Abstract
Endotoxemia participates in the pathogenesis of many liver injuries. Lipopolysaccharide (LPS) was shown to inactivate hepatic methionine adenosyltransferase (MAT), the enzyme responsible for S-adenosylmethionine (SAMe) biosynthesis. SAMe treatment was shown to prevent the LPS-induced increase in tumor necrosis factor-α, which may be one of its beneficial effects. SAMe is also an important precursor of glutathione (GSH) and GSH was shown to ameliorate LPS-induced hepatotoxicity. The aims of this work were to examine changes in SAMe and GSH homeostasis during endotoxemia and the effect of SAMe. Mice received SAMe or vehicle pretreatment followed by LPS and were killed up to18 h afterward. Unexpectedly, we found hepatic SAMe level increased 67% following LPS treatment while S-adenosylhomocysteine level fell by 26%, suggesting an increase in SAMe biosynthesis and/or block in transmethylation. The mRNA and protein levels of MAT1A and MAT2A were increased following LPS. However, despite increased MAT1A expression, MAT activity remained inhibited 18 h after LPS. The major methyltransferase that catabolizes hepatic SAMe is glycine N-methyltransferase, whose expression fell by 65% following LPS. Hepatic GSH level fell more than 50% following LPS, coinciding with a comparable fall in the mRNA and protein levels of glutamate-cysteine ligase (GCL) catalytic (GCLC) and modifier subunits (GCLM). SAMe pretreatment prevented the fall in GCLC and attenuated the fall in GCLM expression and GSH level. SAMe pretreatment prevented the LPS-induced increase in plasma alanine transaminases levels but not the LPS-induced increase in hepatic mRNA levels of proinflammatory cytokines. It further enhanced LPS-induced increase in interleukin-10 mRNA level. Taken together, the hepatic response to LPS is to upregulate MAT expression and inhibit SAMe utilization. GSH is markedly depleted largely due to lower expression of GCL. Interestingly, SAMe treatment prevented the fall in GCL and helped to preserve the GSH store and prevent liver injury.
Keywords: glutamate-cysteine ligase, glutathione, glycine N-methyltransferase, lipopolysaccharide, methionine adenosyltransferase, S-adenosylmethionine
Lipopolysaccharide (LPS) is a major component of the outer membrane of all gram-negative bacteria that can trigger the synthesis and release of proinflammatory cytokines such as tumor necrosis factor-α (TNFα), interleukin 1β (IL-1β), and inducible nitric oxide synthase (iNOS).1,2 Liver plays a central role in the clearing of gut-derived LPS.1 Indeed, endotoxemia frequently occurs in patients with liver cirrhosis and the degree of endotoxemia correlates directly with the degree of liver failure.1 Endotoxemia is also believed to participate in the pathogenesis of many liver diseases including alcoholic liver disease and nonalcoholic steatohepatitis.1,3 To this end it is important to understand hepatic mechanisms that defend against the injury produced by LPS.
S-Adenosylmethionine (SAMe) is known to protect against LPS-induced liver injury.4 Rats fed a methionine- and choline-deficient (MCD) diet were more susceptible to LPS-induced liver injury and had much higher serum TNFα level following LPS challenge, both of which were prevented with exogenous SAMe administration.4 Hepatic SAMe biosynthesis is impaired in patients with chronic liver disease due to decreased expression and activity of the hepatic enzyme methionine adenosyltransferase (MAT).5 SAMe is an important precursor of glutathione (GSH),5 and SAMe treatment of cirrhotic patients resulted in restoration of hepatic GSH levels.6 Importantly, LPS treatment was shown to inactivate hepatic MAT activity, presumably, due to covalent modification of a critical cysteine residue by nitric oxide.7 Thus, it is possible that the fall in SAMe level could further jeopardize hepatic defense against LPS. In this work, hepatic levels of SAMe were unchanged at 6 h after LPS7 but changes in variables important in SAMe homeostasis at a later time point is unknown.
Glutathione is another important variable that determines susceptibility to LPS-induced liver injury.8,9 Endotoxemia is known to lower hepatic GSH levels by several mechanisms that include enhanced sinusoidal GSH efflux,10 inhibition of GSH synthesis at the level of the rate-limiting enzyme glutamate-cysteine ligase (GCL) activity,8 and oxidative stress.2 Exogenous GSH suppressed LPS-induced systemic inflammatory response and reduced mortality.9 In terms of the inhibition in GCL, part of the mechanism may be reduced expression of the GCL catalytic subunit (GCLC) as GCLC mRNA levels fell following LPS.8 However, whether LPS also affects the GCL modifier subunit (GCLM) or the second enzyme of GSH synthesis, GSH synthetase (GS), is unknown. Whether SAMe treatment can raise hepatic GSH level during endotoxemia is also unknown.
Given the importance of SAMe and GSH in the modulation of LPS-induced liver injury, the goals of this work were to evaluate the hepatic response to endotoxemia in terms of SAMe and GSH homeostasis and how SAMe treatment affect these changes. We found that the liver’s response to endotoxemia is to preserve hepatic SAMe store by increasing the expression of MAT genes and decreasing SAMe utilization. GSH synthesis is inhibited due to reduced expression of all three genes involved in GSH synthesis. Interestingly, SAMe pretreatment completely prevented liver injury but had no effect on the induction of TNFα, IL-1β, or iNOS. On the other hand, SAMe pretreatment blocked the LPS-induced lowering of GCLC expression at both the mRNA and protein level. This is a novel action of SAMe that can contribute to its hepatoprotective effect.
MATERIALS AND METHODS
Materials
SAMe in the form of disulfate p-toluenesulfonate dried powder was generously provided by Gnosis SRL (Cairate, Italy), S-adenosylhomocysteine (SAH) and LPS (Escherichia coli 055:B5) were purchased from Sigma Chemical Co. (St Louis, MO, USA). 32P-dCTP (3000 Ci/mmol) was purchased from PerkinElmer Life Sciences (DuPont, Boston, MA, USA). All other reagents were of analytical grade and were obtained from commercial sources.
Animal Experiments
Four-month-old male C57/B6 mice (Harlan, Indianapolis, IN, USA) were fed ad libitum a standard diet (Harland Teklad irradiated mouse diet 7912, Madison, WI, USA) and housed in a temperature-controlled animal facility with 12-h light–dark cycles. Animals were treated humanely and all procedures were in compliance with our institutions guidelines for the use of laboratory animals.
Mice were divided into five experimental groups (n = 4–12 per group), control, SAMe, LPS, and SAMe pretreatment + LPS, and LPS + SAMe post-treatment, respectively. SAMe (50 mg/kg body weight) or vehicle was injected intraperitoneally (i.p.) three times every 12 h. At 30 min after the last SAMe or vehicle injection, LPS (15 mg/kg body weight) was injected i.p. to LPS and SAMe pretreatment + LPS groups. For the LPS + SAMe post-treatment group, animals received SAMe (50 mg/kg body weight) or vehicle i.p. 1, 6, and 12 h after LPS. These five groups of animals were killed 18 h after LPS injection for liver and blood collection. SAMe alone group received SAMe injection as above and vehicle injection at the time of LPS. In a separate experiment, effect of LPS on various parameters was measured 6 h after LPS injection (n = 5 per group). Control animals were injected with saline i.p. as a stress control. Livers were snap frozen immediately in liquid nitrogen. Blood was collected from abdominal aorta by nonheparinized syringe and allowed to clot for 30 min at room temperature. Serum was obtained from the clotted blood with spinning at 1000 g for 10 min (Centrifuge 5417R, Eppendorf, Hamburg, Germany). The liver and serum samples were stored at −80°C until further analyses.
RNA Isolation and Gene Expression Analysis
A portion of frozen liver was homogenized with ice-cold Trizol® (Invitrogen, Carlsbad, CA, USA) using a glass homogenizer immersed in ice. Then, total RNA for northern blot was extracted from the liver-Trizol® homogenate following the manufacturer’s instruction. RNA concentration was determined spectrophotometrically before use and the integrity was checked by electrophoresis with subsequent ethidium bromide staining. Electrophoresis of RNA, gel blotting, and northern hybridization analysis were performed on total RNA using standard procedures as described.11 Specific MAT1A, MAT2A, MAT2β, glycine N-methyltransferase (GNMT), GCLC, GCLM, GS, GAPDH, and 18S rRNA probes were labeled with [32P]dCTP using a random-primer kit (RediPrime DNA Labeling System; Amersham Pharmacia Biotech) as described.12–14 The mouse GAPDH cDNA probe corresponds to nucleotides +221 to +810 of the published mouse GAPDH sequence (accession no. NM_008084). Autoradiography and densitometry (Gel Documentation System, Scientific Technologies, Carlsbad, CA, USA and NIH Image 1.60 software program) were used to quantitate relative RNA. Results of northern blot analysis were normalized to 18S rRNA.
Quantitative real-time PCR also measured mRNA levels. Total RNA was subjected to reverse transcription (RT) by using M-MLV Reverse Transcriptase (Invitrogen). RT product (1 μl) was subjected to quantitative real-time PCR analysis. The primers and TaqMan probes for MAT1A, MAT2A, GCLC, GCLM, GS, TNFα, iNOS, IL-1β, IL-10, GNMT, and Universal PCR Master Mix were purchased from ABI (Foster City, CA, USA). 18SrRNA was used as housekeeping genes as described.15 The ΔCt obtained was used to find the relative expression of these genes according to the formula: relative expression = 2−ΔΔCt, where ΔΔCt = ΔCt of those genes in experimental groups—ΔCt of the same genes in control group.
Western Blot Analysis
Liver homogenates were subjected to western blot analysis as we described.11,12 Equal amounts of protein (15 μg per well) were resolved in 12.5% SDS-polyacrylamide gels. Proteins were electrophoretically transferred to nitrocellulose membranes, blocked with Tris-buffered saline (pH 7.6)/0.1% Tween 20 containing 5% nonfat dried milk, washed with Tris-buffered saline/0.1% Tween 20, and incubated 1.5 h with primary antibodies in Tris-buffered saline/0.1% Tween 20 containing 5% nonfat dried milk. Blots were washed in Tris-buffered saline/0.1% Tween 20 and incubated 45 min with the secondary antibody in Tris-buffered saline/0.1% Tween 20 containing 5% nonfat dried milk. Membranes were probed with antibodies against MATI/III,11 MATII (GenWay Biotech Inc., San Diego, CA, USA), GCLC, GCLM (Novus Biologicals, Littleton, CO, USA), and GNMT.16 To ensure equal loading, protein gels were stained with Coomassie blue and/or membranes were stripped and reprobed with anti-actin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). A horseradish peroxidase-conjugated secondary antibody was used. Blots were developed by enhanced chemiluminescence.
Hepatic SAMe, SAH, and GSH Levels
Hepatic SAMe and SAH levels were measured as described17 and GSH levels were measured as described.18
Hepatic MAT Activity
Hepatic MATI/III activity was measured as we previously described12 with 5 mM methionine concentration.
Serum Alanine Transaminase Levels
Serum alanine transaminase (ALT) level was measured by a commercially available kit (Infinity ALT, Thermo Electron Corp., Waltham, MA, USA) following manufacturer’s instruction.
Statistics
Two-tailed nonpaired Student’s t-test was used for comparisons between two groups, ANOVA followed by Fisher’s test was used for comparison of more than two groups. Significance was defined by P < 0.05.
RESULTS
Effect of SAMe Treatment on Changes in Hepatic SAMe and SAH Levels During Endotoxemia
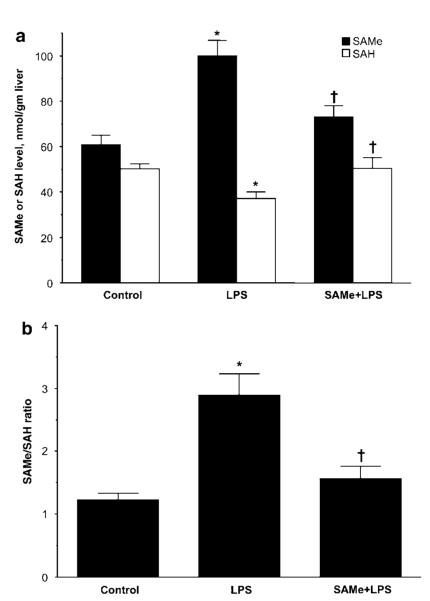
We first determined hepatic SAMe and SAH levels 18 h after LPS administration. Figure 1 shows that hepatic SAMe level increased 67% in LPS-treated mice, whereas SAH fell by 26% (Figure 1a). This translated to a more than doubling of the SAMe/SAH ratio (Figure 1b). SAMe pretreatment attenuated the increase in SAMe and the fall in SAH levels. This unexpected result prompted us to examine factors that are important in determining steady-state hepatic SAMe level, namely biosynthesis and utilization of SAMe.
Figure 1.
Hepatic SAMe and SAH levels during endotoxemia. Hepatic SAMe and SAH levels were measured 18 h following LPS administration and compared to control mice or mice that received SAMe pretreatment as described in ‘Materials and Methods’. (a) SAMe and SAH levels, (b) ratio of SAMe to SAH. *P < 0.005 vs control group, †P < 0.05 vs LPS group, n = 9–10 mice per group.
Effect of SAMe Treatment on Changes in MAT Expression During Endotoxemia
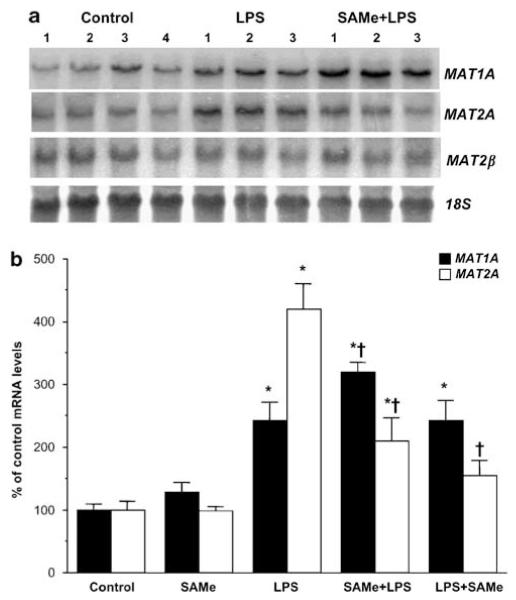
SAMe biosynthesis is catalyzed by the enzyme MAT. In mammals, two genes (MAT1A and MAT2A) encode for catalytic subunits of MAT, whereas MAT2β encodes for a regulatory subunit-β that regulates the activity of MAT2A-encoded isoenzyme.5 MAT1A is largely expressed in normal differentiated liver whereas MAT2A is widely distributed but is minimally expressed in normal hepatocytes.5 Figure 2 shows that 18 h following LPS administration, MAT1A and MAT2A mRNA levels increased 140 and 320% as compared to control, respectively. SAMe pretreatment further increased MAT1A mRNA level by 80% whereas significantly blunting the increase in MAT2A mRNA level. Neither LPS nor SAMe affected MAT2β mRNA levels (densitometry values are: LPS = 95 ± 19% of control, SAMe pretreatment = 123 ± 27% of control). However, while SAMe post-treatment failed to further induce MAT1A mRNA level, it was able to attenuate the LPS-mediated increase in MAT2A mRNA level. SAMe alone did not exert any effect on these genes. Figure 3 shows that the levels of MAT1A- and MAT2A-encoded proteins changed in parallel to mRNA levels.
Figure 2.
Hepatic MAT genes mRNA levels during endotoxemia. (a) Steady-state mRNA levels of MAT1A, MAT2A, and MAT2β were determined by northern blot analysis as described in ‘Materials and Methods’. Membranes were sequentially probed with MAT1A, MAT2A, MAT2β, and 18S rRNA.
(b) Summary of results expressed as % of control mRNA levels from three to eight animals per group. SAMe pretreatment group is labeled SAMe + LPS and SAMe post-treatment group is labeled LPS + SAMe. *P < 0.005 vs control group, †P < 0.05 vs LPS group.
Figure 3.
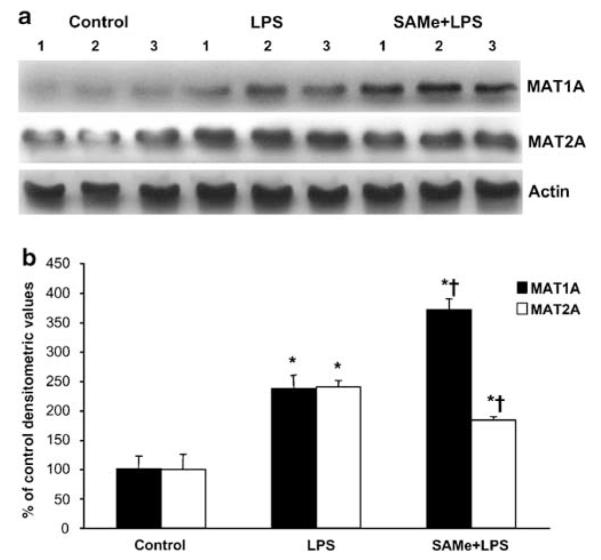
Hepatic MAT1A and MAT2A protein levels during endotoxemia. (a) Steady-state protein levels of MAT1A and MAT2A were determined by western blot analysis as described in ‘Materials and Methods’. Actin was used for housekeeping control. (b) Densitometric values of the western blots are shown normalized to actin. *P < 0.05 vs control group, †P < 0.05 vs LPS.
GNMT Expression Falls During Endotoxemia
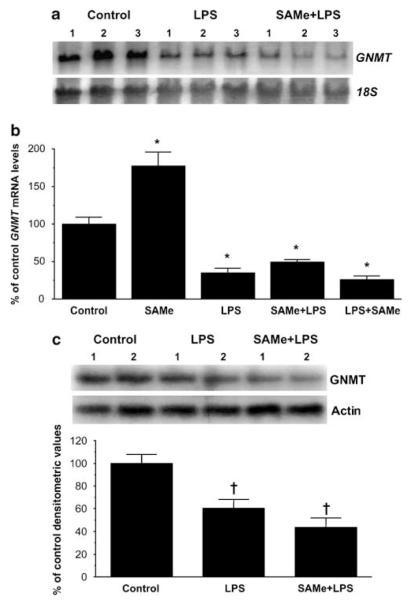
In liver, the main enzyme responsible for SAMe catabolism is GNMT.5 We found that following LPS, GNMT mRNA levels fell by 65%, which was not affected by SAMe pre- or post-treatment (Figure 4a). Interestingly, SAMe treatment alone nearly doubled the GNMT mRNA level (Figure 4b). Comparable changes in GNMT protein levels were also observed (Figure 4c).
Figure 4.
Hepatic GNMT mRNA and protein levels during endotoxemia. (a) Steady-state mRNA levels of GNMT were determined by northern blot analysis as described in ‘Materials and Methods’. Membranes were sequentially probed with GNMT and 18S rRNA. (b) Summary of results expressed as % of control GNMT mRNA levels as determined by both northern blot analysis and real-time PCR. SAMe pretreatment group is labeled SAMe + LPS and SAMe post-treatment group is labeled LPS + SAMe. (c) Steady-state protein levels of GNMT were determined by western blot analysis and densitometric values are shown below the western blot. *P < 0.005 vs control group, †P < 0.01 vs control group, n = 4–8 per group.
Effect of SAMe Treatment on GSH Levels and GSH Synthetic Enzymes During Endotoxemia
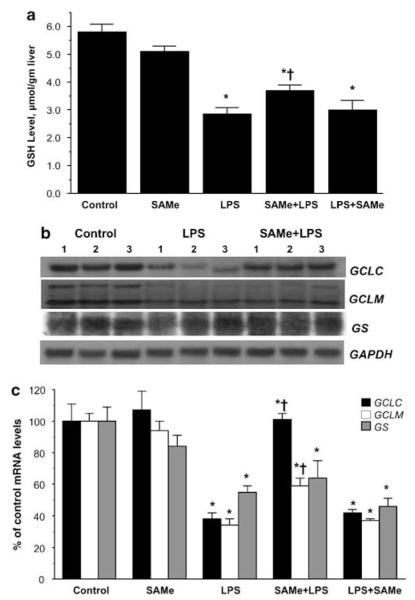
Previous study showed LPS-lowered GSH levels and the mRNA levels of the GCLC.8 However, the method used for mRNA level measurement was semiquantitative and whether the modifier subunit of GCL or the second enzyme GS are affected was not examined. Our results are in agreement in terms of the fall in the levels of GSH and GCLC mRNA following LPS (Figure 5). In addition, we found the mRNA levels of the modifier subunit GCLM and GS were also reduced after LPS administration (Figure 5b and c). Interestingly, SAMe pretreatment completely prevented the fall in GCLC mRNA levels, whereas it attenuated the fall in GCLM mRNA level and had no effect on GS mRNA level (Figure 5c). On the other hand, SAMe post-treatment had no protective effect (Figure 5c). Comparable changes in GCLC and GCLM protein levels were also observed (Figure 6). SAMe pretreatment also blunted significantly the fall in GSH level (Figure 5a).
Figure 5.
Hepatic GSH and mRNA levels of GSH synthetic enzymes during endotoxemia. (a) GSH levels during endotoxemia. GSH levels were measured as described in ‘Materials and Methods’. *P < 0.0005 vs control group, †P < 0.05 vs LPS group, n = 4–9 per group. (b) Steady-state mRNA levels of GCLC, GCLM, and GS were determined by northern blot analysis as described in ‘Materials and Methods’. GAPDH was used as the housekeeping gene. (c) Summary of results expressed as % of control mRNA levels as determined by both northern blot analysis and real-time PCR. SAMe pretreatment group is labeled SAMe + LPS and SAMe post-treatment group is labeled LPS + SAMe. *P < 0.005 vs control group, †P < 0.05 vs LPS group, n = 4–8 per group.
Figure 6.
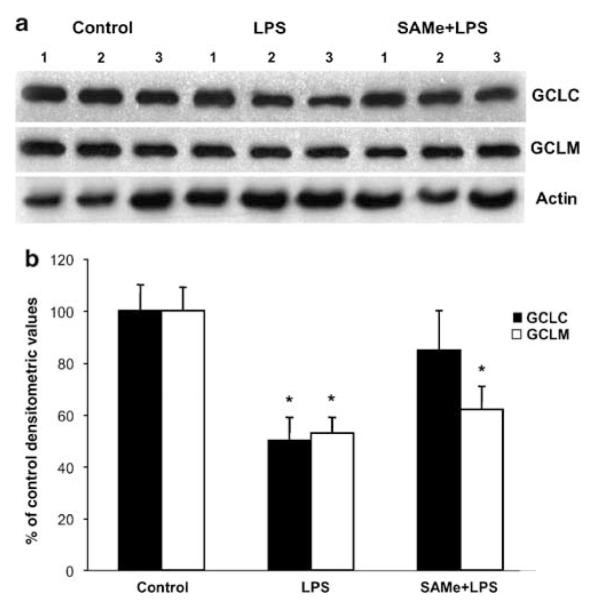
Hepatic GCLC and GCLM protein levels during endotoxemia. (a) Steady-state protein levels of GCLC and GCLM were determined by western blot analysis as described in ‘Materials and Methods’. Actin was used for housekeeping control. (b) Densitometric values of the western blots are shown normalized to actin. *P < 0.05 vs control group.
SAMe Pretreatment Protects Against LPS-Induced Liver Injury
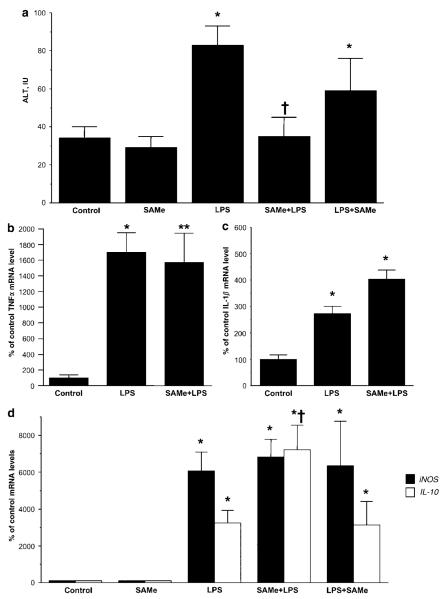
Previous study showed that exogenous SAMe protected against LPS-induced liver injury.4 Consistently, we also found SAMe pretreatment completely prevented the LPS-induced increase in serum ALT level (Figure 7a). However, SAMe pretreatment had no effect on the LPS-induced increase in hepatic expression of TNFα (Figure 7b) or IL-1β (Figure 7c). SAMe pretreatment also did not prevent LPS-induced iNOS expression but it further enhanced IL-10 expression (Figure 7d). On the other hand, SAMe post-treatment was unable to influence either iNOS or IL-10 mRNA levels (Figure 7d). SAMe post-treatment also was unable to protect from LPS-induced ALT elevation (Figure 7a).
Figure 7.
Effect of LPS and SAMe treatment on serum ALT levels and hepatic mRNA levels of cytokines. (a) Serum ALT levels were measured as described in ‘Materials and Methods’. *P < 0.001 vs control group, †P < 0.01 vs LPS group, n = 4–14 per group. (b) Hepatic TNFα mRNA levels were measured using real-time PCR as described in ‘Materials and Methods’. (c) Hepatic interleukin (IL)-1β mRNA levels were measured using real-time PCR. (d) Hepatic iNOS and IL-10 mRNA levels were measured using real-time PCR. SAMe pretreatment group is labeled SAMe + LPS and SAMe post-treatment group is labeled LPS + SAMe. For cytokine mRNA levels, *P < 0.01 vs control group and **P < 0.05 vs control group, †P < 0.05 vs LPS group, n = 4–8 per group.
Time Course of Changes During Endotoxemia
Table 1 summarizes changes in various parameters at 6 and 18 h following LPS administration. Note that MAT genes are already induced at the mRNA level at 6 h but were further induced by 18 h. GNMT and GSH synthetic enzymes were nearly maximally inhibited at the mRNA level by 6 h. Both pro- and anti-inflammatory cytokines are induced early on and remained elevated at 18 h. GSH level was reduced by 40% at 6 h. Previously study showed inhibition in hepatic MAT activity at 6 h.7 Despite the fact that the expression of enzymes for SAMe biosynthesis is induced greatly at 18 h, hepatic MAT activity remained inhibited (Table 1).
Table 1.
LPS-induced changes in hepatic metabolite, gene expression, and enzyme activity
| 6 h after LPS | 18 h after LPS | |
|---|---|---|
|
|
||
| % of control value | % of control value | |
| GSH (μmol/gm) | 60 ± 3* | 49 ± 8.4* |
| mRNA levels | ||
| MAT1A | 155 ± 13** | 242 ± 30† |
| MAT2A | 181 ± 19** | 420 ± 40* |
| GNMT | 48 ± 7* | 35 ± 6* |
| GCLC | 52 ± 5** | 38 ± 4† |
| GCLM | 38 ± 5* | 34 ± 4* |
| GS | 62 ± 10** | 55 ± 4† |
| iNOS | 22941 ± 9005** | 6057 ± 1029* |
| IL-10 | 2189 ± 486† | 3234 ± 693* |
| IL-1β | 1892 ± 348† | 262 ± 17† |
| Enzyme activity | ||
| MATI/III | 53.5 ± 3.2* | 58 ± 11** |
Abbreviations: GCLC, glutamate-cysteine ligase catalytic; GCLM, glutamatecysteine ligase catalytic and modifier subunit; GNMT, glycine N-methyltransferase; GS, glutathione synthetase; GSH, glutathione; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MAT, methionine adenosyltransferase.
Results are mean ± s.e. from 5 to 12 determinations for each group. Hepatic GSH levels, mRNA levels, and MATI/III activity were measured as described in ‘Materials and Methods’ 6 and 18 h following LPS administration and expressed as % of control values.
P < 0.001,
P < 0.05,
P < 0.01 vs control.
DISCUSSION
Liver injury frequently accompanies septic shock, a pathological condition triggered by LPS.1,7 Liver also play a major role in clearing gut-derived LPS.1 Endotoxemia occurs when liver function is impaired and endotoxemia can further contribute to liver injury.1,3 The current study is focused on two variables known to modulate LPS-induced liver injury, namely SAMe and GSH. LPS has been shown to inactivate the enzyme responsible for hepatic SAMe biosynthesis7 and inhibit the expression of the rate-limiting enzyme for GSH synthesis.8 Administration of either SAMe or GSH has been shown to ameliorate LPS-induced liver injury.4,9 However, inactivation of the hepatic MAT enzyme occurred at an early time point (6 h) following LPS administration and how the liver respond at a later time point remains unknown. Likewise, depletion in hepatic GSH was thought to occur due to oxidative stress,2 enhanced sinusoidal GSH efflux,10 and lower GCL activity8 but a more detailed examination of the enzymes involved in GSH synthesis has not been performed. Finally, SAMe administration was shown to protect rats fed a MCD diet against LPS-induced liver injury and blunt the increase in TNFα.4 However, MCD diet lowers both hepatic SAMe and choline levels4 and whether SAMe protects in animals fed a normal diet against LPS-induced liver injury is unknown. The current study was undertaken to fill in some gaps in the knowledge of changes in SAMe and GSH homeostasis during endotoxemia and the effect of SAMe treatment on these changes.
As LPS administration was shown to inactivate hepatic MAT by nitrosylation,7 we were surprised to find that hepatic SAMe levels were actually markedly elevated at 18 h after LPS administration. This was accompanied by reduced SAH levels, suggesting either there is increased biosynthesis of SAMe and/or decreased SAMe utilization. Interestingly, a similar increase in hepatic SAMe level 24 h after LPS treatment occurred in rats fed a methionine and choline sufficient diet but the mechanism of this increase was not examined.4 We found that both MAT1A and MAT2A mRNA and protein levels were markedly elevated at 18 h, consistent with increased capacity to biosynthesize SAMe. SAMe pretreatment further increased MAT1A mRNA levels whereas suppressing the increase in MAT2A mRNA levels. This is consistent with our previous report demonstrating SAMe maintains MAT1A expression and inhibits MAT2A expression.19,20 However, those studies were carried out in vitro and this is the first demonstration of SAMe’s ability to regulate these two genes in vivo.
Despite the increase in MAT1A gene expression, MATI/III activity remained inhibited at 18 h following LPS. Thus, the elevation in hepatic SAMe level observed at 18 h cannot be due to increased biosynthesis. Although SAMe pretreatment was able to further enhance MAT1A expression, SAMe administration after LPS was ineffective. This was not the case for MAT2A, where both pre- and post-treatment of SAMe was able to significantly attenuate LPS-induced MAT2A expression. The implications of this observation is unclear at present but clearly point to very different mechanisms are involved in how SAMe modulate the expression of these two genes in the setting of endotoxemia.
We next examined whether SAMe utilization is inhibited during endotoxemia. In liver, the most important enzyme that catabolizes SAMe is GNMT.5,14 Mice deficient in GNMT have markedly elevated hepatic SAMe levels,14 attesting to the importance of this enzyme in SAMe removal. Interestingly, LPS administration led to a 50% fall in GNMT expression at the mRNA level as early as 6 h afterward. Comparable fall in GNMT protein level also occurred. Thus, during endotoxemia the liver responds by upregulating MAT genes to compensate for the inhibition in MAT activity and at the same time decrease SAMe utilization. This effectively creates a block at the level of SAMe conversion to SAH, which can also reduce substrate availability for GSH synthesis.21 SAMe pretreatment tended to prevent the mark increase in SAMe or the fall in SAH, which can only be explained by an activation in the SAMe utilization pathway. However, SAMe treatment did not prevent the fall in GNMT expression. It remains possible that the activity of GNMT may have increased or alternatively, SAMe may have induced the expression and/or activity of other methyltransferases. This remains to be examined.
SAMe treatment alone had no influence on any of the parameters examined with the exception of GNMT mRNA level, where it increased by 80%. This effect has never been reported previously and may be consistent with the notion that GNMT’s main function is to maintain a constant SAMe level14 so that SAMe administration is likely to induce its expression to prevent accumulation. The molecular mechanism of how SAMe induces GNMT expression remains to be elucidated.
GSH is the main nonprotein thiol in mammalian cells that defends against oxidative stress.21 GSH synthesis occurs by two enzymatic steps: the formation of γ-glutamylcysteine from glutamate and cysteine catalyzed by GCL, and formation of GSH from γ-glutamylcysteine and glycine catalyzed by GS.21 GCL, the rate-limiting enzyme, is made up of a catalytic and a modifier subunit.22 Both subunits are subjected to regulation at multiple levels but in general, oxidative stress upregulate both GCLC and GCLM.21 The second enzyme GS is also regulated transcriptionally and coordinated upregulation of GCL and GS further enhances the GSH synthetic capacity.23 Thus, it is important to examine all three genes when changes in GSH synthesis occur. We found that during endotoxemia, all three genes are downregulated at the mRNA level and both GCLC and GCLM also at the protein level. The degree of lowering in GCLC (62% reduced) and GCLM (66% reduced) expression suggests this is important in the depletion of GSH that occurs during endotoxemia. This is consistent with the observation that cysteine precursor failed to normalize hepatic GSH content during endotoxemia,8 supporting the notion that the problem lies at the activity of the rate-limiting enzyme rather than precursor availability.
Unexpectedly, SAMe pretreatment completely blocked the inhibition in GCLC, attenuated the fall in GCLM but not GS expression. SAMe given after LPS had no such protective effect. SAMe pretreatment (but not post-treatment) also blunted significantly the fall in GSH content. This suggests in mouse liver GCLC may be limiting so that higher GCLC expression can lead to higher GSH biosynthesis. This action of SAMe has never been reported and the molecular mechanism is unclear. Previous report showed that the fall in hepatic GSH during endotoxemia could be blocked by inhibiting nitric oxide synthesis, suggesting that the increase in iNOS that occur during endotoxemia might play a role.8 This is intriguing because SAMe had been shown to block LPS-induced iNOS production.24 However, SAMe did not prevent the increase in iNOS expression under our experimental conditions so that this cannot be the explanation. Differences in our results may be related to different dosing of SAMe and animals used as well as the time point studied. In the study by Majano et al,24 Wistar rats were given SAMe 30 min before and 3 h after LPS and all animals were killed 6 h after LPS. Our end point was much later and SAMe was not given after LPS administration. Further work will be required to elucidate how SAMe prevented the fall in GCLC and GCLM expression. This novel action of SAMe may be another mechanism of its hepatoprotective effect.
SAMe pretreatment completely prevented the increase in serum ALT level induced by LPS. This is in agreement with its hepatoprotective effect in animals fed with the MCD diet that were challenged by LPS.4 However, the protection occurred despite lack of influence on the expression of TNFα, IL-1β or iNOS, proinflammatory cytokines that have been implicated in the pathogenesis of liver injury.1,2 This apparent contradiction to previously published results4 may be explained by the difference in the models and dosing of SAMe. Thus, Chawla et al4 studied the effect of SAMe (170 μmol/kg per day, or about 68 mg/kg per day, for 16 days) in rats fed a MCD diet and under this experimental design, SAMe prevented the increase in TNFα attributed to the MCD diet. However, whether it would prevent the increase in TNFα in animals on a normal diet was not assessed. Our design was of acute pretreatment in mice on a normal diet. It also suggests the hepatoprotective effect in this setting has nothing to do with the expression of proinflammatory cytokines. One possible mechanism for the hepatoprotection is the preservation of GCL expression and hepatic GSH level. Another protective mechanism may involve SAMe’s ability to further induce IL-10, an important anti-inflammatory cytokine.25 Absence or decreased IL-10 predispose to hepatotoxicity whereas exogenous IL-10 is hepatoprotective in multiple experimental models of liver injury.25 Consistent with previous report,25 SAMe pretreatment further enhanced IL-10 production. However, SAMe given after LPS was ineffective. It is also likely that SAMe pretreatment may preserve mitochondrial function, which was demonstrated in the setting of acute alcohol-induced liver injury.26 However, we cannot exclude the high likelihood that other yet to be recognized molecular mechanisms are also involved.
In summary, endotoxemia elicits a series of responses from the liver leading to marked alterations in SAMe and GSH homeostasis with increased expression of MAT genes but decreased MAT activity, SAMe utilization and GSH synthesis. This translated to high hepatic SAMe but low GSH levels. The increase in SAMe may preserve methylation reactions. SAMe pretreatment tended to activate methylation reactions so that there is less accumulation of SAMe. At the same time, it also prevents the fall in GCL expression, helping to preserve the GSH store. This may be an important mechanism of SAMe’s hepatoprotective effect during endotoxemia.
ACKNOWLEDGEMENTS
This work was supported by NIH Grants DK51719 and DK45334 (to SC Lu), AA13847 and AT-1576 (to SCL and JMM); DK15289 (to CW), PN I + D SAF 2005-00855, HEPADIP-EULSHM-CT-205, and ETORTEK 2005 (to JMM). Ainhoa Iglesias Ara is a recipient of the Postdoctoral Fellowship supported by the CIC bioGUNE, Centro de Investigación Cooperativa en Biosciencías.
References
- 1.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Walker LM, Hinson JA, et al. Oxidant stress in rat liver after lipopolysaccharide administration: effect of inducible nitric-oxide synthase inhibition. J Pharmacol Exp Ther. 2000;293:968–972. [PubMed] [Google Scholar]
- 3.Yang SQ, Lin HZ, Lane MD, et al. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla RK, Watson WH, Eastin CE, et al. S-adenosylmethionine deficiency and TNFα in lipopolysaccharide-induced hepatic injury. Am J Physiol Gastrointest Liver Physiol. 1998;38:G125–G129. doi: 10.1152/ajpgi.1998.275.1.G125. [DOI] [PubMed] [Google Scholar]
- 5.Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 6.Vendemiale G, Altomare E, Trizio T, et al. Effects of oral S-adenosyl-l-methionine on hepatic glutathione in patients with liver disease. Scand J Gastroenterol. 1989;24:407–415. doi: 10.3109/00365528909093067. [DOI] [PubMed] [Google Scholar]
- 7.Avila MA, Mingorance J, Martínez-Chantar ML, et al. Regulation of rat liver S-adenoysylmethionine synthetase during septic shock: role of nitric oxide. Hepatology. 1997;25:391–396. doi: 10.1002/hep.510250222. [DOI] [PubMed] [Google Scholar]
- 8.Payabvash S, Ghahremani MH, Goliaei A, et al. Nitric oxide modulates glutathione synthesis during endotoxemia. Free Radic Biol Med. 2006;41:1817–1828. doi: 10.1016/j.freeradbiomed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Sun S, Zhang H, Xue B, et al. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm Res. 2006;55:504–510. doi: 10.1007/s00011-006-6037-7. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H. Enhanced sinusoidal glutathione efflux during endotoxin-induced oxidant stress in vivo. Am J Physiol Gastrointest Liver Physiol. 1992;263:G60–G68. doi: 10.1152/ajpgi.1992.263.1.G60. [DOI] [PubMed] [Google Scholar]
- 11.Lu SC, Alvarez L, Huang ZZ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang HP, Magilnick N, Noureddin M, et al. Effect of hepatocyte growth factor on methionine adenosyltransferase genes and growth is cell density-dependent in HepG2 cells. J Cell Physiol. 2007;210:766–773. doi: 10.1002/jcp.20891. [DOI] [PubMed] [Google Scholar]
- 13.Yang HP, Magilnick N, Ou XP, et al. Tumor necrosis alpha induces coordinated activation of rat GSH synthetic enzymes via nuclear factor kappa B and activator protein-1. Biochem J. 2005;391:399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luka Z, Capdevila A, Mato JM, et al. A glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgenic Res. 2006;15:393–397. doi: 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veal N, Hsieh CL, Xiong S, et al. Inhibition of lipopolysaccharide-stimulated TNF-alpha promoter activity by S-adenosylmethionine and 5′-methylthioadenosine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G352–G362. doi: 10.1152/ajpgi.00316.2003. [DOI] [PubMed] [Google Scholar]
- 16.Yeo EJ, Wagner C. Tissue distribution of glycine N-methyltransferase, a major folate-binding protein in the liver. Proc Natl Acad Sci, USA. 1994;91:210–214. doi: 10.1073/pnas.91.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Xia M, Lin M, et al. Role of methionine adenosyltransferase 2A and S-adenosylemthionine in mitogen-induced growth of human colon cancer cells. Gastroenterology. 2007;133:207–218. doi: 10.1053/j.gastro.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 18.Huang ZZ, Chen CJ, Zeng ZH, et al. Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver regeneration. FASEB J. 2001;15:19–21. doi: 10.1096/fj.00-0445fje. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Trevijano ER, Latasa MU, Carretero MV, et al. S-Adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511–2518. doi: 10.1096/fj.00-0121com. [DOI] [PubMed] [Google Scholar]
- 20.Yang HP, Huang ZZ, Zeng ZH, et al. Role of promoter methylation in increased methionine adenosyltransferase 2A expression in human liver cancer. Am J Physiol Gastrointest Liver Physiol. 2001;280:G184–G190. doi: 10.1152/ajpgi.2001.280.2.G184. [DOI] [PubMed] [Google Scholar]
- 21.Lu SC. Regulation of hepatic glutathione synthesis: current concept and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 22.Huang C, Chang L, Anderson ME, et al. Catalytic and regulatory properties of the heavy subunit of rat kidney γ-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- 23.Huang ZZ, Yang HP, Chen CJ, et al. Inducers of γ-glutamylcysteine synthetase and their effects on glutathione synthetase expression. Biochim Biophys Acta. 2000;1493:48–55. doi: 10.1016/s0167-4781(00)00156-1. [DOI] [PubMed] [Google Scholar]
- 24.Majano PL, García-Monzón C, García-Trevijano ER, et al. S-adenosylmethionine modulates inducible nitric oxide synthase gene expression in rat liver and isolated hepatocytes. J Hepatol. 2001;35:692–699. doi: 10.1016/s0168-8278(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 25.Song Z, Barve S, Chen T, et al. S-adenosylmethionine (AdoMet) modulates endotoxin stimulated interleukin-10 production in monocytes. Am J Physiol. 2003;284:G949–G955. doi: 10.1152/ajpgi.00426.2002. [DOI] [PubMed] [Google Scholar]
- 26.Song Z, Zhou Z, Chen T, et al. S-Adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]