Abstract
A new phenotype of multiple organ failure has appeared: Persistent Inflammatory, Immunosuppressed, Catabolic Syndrome (PICS). Comorbidities and age >65 years have been established as the leading risk factors for PICS. As the percentage of elderly people continues to increase the prevalence of PICS in our ICUs will surely grow. Malnutrition (despite appropriate supplementation), recurrent nosocomial infections, frailty, ventilator dependence, and an indolent death depicts the central theme that plagues PICS patients. Aligned with the recently awarded P50 grant by NIGMS entitled, “PICS: A New Horizon for Surgical Critical Care”, and the University Of Florida’s Sepsis and Critical Illness Research Center will investigate the genetic make-up of PICS patients, better understand frailty and the implication in trauma patients, and hopefully elucidate new therapies. Currently, there are no therapies to combat PICS aside from nutritional inference elaborated after reviewing the literature on Burns, Cachexia, and Sarcopenia.
Keywords: Persistent, Inflammation, Immunosuppression, Catabolism, Syndrome, PICS, nutrition, Multiple Organ Failure, MOF
Introduction
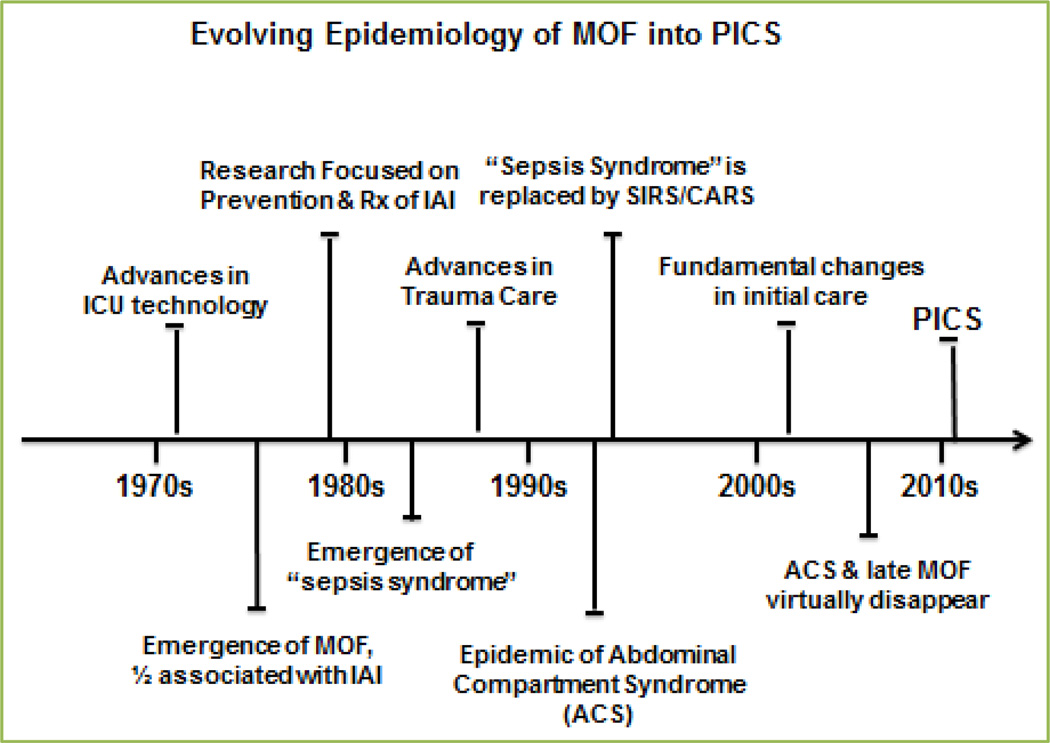
Surgical intensive care units (ICUs) initiated in the 1970s resulted in more patients surviving single organ failure. When discussing organ failure there is a certain paradigm that has become clear: once one phenotype of organ failure is treated a new phenotype replaces the existing one. As ICU care improved though the 1970s–1980s, a significant number of single organ failure patients progressed to developed multiple organ failure (MOF)[1]. This became an epidemic by the mid-1980s unfortunately carrying a prohibitive mortality of > 80% for the full blown syndrome, currently it remains >40% mortality[2]. Over the last 35 years ICU care has improved dramatically, and as a consequence the epidemiology of MOF has evolved considerably producing newer phenotypes and pathophysiology. MOF is still the leading cause of surgical ICU deaths, however, fewer patients are dying in the early phase of MOF[2]. Fewer deaths, coupled with decreasing late onset MOF, has made prolonged ICU stays more common. Certainly, as one phenotype disappeared, another becomes overt.
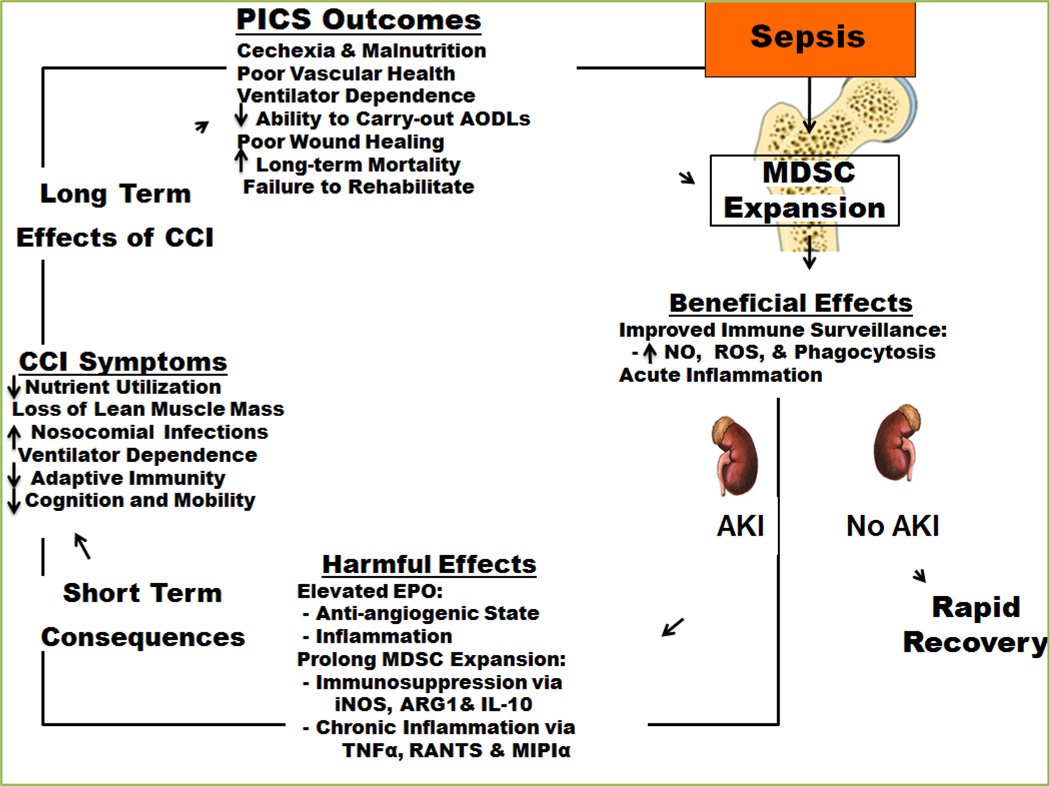
Most recently, the new phenotype of MOF is a smoldering inflammatory state, increased susceptibility to infection, and lean muscle protein loss refractory to nutritional intervention (similar to cachexia and sarcopenia) has been described. This syndrome has been coined the Persistent Inflammatory, Immunosuppression, Catabolism Syndrome (PICS) (Figure 1). Early on it was recognized that despite the MOF phenotype, these high risk patients experienced significant hypermetabolism and expansion of immature cell lineage called myeloid derived suppressor cells (MDSCs), which both appear to play a key role in the pathogenesis of end organ dysfunction[3–5].
Figure 1. Clinical Progression to PICS.
As the predominant phenotypes of MOF evolve into PICS (Figure 2), so has the rationale for various therapeutic interventions. The purpose of this manuscript is to a) introduce the newest phenotype of MOF: Persistent Inflammatory, Immunosuppression, Catabolism Syndrome (PICS), b) describe the underlying pathophysiology of chronic critical illness (defined as ICU stay > 14 days), and c) discuss potential therapeutic interventions.
Figure 2. Schematic depiction of the evolution of MOF to PICS.
Evolution of Multiorgan Failure to Persistent Inflammatory Immunosuppression Catabolism Syndrome
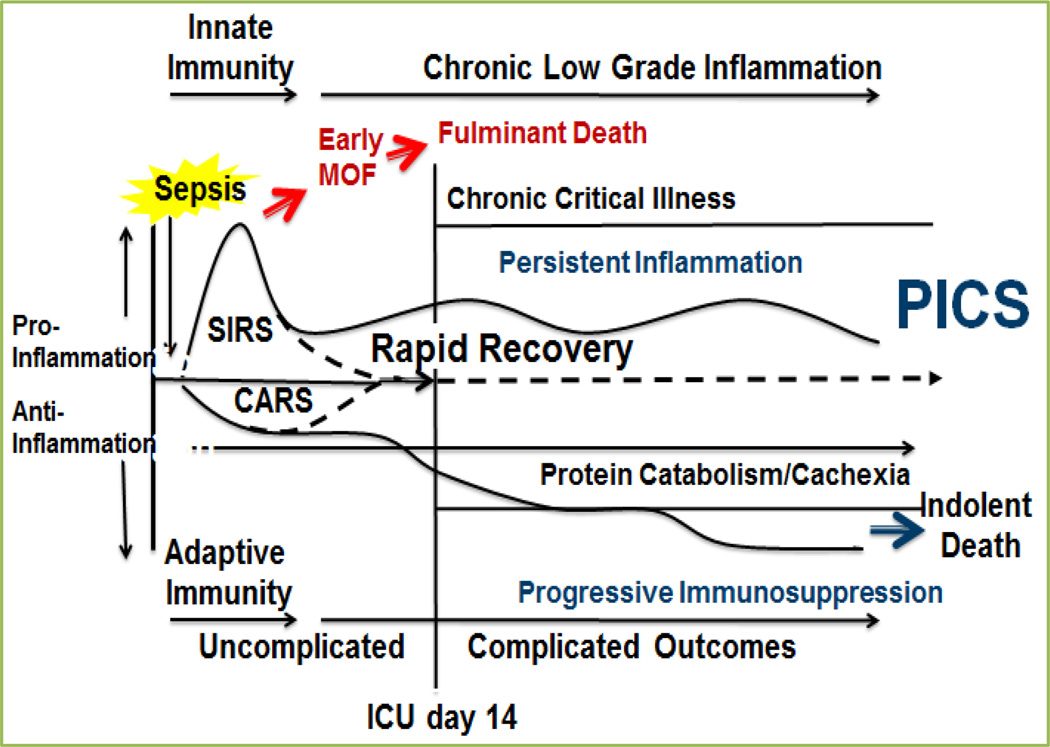
In understanding the pathophysiology of MOF to PICS there has to be a discussion and understanding of the etiology of multiple organ failure from Systemic Inflammatory Response Syndrome (SIRS), Compensatory Anti-Inflammatory Response Syndrome (CARS), and the evolution of Chronic Critical Illness (CCI) into PICS (Figure 3).
Figure 3. How PICS becomes the final outcome.
(MOF=Multiple Organ Failure. SIRS=Systemic Inflammatory Response Syndrome. CARS= Compensatory Anti-Inflammatory Response Syndrome. PICS= Persistent Inflammation, Immunosuppression, Catabolism Syndrome.)
SIRS
Initially when a patient suffers a traumatic injury, undergoes surgery, critical illness like sepsis (sepsis syndrome), or intense inflammatory process like pancreatitis the insult triggers a systemic response: SIRS.[6, 7] “Systemic inflammatory response syndrome (SIRS) is the clinical expression of the action of complex intrinsic mediators of the acute phase reaction. SIRS then can compromise the function of various end organ systems resulting in MOF. SIRS and MOF are graded expressions of the inflammation associated with acute illness”[8].
The SIRS response is defined as satisfying two of four criteria according to Bone et al at the American College of Chest Physician and Society of Critical Care Medicine in 1992[6]: temperature 38°C or 36°C; heart rate 90 beats/min; respiratory rate 20 breaths/min or PaCO2 32 torr (4.3 kPa); WBC 12,000 cells/mm3, 4000cells/mm3 or 10% immature (band) forms
If the SIRS response is robust enough early onset MOF can result and even death, but often times ICU care predominates and the patients survive the initial insult. To better comprehend the epidemiology of postinjury MOF, Frederick Moore, M.D and investigators at University of Colorado Trauma Research Center (UCTRC) developed the Denver MOF score and a clinical database to characterize postinjury MOF. With ongoing analysis, it was observed that MOF was a bimodal phenomenon, and using the best available information, another paradigm for MOF was proposed.[9] In 1996, the prospective study evaluated 457 high-risk trauma patients who survived more than 48 hours. 70 (15%) developed MOF, and of these 70 patients, 27 (39%) patients suffered early onset MOF (defined as end organ failure within 72hrs of inciting insult), while the majority, 43 (61%) patients presented later in their clinical course.[9] At presentation, early MOF had more cardiac dysfunction, while late MOF had greater hepatic failure. According to Moore, shock correlated more frequently with early MOF, while advanced age was a more important risk factor for late MOF[9]. Both early and late MOF had a similar high incidence of major infections, but these infections appeared to be more important in precipitating late MOF[9]. Thus, Moore and colleagues posed that MOF has a bimodal phenomenon, where by early onset MOF typically results from a robust SIRS response and late onset MOF is elicited by a second hit infection in immunosuppressed, critically ill patients that depict the phenotype CARS.
This theory was well accepted at the time, but current literature has established a different paradigm. It is now understood and accepted that the both SIRS and CARS can be triggered as a simultaneous response. Each having implications in the clinical course of the patient, as well as, precipitating MOF. Discussed next CARS is not a syndrome that occurs in response to SIRS, but rather occurs with SIRS.
CARS
Originally, Bone et al postulated that as time proceeds certain aspects of SIRS are intentionally down-regulated to minimize autogenous tissue injury[10]. As a consequence, critically ill patients can develop severe immunosuppression as a dysfunction of adaptive immune system (CARS), thus setting the stage for late nosocomial infections that can precipitate late onset MOF[10]. According to Ward et al., “CARS, similar to SIRS, is a complex and incompletely defined pattern of immunologic responses to severe insult. The difference was that while SIRS was a pro-inflammatory syndrome that seemed tasked with killing infectious organisms through activation of the immune system, CARS was a systemic deactivation of the immune system tasked with restoring homeostasis from an inflammatory state. Additionally, it has a distinct set of cytokines and cellular responses and has a powerful influence on clinical outcomes in sepsis”[11].
The paradigm that SIRS results as a consequence of the innate inflammatory response (principally PMNs)[12, 13], and CARS represents the adaptive immune response (principally lymphocytes)[14–16] has been accepted. Thus, it was found that late MOF is actually a lingering state of CARS where infections and poor wound healing are symptoms of underlying pathophysiology[15]. By the late 1990s the SIRS/CARS paradigm became the popular mechanistic explanation for the immunologic trajectory of a complicated ICU course, but as these patients lingered in the ICU for great lengths of time the manageable MOF with catabolic state, smoldering inflammation, and on-going immunosuppression produced the newest described phenotype: PICS.
According to Moore et al., the perception that trauma patients become severely immunosuppressed was not new, but the link between the severity of delayed immunosuppression and the severity of early SIRS (which could be amplified by second hits) was novel.[17] This notion was generated from focused observational clinical studies; showing that high-risk patients develop an early vulnerable window of neutrophil (PMN) priming, activation, and sequestration, which are pivotal mechanisms in early MOF[12, 13]. Next there’s a period when circulating PMNs become functionally ineffective and bone marrow granulopoiesis becomes suppressed determining the degree of immunosuppression[18–20]. Re-iterated again, should the state of inflammation (SIRS) and immunosuppression (CARS) persists, the patient progresses to a phenotype clinically consistent with chronic critical illness, smoldering inflammation, immunosuppression, and catabolism (PICS)[21]. Unfortunately, with the advances in ICU technology the PIC syndrome is increasing in prevalence[22].
Persistent Inflammatory Immunosuppression Catabolism Syndrome
There is a considerable portion of patients who survive acute critical illness and MOF who entered into chronic critical illness (CCI - defined as being in the ICU for > 14 days with ongoing low grade organ dysfunction). In the surgical ICU a substantial portion of CCI patients developed PICS[3, 23]. Gentile and Moore et al, characterized PICS as: ICU stay >14days, C-reactive protein 150Kg/dL, Total lymphocyte count <0.80 × 109/L, Weight loss >10% during hospitalization or body mass index<18, Creatinine height index <80%, Albumin level <3.0 g/dL, Prealbumin level <10 mg/dL, Retinol binding protein level <10 µg/dL[3, 23].
The patient populations most vulnerable to PICS and CCI are those patient >65 years of age and those with poor premorbid health status[21–26]. PICS patients were observed to clinically lose tremendous amounts of lean body mass despite optimal nutrition causing profound weakness, suffer from recurrent nosocomial infections, typically develop decubitus ulcers, have poor wound healing, and become what intensivist refer to as “rocks”(Table 1)[27–29]. Additionally, these patients tend to suffer from significant levels of pain, dyspnea, psychological distress, thirst, fatigue, delirium, and distress related to impaired communication[28, 30–34]. These symptoms not only stem from the protracted course of their hospital stay, but also exposure to ICU interventions that cause distressing symptoms[28, 30, 31, 35].
Table 1.
New Predominant Phenotype of MOF Emerges
| Prolonged ICU stays - manageable organ dysfunction |
| Recurrent inflammatory insults & nosocomial infections |
| Persistent acute phase response - very high CRPs |
| Neutrophilia and lymphopenia |
| Cachexia despite good nutrition - a wasting disease |
| Poor wound healing & decubitus ulcers |
| Transfer to LTACs for indolent death |
| Sepsis recidivism |
PICS victims are typically discharged alive to long-term acute care facilities where they failed to rehabilitate and experience dismal long-term outcomes (defined as being dead or fully functionally dependent at one year post-discharge)[29, 36]. Long term quality of life would be dreary; many PICS patient suffer from depression, cognitive impairment, complex physiologic abnormalities, organ dysfunction, neuroendocrine deficits, and immunologic dysfunction[24, 25, 28, 29, 37–41]. Moreover, surviving PICS patient have exuberant financial toll on Healthcare systems[24, 42] and caregivers (strained relationships, depressed mood, adverse psychological responses, and underlying stress)[43, 44].
Ultimately, with the increasing incidence of PICS and the long term implications these patients have on caregivers, healthcare systems, and the victims themselves requires immediate attention[22, 37]. The Census Bureau estimates that between 2000 and 2025 the elderly population will grow by ~80%[45]. Thus, the University of Florida, Health Sepsis and Critical Illness Research Center (UF SCIRC) will be investigating the genomic make up of PICS, trying to predict patients at high-risk of developing PICS, and possible interventions. Through a recently awarded a P50 grant by NIGMS entitled, “PICS: A New Horizon for Surgical Critical Care” the funding will provide a strong foundation to make this achievement possible (Figure 3).
Malnutrition
The persistent smoldering inflammatory and catabolic state, as described above, produces a “cachexia” phenotype for which current ICU interventions are ineffective, rendering PICS extremely difficult to treat. Stopping this process, slowing the progression, or even reversing PICS presents many challenges. Currently, there is no existing literature for nutritional support in PICS, but, as defined earlier, one of the uniting characteristic of these patients is profound malnutrition. Weight loss >10% throughout hospital stay, Body Mass Index <18, Creatinine Height Index <80%, Albumin <3.0 g/dL, Prealbumin <10 mg/dL, or Retinol Binding Protein <10 µg/dL all define the clinical deterioration these patients experience[3, 23]. Unfortunately, the malnutrition PICS patients experience is refractory to nutritional supplementation. As discussed later, this mimics three disease states that progress similar to PICS in their clinical presentation concurrent with a state of persistent inflammation including a) aging sarcopenia, b) major burns, and c) cancer cachexia. From these three pathophysiologic states physicians can make inferences regarding adequate/optimal nutritional support for patients vulnerable to PICS.
Implied Nutritional Strategies for Combating PICS
Sarcopenia
We believe that PICS, not only dramatically exacerbates aging sarcopenia, but in many ways, imitates the pathophysiology of aging sarcopenia. Rationally, PICS and sarcopenia are both disease processes that afflict the aging population. Thus, the catabolic nature of PICS, inevitably accelerates sarcopenia. Various strategies have been employed to combat sarcopenia and indirectly PICS. These strategies involve “anabolic nutrition”. There have been a plethora of trials and studies that used supplemental hormone replacement with inconsistent results. These include testosterone[46–49], oxandralone[50], growth hormone[51], insulin-like growth factor-1[52], and dehydroepiandrosterone[53–55]. Moreover, we caution wide spread use in PICS patient until further research elucidates appropriate indications, as some hormonal supplements caused harm in critically ill patients.
According to Fiatarone et al, the two most persuasive strategies to contest sarcopenia, and thereby, PICS are resistance exercise and anabolic nutrition. In Dr. Fiatarone’s article he defines anabolic nutrition as targeting 0.8–1.5 g/kg/d of daily protein consumption[56–58]. Reinforcing Fiatarone’s claim, Paddon-Jones et al, established that daily protein consumption and dietary derived amino acids potentially slows or prevent muscle protein catabolism[59–61]. Future research identifying therapeutic interventions for PICS certainly involves aggressive early mobilization, physical therapy, and active exercise early in the ICU course of high risk PICS patients. In keeping with this mentality, Niveus Medical has designed a device for early mobility intervention to help prevent ICU acquired weakness. The intervention can be used to prevent atrophy and weakness on day 1 of the patient stay, and can be deployed by a bedside nurse in less than 5 minutes per day, even in sedated, critically ill patient.
Finally, Morely et al, and The Society of Sarcopenia, Cachexia, and Wasting Disease employed a multi-modality approach similar to Paddon-Jones to treat sarcopenia and other wasting syndromes. These strategies included providing supplemental nutrition, with a combined approach of exercise, possibly increasing supplemental leucine and creatine, and appropriate anabolic nutrition could at least slow the progression of sarcopenia in the aging population[62].
Burn
Burn injury >30% total body surface area causes profound muscle catabolism, according to Hart et al[63, 64]. Anabolic supplementation has been used to reverse muscle breakdown and protein loss sustained after burn. Hart and Herndon et al recount, “The hypermetabolic response to major burn injury is associated with increased energy expenditure, insulin resistance, immunodeficiency, and whole body catabolism that persists for months after injury”[65].
Herndon et al, has studied the effects of five anabolic supplements in pediatric burn patients including a) growth hormone[65], b) intensive insulin therapy[66, 67], c) oxandralone[50, 68], d) propranolol[69] and e) exercise programs[70]. Hart and Herndon et al, established that the human growth hormone could be a “potent anabolic agent and salutary modulator of posttraumatic metabolic responses”[65]. The conclusion was that human derived growth hormone in the pediatric burn population, when compared to a placebo, could substantially reduce muscle catabolism and osteopenia. In fact, growth hormone could increase lean muscle mass, overall weight, and height at 9 and 12 month follow-up[65].
Herndon and Jeschke have also demonstrated that strict glucose control (80–160mg/dl) in >30% TBSA, pediatric burn patients can significantly increase in bone mineralization and muscle strength in this population (p= 0.05)[64, 66, 67]. In multiple other studies, Herndon and colleagues demonstrated that oxandrolone considerably decreased resting energy expenditure and increased insulin-like growth factor-1 secretion during the first year after burn injury. These effects of oxandrolone, in combination with exercise, increased lean body mass and muscle strength[68, 71–75].
Finally, Herndon predicted a reduction in cardiac work, resting energy expenditure, and lipolysis in severely burned children treated with propranolol, which could possibly decrease skeletal muscle catabolism[69]. Herndon’s hypothesis was supported in a study that demonstrated two weeks of propranolol administration significantly reduced oxygen consumption and resting energy expenditure. In fact, “stable isotope studies revealed an astounding improvement in the net skeletal muscle protein balance that was the result of a reduction in burn-induced proteolysis with an unexpected increase in muscle anabolism following propranolol administration”[69]. These effects all represent strategic interventions that could potentially decrease the catabolism PICS patients suffer from.
Cancer Cachexia
According to Delano and Moldawer et al, “cachexia represents a complex metabolic state characterized by progressive weight loss, muscle loss, muscle atrophy, and anemia secondary to preferential use and depletion of host adipose tissue and skeletal muscle stores, mediated mainly by cytokines and hormones, with smaller contribution from tumor-derived compounds and neuropeptides. The abnormalities associated with cachexia in cancer, sepsis, and now PICS are manifested by defects in carbohydrate, lipid, and protein metabolism mediated in part by the cytokines TNF-α, IL-1, IL-6, and IFN-γ”[76].
There is a well-known association between significant injury and hyperglycemia as a result of increased glycolytic pathways and insulin resistance[77]. This phenomenon was first described in the 19th century by Bernard, and is similar to the cancer-associated glucose alterations described by Warburg in the 1920’s[77, 78]. Furthermore, these alterations in glucose metabolism are also observed in sepsis and PICS, but are believed to be multifactorial; being influenced by the hormonal/cytokine stress response setting the stage for muscle wasting from protein catabolism explained below[76].
Normal physiology during early starvation utilizes amino acids from the breakdown of skeletal muscle, which decreases the total body protein stores. This subsides as the body preferentially switches to metabolizing free fatty acids and ketone bodies from lipid metabolism for energy production[76]. The preferential use of ketone bodies instead of amino acids as fuel is imperative for preservation of nitrogen balance and conservation of lean body mass. It seems in any cachectic-like state and at times of stress, the body losses the ability to maintain nitrogen balance, culminating in protein depletion[76]. Whether from trauma, sepsis, cancer, or PICS, protein wasting is thought to revolve around increased whole-body protein turnover, increased muscle protein breakdown, decreased protein synthesis, and increased hepatic protein synthesis mediated by cytokines[79–82].
Thus, patients with cancer cachexia and cachexia of sepsis (i.e.PICS) exhibit progressive catabolism, ultimately, resulting in the development of asthenia or weakness and lack of strength[83]. Unfortunately, blocking these cytokines has not proven clinically significant in reversing the cachectic state, largely due to the overlapping activities of several inflammatory cytokines[76, 84].
In hope to better understanding PICS on a cellular and humoral level, Drs. Winfield and Moldawer et al, recently focused efforts on elaborating bone marrow derived MDSCs that expands dramatically with tumors and sepsis[84]. In response to stress the bone marrow attempts to preserve innate immunity (i.e. granulocytes, monocytes and dendritic cells) by mobilizing MDSCs and trying to shut down adaptive immunity (i.e. lymphocyte production) and erythropoiesis. These immature MDCSs are metabolically active and secrete large quantities of inflammatory cytokines and chemokines, which promote an acute phase response including protein catabolism[84]. Their expansion in both cancer and sepsis is temporally associated with the development of cachexia[84]. Unfortunately, in PICS this elaboration of MDSC’s by the bone marrow does not such down and stays active promoting the new phenotype. There could potentially be therapies directed at these cells, and prove beneficial for both cancer and sepsis cachexia (i.e. PICS). Ultimately we believe that treating PICS patients is going to be a multimodality approach and not just “one silver bullet”.
Acknowledgement
The investigators acknowledge the contribution of the Center for Sepsis and Critical Illness Award # P50 GM-111152 from the National Institute of General Medical Sciences
Footnotes
Conflict of Interest and Financial Disclosure Statement
No conflict of or competing interests have been declared and there were no funds provided for this manuscript.
References
- 1.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerra FB. Hypermetabolism, organ failure, and metabolic support. Surgery. 1987;101:1–14. [PubMed] [Google Scholar]
- 5.Border JR, Chenier R, McManamy RH, La Duca J, Seibel R, Birkhahn R, et al. Multiple systems organ failure: muscle fuel deficit with visceral protein malnutrition. Surg Clin North Am. 1976;56:1147–1167. doi: 10.1016/s0039-6109(16)41035-2. [DOI] [PubMed] [Google Scholar]
- 6.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992;268:3452–3455. [PubMed] [Google Scholar]
- 7.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 8.Nystrom PO. The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother. 1998;41(Suppl A):1–7. doi: 10.1093/jac/41.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 9.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. discussion 10–2. [DOI] [PubMed] [Google Scholar]
- 10.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617–625. viii. doi: 10.1016/j.ccm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botha AJ, Moore FA, Moore EE, Fontes B, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation states: therapeutic challenges. Shock. 1995;3:157–166. doi: 10.1097/00024382-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Botha AJ, Moore FA, Moore EE, Sauaia A, Banerjee A, Peterson VM. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J Trauma. 1995;39:411–417. doi: 10.1097/00005373-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 15.Mannick JA. 15th Annual Semmelweis Lecture Surgical Infection Society-Europe. Injury-induced immune dysfunction: is the lymphocyte irrelevant? Surg Infect (Larchmt) 2002;3:297–302. doi: 10.1089/109629602762539526. [DOI] [PubMed] [Google Scholar]
- 16.Miller-Graziano CL, Szabo G, Griffey K, Mehta B, Kodys K, Catalano D. Role of elevated monocyte transforming growth factor beta (TGF beta) production in posttrauma immunosuppression. J Clin Immunol. 1991;11:95–102. doi: 10.1007/BF00917745. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA, Moore EE. The evolving rationale for early enteral nutrition based on paradigms of multiple organ failure: a personal journey. Nutr Clin Pract. 2009;24(3):297–304. doi: 10.1177/0884533609336604. [DOI] [PubMed] [Google Scholar]
- 18.Faist E, Baue AE, Dittmer H, Heberer G. Multiple organ failure in polytrauma patients. J Trauma. 1983;23:775–787. doi: 10.1097/00005373-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Faist E, Mewes A, Baker CC, Strasser T, Alkan SS, Rieber P, et al. Prostaglandin E2 (PGE2)-dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma. 1987;27:837–848. doi: 10.1097/00005373-198708000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–459. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 21.Nierman DM. A structure of care for the chronically critically ill. Crit Care Clin. 2002;18:477–491. v. doi: 10.1016/s0749-0704(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 22.Daly BJ, Douglas SL, Kelley CG, O'Toole E, Montenegro H. Trial of a disease management program to reduce hospital readmissions of the chronically critically ill. Chest. 2005;128:507–517. doi: 10.1378/chest.128.2.507. [DOI] [PubMed] [Google Scholar]
- 23.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:21–29. doi: 10.1097/TA.0b013e3182ab1ab5. discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson SS, Bach PB. The epidemiology and costs of chronic critical illness. Crit Care Clin. 2002;18:461–476. doi: 10.1016/s0749-0704(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 25.Daly BJ, Douglas SL, Gordon NH, Kelley CG, O'Toole E, Montenegro H, et al. Composite outcomes of chronically critically ill patients 4 months after hospital discharge. Am J Crit Care. 2009;18:456–464. doi: 10.4037/ajcc2009580. quiz 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seneff MG, Zimmerman JE, Knaus WA, Wagner DP, Draper EA. Predicting the duration of mechanical ventilation. The importance of disease and patient characteristics. Chest. 1996;110:469–479. doi: 10.1378/chest.110.2.469. [DOI] [PubMed] [Google Scholar]
- 27.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370:1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JE, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Crit Care Med. 2004;32:1527–1534. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 29.Nelson JE, Mercado AF, Camhi SL, Tandon N, Wallenstein S, August GI, et al. Communication about chronic critical illness. Arch Intern Med. 2007;167:2509–2515. doi: 10.1001/archinte.167.22.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puntillo KA. Pain experiences of intensive care unit patients. Heart Lung. 1990;19:526–533. [PubMed] [Google Scholar]
- 31.Puntillo KA, White C, Morris AB, Perdue ST, Stanik-Hutt J, Thompson CL, et al. Patients' perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care. 2001;10:238–251. [PubMed] [Google Scholar]
- 32.Desbiens NA, Mueller-Rizner N, Connors AF, Jr, Wenger NS, Lynn J. The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment. J Pain Symptom Manage. 1999;17:248–255. doi: 10.1016/s0885-3924(98)00149-3. [DOI] [PubMed] [Google Scholar]
- 33.Desbiens NA, Wu AW, Broste SK, Wenger NS, Connors AF, Jr, Lynn J, et al. Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatmentm. Crit Care Med. 1996;24:1953–1961. doi: 10.1097/00003246-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277–282. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 36.Thirugnanam S, Herridge MS. Physical consequences of critical illness. Br J Hosp Med (Lond) 2007;68:477–481. doi: 10.12968/hmed.2007.68.9.27168. [DOI] [PubMed] [Google Scholar]
- 37.Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: a population-based study. J Intensive Care Med. 2006;21:173–182. doi: 10.1177/0885066605282784. [DOI] [PubMed] [Google Scholar]
- 38.Carson SS, Garrett J, Hanson LC, Lanier J, Govert J, Brake MC, et al. A prognostic model for one-year mortality in patients requiring prolonged mechanical ventilation. Crit Care Med. 2008;36:2061–2069. doi: 10.1097/CCM.0b013e31817b8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van den Berghe G. Neuroendocrine pathobiology of chronic critical illness. Crit Care Clin. 2002;18:509–528. doi: 10.1016/s0749-0704(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 40.Douglas SL, Daly BJ, Gordon N, Brennan PF. Survival and quality of life: short-term versus long-term ventilator patients. Crit Care Med. 2002;30:2655–2662. doi: 10.1097/00003246-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159:1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 42.Services UDoHH. Research, statistics and data. 2009 [Google Scholar]
- 43.Im K, Belle SH, Schulz R, Mendelsohn AB, Chelluri L Investigators Q-M. Prevalence and outcomes of caregiving after prolonged (> or =48 hours) mechanical ventilation in the ICU. Chest. 2004;125:597–606. doi: 10.1378/chest.125.2.597. [DOI] [PubMed] [Google Scholar]
- 44.Douglas SL, Daly BJ. Caregivers of long-term ventilator patients: physical and psychological outcomes. Chest. 2003;123:1073–1081. doi: 10.1378/chest.123.4.1073. [DOI] [PubMed] [Google Scholar]
- 45.Bureau UDoCEaSAUC, editor. The United States in International Context: 2000. Washington, DC: 2000. [Google Scholar]
- 46.Sheffield-Moore M, Paddon-Jones D, Casperson SL, Gilkison C, Volpi E, Wolf SE, et al. Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrinol Metab. 2006;91:3844–3849. doi: 10.1210/jc.2006-0588. [DOI] [PubMed] [Google Scholar]
- 47.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 48.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 49.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 50.Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84:2705–2711. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 51.Zachwieja JJ, Yarasheski KE. Does growth hormone therapy in conjunction with resistance exercise increase muscle force production and muscle mass in men and women aged 60 years or older? Phys Ther. 1999;79:76–82. [PubMed] [Google Scholar]
- 52.Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol. 1997;272:E94–E99. doi: 10.1152/ajpendo.1997.272.1.E94. [DOI] [PubMed] [Google Scholar]
- 53.Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 54.Rawson ES. Risks and benefits of supplement use. Curr Sports Med Rep. 2005;4(4):185–187. doi: 10.1097/01.csmr.0000306204.34068.76. [DOI] [PubMed] [Google Scholar]
- 55.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, et al. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007;56:753–766. doi: 10.2337/db06-1504. [DOI] [PubMed] [Google Scholar]
- 56.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 57.Frontera WR, Meredith CN, O'Reilly KP, Evans WJ. Strength training and determinants of VO2max in older men. J Appl Physiol (1985) 1990;68:329–333. doi: 10.1152/jappl.1990.68.1.329. [DOI] [PubMed] [Google Scholar]
- 58.Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, et al. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288:E922–E929. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- 59.Paddon-Jones D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J Nutr. 2006;136:2123–2126. doi: 10.1093/jn/136.8.2123. [DOI] [PubMed] [Google Scholar]
- 60.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 61.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 62.Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11:391–396. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart DW, Herndon DN, Klein G, Lee SB, Celis M, Mohan S, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–834. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeschke MG, Kraft R, Emdad F, Kulp GA, Williams FN, Herndon DN. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann Surg. 2010;252:521–527. doi: 10.1097/SLA.0b013e3181f2774c. discussion 27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeschke MG, Kulp GA, Kraft R, Finnerty CC, Mlcak R, Lee JO, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182:351–359. doi: 10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porro LJ, Herndon DN, Rodriguez NA, Jennings K, Klein GL, Mlcak RP, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214:489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. discussion 02–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 70.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985) 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 71.Dasu MR, Herndon DN, Nesic O, Perez-Polo JR. IGF-I gene transfer effects on inflammatory elements present after thermal trauma. Am J Physiol Regul Integr Comp Physiol. 2003;285:R741–R746. doi: 10.1152/ajpregu.00046.2003. [DOI] [PubMed] [Google Scholar]
- 72.Elijah IE, Branski LK, Finnerty CC, Herndon DN. The GH/IGF-1 system in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:759–767. doi: 10.1016/j.beem.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeschke MG, Barrow RE, Hawkins HK, Yang K, Hayes RL, Lichtenbelt BJ, et al. IGF-I gene transfer in thermally injured rats. Gene Ther. 1999;6:1015–1020. doi: 10.1038/sj.gt.3300923. [DOI] [PubMed] [Google Scholar]
- 74.Jeschke MG, Barrow RE, Suzuki F, Rai J, Benjamin D, Herndon DN. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8:238–246. [PMC free article] [PubMed] [Google Scholar]
- 75.Jeschke MG, Herndon DN, Vita R, Traber DL, Jauch KW, Barrow RE. IGF-I/BP-3 administration preserves hepatic homeostasis after thermal injury which is associated with increases in no and hepatic NF-kappa B. Shock. 2001;16:373–379. doi: 10.1097/00024382-200116050-00009. [DOI] [PubMed] [Google Scholar]
- 76.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 77.Bernard C. Lecons sur le Diabete et la Glycogenase Animale. Paris: Bailliere; 1877. [Google Scholar]
- 78.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norton JA, Stein TP, Brennan MF. Whole body protein synthesis and turnover in normal man and malnourished patients with and without known cancer. Ann Surg. 1981;194:123–128. doi: 10.1097/00000658-198108000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lundholm K, Bennegard K, Eden E, Svaninger G, Emery PW, Rennie MJ. Efflux of 3-methylhistidine from the leg in cancer patients who experience weight loss. Cancer Res. 1982;42:4807–4811. [PubMed] [Google Scholar]
- 81.Lundholm K, Bylund AC, Holm J, Schersten T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976;12:465–473. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- 82.Warren RS, Jeevanandam M, Brennan MF. Protein synthesis in the tumor-influenced hepatocyte. Surgery. 1985;98:275–282. [PubMed] [Google Scholar]
- 83.Kress JP. Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37(10 Suppl):S442–S447. doi: 10.1097/CCM.0b013e3181b6f9c0. [DOI] [PubMed] [Google Scholar]
- 84.Winfield RD, Delano MJ, Pande K, Scumpia PO, Laface D, Moldawer LL. Myeloid-derived suppressor cells in cancer cachexia syndrome: a new explanation for an old problem. JPEN J Parenter Enteral Nutr. 2008;32:651–655. doi: 10.1177/0148607108325075. [DOI] [PMC free article] [PubMed] [Google Scholar]