S, Parameswaran N, Amalfitano A. beta-Arrestins modulate Adenovirus-vector-induced innate immune responses: differential regulation by beta-arrestin-1 and beta-arrestin-2. Virus Res. 2010;147:123–134. doi: 10.1016/j.virusres.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li H, Sun X, LeSage G, Zhang Y, Liang Z, Chen J, Hanley G, He L, Sun S, Yin D. beta-arrestin 2 regulates Toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130:556–563. doi: 10.1111/j.1365-2567.2010.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li H, Chen L, Zhang Y, Lesage G, Wu Y, Hanley G, Sun S, Yin D. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a beta-arrestin 2 dependent manner. J Neuroimmunol. 2011;233:73–79. doi: 10.1016/j.jneuroim.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, Benovic JL. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 119.Tipping M, Kim Y, Kyriakakis P, Tong M, Shvartsman SY, Veraksa A. beta-arrestin Kurtz inhibits MAPK and Toll signalling in Drosophila development. EMBO J. 2010;29:3222–3235. doi: 10.1038/emboj.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W, Xu M, Zhang YY, He B. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol Sin. 2009;30:1522–1528. doi: 10.1038/aps.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kizaki T, Shirato K, Sakurai T, Ogasawara JE, Oh-ishi S, Matsuoka T, Izawa T, Imaizumi K, Haga S, Ohno H. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol Immunol. 2009;46:1195–1203. doi: 10.1016/j.molimm.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 122.Fox JM, Letellier E, Oliphant CJ, Signoret N. TLR2-dependent pathway of heterologous down-modulation for the CC chemokine receptors 1, 2, and 5 in human blood monocytes. Blood. 2011;117:1851–1860. doi: 10.1182/blood-2010-05-287474. [DOI] [PubMed] [Google Scholar]
- 123.Zhuang LN, Hu WX, Xin SM, Zhao J, Pei G. Beta-arrestin-1 protein represses adipogenesis and inflammatory responses through its interaction with peroxisome proliferator-activated receptor-gamma (PPARgamma) J Biol Chem. 2011;286:28403–28413. doi: 10.1074/jbc.M111.256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 125.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kawamata Y, Imamura T, Babendure JL, Lu JC, Yoshizaki T, Olefsky JM. Tumor necrosis factor receptor-1 can function through a G alpha q/11-beta-arrestin-1 signaling complex. J Biol Chem. 2007;282:28549–28556. doi: 10.1074/jbc.M705869200. [DOI] [PubMed] [Google Scholar]
- 127.Mo W, Zhang L, Yang G, Zhai J, Hu Z, Chen Y, Chen X, Hui L, Huang R, Hu G. Nuclear beta-arrestin1 functions as a scaffold for the dephosphorylation of STAT1 and moderates the antiviral activity of IFN-gamma. Mol Cell. 2008;31:695–707. doi: 10.1016/j.molcel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 128.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 129.Lee NY, Kirkbride KC, Sheu RD, Blobe GC. The transforming growth factor-beta type III receptor mediates distinct subcellular trafficking and downstream signaling of activin-like kinase (ALK)3 and ALK6 receptors. Mol Biol Cell. 2009;20:4362–4370. doi: 10.1091/mbc.E09-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mythreye K, Blobe GC. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci U S A. 2009;106:8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lovgren AK, Kovacs JJ, Xie T, Potts EN, Li Y, Foster WM, Liang J, Meltzer EB, Jiang D, Lefkowitz RJ, Noble PW. beta-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3:74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J. 2005;387:573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tasken K, Stokka AJ. The molecular machinery for cAMP-dependent immunomodulation in T-cells. Biochem Soc Trans. 2006;34:476–479. doi: 10.1042/BST0340476. [DOI] [PubMed] [Google Scholar]
- 134.Bitto A, Minutoli L, David A, Irrera N, Rinaldi M, Venuti FS, Squadrito F, Altavilla D. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care. 2012;16:R32. doi: 10.1186/1364-8535-16-R32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Fan H, Bitto A, Zingarelli B, Luttrell LM, Borg K, Halushka PV, Cook JA. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, Dumenil G, Mege RM, Weksler BB, Romero IA, Couraud PO, Nassif X. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tourret J, Finlay BB. A receptor for meningococcus: eliciting beta-arrestin signaling for barrier breaching. Dev Cell. 2011;20:7–8. doi: 10.1016/j.devcel.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 138.Lecuyer H, Nassif X, Coureuil M. Two strikingly different signaling pathways are induced by meningococcal type IV pili on endothelial and epithelial cells. Infection and immunity. 2012;80:175–186. doi: 10.1128/IAI.05837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 140.Papp M, Li X, Zhuang J, Wang R, Uhal BD. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282:L713–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 141.Uhal BD, Li X, Xue A, Gao X, Abdul-Hafez A. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1–7/Mas axis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L269–L274. doi: 10.1152/ajplung.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 145.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 146.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, Li Y, Tager AM, Goetinck PF, Luster AD, Noble PW. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 148.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hennenberg M, Trebicka J, Biecker E, Schepke M, Sauerbruch T, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495–506. doi: 10.1002/hep.21502. [DOI] [PubMed] [Google Scholar]
- 150.Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest. 2009;39:906–913. doi: 10.1111/j.1365-2362.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 151.Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- 152.Lombardi MS, Kavelaars A, Cobelens PM, Schmidt RE, Schedlowski M, Heijnen CJ. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J Immunol. 2001;166:1635–1640. doi: 10.4049/jimmunol.166.3.1635. [DOI] [PubMed] [Google Scholar]
- 153.Li P, Cook JA, Gilkeson GS, Luttrell LM, Wang L, Borg KT, Halushka PV, Fan H. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: Isoform specific regulation of inflammation. Mol Immunol. 2011;49:64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu H, Wei W, Song L, Zhang L, Chen Y, Hu X. Paeoniflorin induced immune tolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergic receptor desensitization in rats with adjuvant arthritis. Int Immunopharmacol. 2007;7:662–673. doi: 10.1016/j.intimp.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 155.Ohguro H, Chiba S, Igarashi Y, Matsumoto H, Akino T, Palczewski K. Beta-arrestin and arrestin are recognized by autoantibodies in sera from multiple sclerosis patients. Proc Natl Acad Sci U S A. 1993;90:3241–3245. doi: 10.1073/pnas.90.8.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forooghian F, Cheung RK, Smith WC, O'Connor P, Dosch HM. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol. 2007;27:388–396. doi: 10.1007/s10875-007-9091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsutsui S, Vergote D, Shariat N, Warren K, Ferguson SS, Power C. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J. 2008;22:786–796. doi: 10.1096/fj.07-9002com. [DOI] [PubMed] [Google Scholar]
- 158.Vroon A, Lombardi MS, Kavelaars A, Heijnen CJ. Changes in the G-protein-coupled receptor desensitization machinery during relapsing-progressive experimental allergic encephalomyelitis. J Neuroimmunol. 2003;137:79–86. doi: 10.1016/s0165-5728(03)00050-x. [DOI] [PubMed] [Google Scholar]
- 159.Colombo G, Sordi A, Lonati C, Carlin A, Turcatti F, Leonardi P, Gatti S, Catania A. Treatment with alpha-melanocyte stimulating hormone preserves calcium regulatory proteins in rat heart allografts. Brain Behav Immun. 2008;22:817–823. doi: 10.1016/j.bbi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 160.Deshpande DA, Theriot BS, Penn RB, Walker JK. Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–2141. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang WC, Mihlbachler KA, Brunnett AC, Liggett SB. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway beta2-adrenergic receptor physiologic signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15007–15012. doi: 10.1073/pnas.0906034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dickey BF, Walker JK, Hanania NA, Bond RA. beta-Adrenoceptor inverse agonists in asthma. Curr Opin Pharmacol. 2010;10:254–259. doi: 10.1016/j.coph.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Manson ME, Corey DA, White NM, Kelley TJ. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. American journal of physiology. Lung cellular and molecular physiology. 2008;295:L809–L819. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Manson ME, Corey DA, Rymut SM, Kelley TJ. beta-arrestin-2 regulation of the cAMP response element binding protein. Biochemistry. 2011;50:6022–6029. doi: 10.1021/bi200015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Engelhardt S. Alternative signaling: cardiomyocyte beta1-adrenergic receptors signal through EGFRs. J Clin Invest. 2007;117:2396–2398. doi: 10.1172/JCI33135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Noor N, Patel CB, Rockman HA. Beta-arrestin: a signaling molecule and potential therapeutic target for heart failure. J Mol Cell Cardiol. 2011;51:534–541. doi: 10.1016/j.yjmcc.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci U S A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY. mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol. 2010;77:102–109. doi: 10.1124/mol.109.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kefas B, Comeau L, Floyd DH, Seleverstov O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca EA, Lee J, Fine H, Abounader R, Lawler S, Purow B. The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J Neurosci. 2009;29:15161–15168. doi: 10.1523/JNEUROSCI.4966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, 3rd, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma'ayan A, Gygi SP, Lefkowitz RJ. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci U S A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]