Abstract
Background
Obese patients display differences in vancomycin drug disposition, which may complicate attainment of appropriate serum vancomycin concentrations (SVCs). This study was conducted to determine if obesity leads to trough SVCs above the therapeutic range.
Methods
This retrospective cohort study sought to determine the rate and predictors of high (i.e. > 20 mg/L) serum trough levels according to level of obesity.
Results
Increasing BMI predicted SVCs > 20 mg/L after controlling for dose, age, and serum creatinine. Obese patients had significantly higher mean trough SVCs compared to non-obese patients (16.5 mg/L vs 12.1 mg/L, p=0.004) and a significantly higher proportion of obese patients had trough SVCs > 20 mg/L (18.9% vs 4.2%, p=0.03).
Conclusion
Increasing obesity predicted higher probabilities of SVCs > 20 mg/L. Development of alternative dosing and management strategies for vancomycin may be necessary to account for pharmacokinetic changes associated with obesity.
Keywords: pharmacokinetics, antibacterials, antibiotic therapy
1. Introduction
Obesity has more than doubled worldwide since 1980 and it is estimated that 60% of the world’s population will be classified as overweight or obese by the year 2030.[1] Given this trend, it is becoming increasingly important to characterize the effect of obesity on drug disposition. A growing body of research has indicated that obese individuals have altered pharmacokinetic parameters, as compared to normal-weight individuals[2–8] Studies of vancomycin have indicated that obese patients have a larger volume of distribution (VD), increased clearance and altered protein binding, which results in a reduced free fraction of vancomycin in the serum.[8] These pharmacokinetic alterations have implications on dosing and attainment of therapeutic serum vancomycin concentrations (SVCs).
Consensus guidelines recommend using total body weight (TBW) to dose vancomycin, because data has indicated TBW predicts vancomycin clearance in obese individuals.[9] However, in simple one-compartment models, drug disposition (i.e. volume of distribution) can be mistaken as clearance.[10] The proper dosing algorithm for obese patients remains a controversial topic, despite the consensus among researchers that obesity can alter drug disposition and complicate the achievement of desired vancomycin concentrations. One study demonstrated that an adjusted body weight (ABW) was more accurate at predicting vancomycin clearance in obese individuals and should therefore be used when dosing.[5] Another study reported utilizing TBW and vancomycin doses of 15–20 mg/kg every 8–12 hours. Here vancomycin trough SVCs exceeded 20 mg/L in approximately 50% of their obese patient population.[11] As a result of this observation, the authors created a revised protocol for obese patients utilizing lower maintenance doses of 10 mg/kg every 12 hours or 15 mg/kg every 24 hours. When compared with the original dosing protocol, the revised protocol resulted in a significantly higher proportion of patients attaining trough SVCs in the target range of 10–20 mg/L and above-target SVCs were significantly less frequent with the revised protocol. However, utilizing the revised protocol’s lower dosing also resulted in a significantly higher rate of below-target SVCs when compared with the original protocol (23% vs 9%, P=0.033). This observation is clinically concerning given that other recent studies have indicated that targeting higher trough SVCs of 15–20 mg/L in patients with complicated, serious infections is associated with significantly higher success rates compared to less aggressive traditional dosing.[12]
Considering the controversy surrounding dosing strategies necessary to obtain optimal SVCs in obese patients, we conducted a study investigating the effect of obesity and level of obesity on trough SVCs. The purpose of this study was to determine if body mass index (BMI) category or obesity classification was associated with vancomycin SVCs above the therapeutic range. Secondary objectives included characterizing clinical and safety outcomes associated with targeting trough SVCs > 15 mg/L.
2. Patients and methods
This retrospective study was conducted on a cohort of patients treated with intravenous (IV) vancomycin during routine practice at an institution that utilizes a pharmacist-managed dosing protocol. This study sought to classify the existence of trough SVCs above the desired therapeutic range (i.e. > 20 mg/L) according to BMI classification and obesity status. All patients were initiated with a vancomycin dose of 15 mg/kg TBW with a maximum initial dose of 2000 mg and subsequent dosing interval based on calculated creatinine clearance (CLCr).[13] Full details of the dosing protocol can be found in table 1. The dosing scheme utilized was in accordance with hospital guidelines. At the study institution, loading doses were not routinely administered. Patients were abstracted from pharmacy reports of all inpatient orders for vancomycin during the period of July through November 2011. This study was approved by the appropriate Institutional Review Boards.
Table 1.
Vancomycin dosing protocol (Adapted from reference [26])
| Initial dose of 15 mg/kg using actual body weight administered at intervals based on estimated creatinine clearance (CLcr) | |
|---|---|
| CLcr (ml/min)1 | Dosing Interval |
| 101 – 120 + | Every 8 hours |
| 70 – 100 | Every 12 hours |
| 40 – 69 | Every 24 hours |
| 30 – 39 | Every 36 hours |
| 20 – 29 | Every 48 hours |
| < 10 | Random* |
Random doses of vancomycin administered based on random serum vancomycin concentrations with doses given when serum concentrations were < 20 mg/L
Patients were included if they were 18 years of age or older with a BMI of at least 18 kg/m2. In addition, patients were required to have a documented or clinically suspected infection with a corresponding goal trough SVC of 15–20 mg/L such as pneumonia, meningitis, endocarditis, osteomyelitis or bacteremia, consistent with American Society of Health-System Pharmacists (ASHP) and Infectious Diseases Society of America (IDSA) recommendations.[9, 14] Patients with sepsis of unknown etiology were also included if at least two of the systemic inflammatory response criteria were met: body temperature < 36°C or > 38°C, heart rate > 90 beats per minute, respiration rate > 20 breaths per minute or PaCO2 < 32mmHg, or white blood cell count > 12,000 cells/mL3 of blood, < 4,000 cells/mL3 of blood or > 10% bands, or as suspected by the ordering physician.[15] To ensure that patients were approaching “steady-state” concentrations, a minimum of four IV vancomycin doses were required for patient inclusion. Trough SVC measurement no more than two hours prior to the fourth or subsequent dose was done as recommended by current guidelines for therapeutic drug monitoring of vancomycin.[9] Patients were excluded if they were younger than 18 years of age, had a CLcr less than 40 mL/min, received vancomycin within the 48 hours prior to their hospital admission, were administered less than four doses of vancomycin, or received vancomycin for treatment of an infection with a goal trough SVC < 15 mg/L.
Body mass index was calculated and patients were classified as obese (BMI ≥ 30 kg/m2) or non-obese (BMI < 30 kg/m2).[1] Patients were further characterized into World Health Organization obesity classes.[16] Comparisons planned a-priori included non-overweight individuals compared to each categorical group of overweight individuals (i.e. BMI < 24.99 kg/m2 vs. BMI 25–29.99, 30–34.99, 35 to 39.99, and ≥ 40 kg/m2). Physician report of comorbid diabetes, hypertension, congestive heart failure, sepsis or ascites was documented. Albumin was documented if available; hypoalbuminemia was defined as serum albumin < 3 g/dL. The type of documented or suspected infection was also characterized. Each vancomycin dose and time of administration were recorded, and renal function at each dose was calculated with the modified Cockcroft-Gault method* using an adjusted body weight in patients > 20% over their ideal body weight.[13, 17]
*Modifed Cockcroft-Gault equation:
Adjusted body weight = Ideal body weight + 0.4 × (Total body weight − ideal body weight)
Serum creatinine values < 0.8 mg/dL were rounded to 0.8 mg/dL [18] and acute kidney injury was defined as a 1.5-fold increase in serum creatinine from baseline (measured on the first day of vancomycin therapy).[19] Concurrent use of other nephrotoxic medications was documented if administered any time after the first vancomycin dose was given. Clinical response was defined as a normal or down-trending white blood cell count (4,000–10,000 cells/ mL3) and absence of fever (temperature < 38°C) for at least 24 hours after the initiation of vancomycin. Severity of illness was characterized with Acute Physiology And Chronic Health Evaluation II (APACHE II) [20] and Sequential Organ Failure Assessment (SOFA) [21] scores. Hospital length-of-stay and in-hospital mortality were also recorded.
Secondarily, we sought to classify the likely percent of steady-state achieved in order to ensure that trough concentrations were comparable. Since obtaining greater than one serum concentration after a vancomycin dose is not standard of care at the study institution, volume of distribution and vancomycin clearance were estimated based on a modification [5] of the method described by Leonard and Boro [22] using an ABW to estimate vancomycin clearance and half-life. Approximation of the percent of steady-state* achieved was calculated.
* Percent of Steady State Achieved:
Bi-variate statistical analyses were conducted using SPSS version 19.0 (SPSS, Inc., 2010, Chicago, IL). Continuous variables were evaluated using descriptive statistics and analyzed with the independent-sample Student’s t-test or Mann-Whitney U test when appropriate. Categorical variables were analyzed with the Pearson chi-square test or Fisher’s exact test when appropriate. Multivariate statistics were performed using STATA v.13 (Statacorp, College Station, TX). The multivariate model attempted to predict risk factors for trough SVCs greater than 20 mg/L. Variables were log transformed as necessary so as to not violate parametric rules. To find the most parsimonious and explanatory model, relevant confounders were controlled using a forward stepwise procedure by adding variables with a plausible relationship to the dependent outcomes. Variables of interest, such as BMI category and baseline serum creatinine value were forced into the model. Models were assessed for optimization and compared against the model with one additional variable with ultimate inclusion dictated by the lowest Bayesian and Akaike Information Criterion for the nested models. Statistical significance was defined as a p value < 0.05 for all tests.
3. Results
A total of 1,543 patients with vancomycin orders were screened for inclusion. The primary reason for exclusion was receipt of less than 4 doses of IV vancomycin, leaving 108 eligible patients included in the analyses. The cohort was primarily male (62%), and 34% of the population was obese. The mean age of the cohort was 60 years and 76% were African American. Overall, 65% did not reach a trough SVC of at least 15 mg/L at the time of assessment, and 9.3% had trough SVCs > 20 mg/L.
The characteristics of non-obese versus obese patients are shown in table 2. Non-obese patients were more likely to be male (76.1% vs 35.1%, p < 0.001). There were no significant differences between the two groups with regard to age, race, comorbidities or type of infection. While all patients were close to their vancomycin steady-state trough concentration, non-obese patients were nearer this goal (mean of 97.5% (2.6% SD) vs. 94.6% (4.4% SD), p < 0.001). There was a trend toward diabetes being more prevalent in obese patients (43.2% vs 25.4%, p=0.057). Renal function at baseline and at the time of the trough SVC was similar between non-obese and obese patients. There were no significant differences in APACHE II or SOFA scores.
Table 2.
Patient characteristics of obese versus non-obese patients (N = 108)
| Obese (n=37) |
Non-Obese (n=71) |
P-value | |
|---|---|---|---|
| Age, mean (SD) | 59.3 (14.0) | 59.6 (15.4) | 0.27 |
| Male, n (%) | 13 (35.1) | 54 (76.1) | < 0.001 |
| Ethnicity, n (%) | 0.70 | ||
| Caucasian | 4 (10.8) | 6 (8.5) | |
| African American | 29 (78.4) | 53 (74.6) | |
| Asian | 0 (0) | 3 (4.2) | |
| Hispanic | 3 (8.1) | 8 (11.3) | |
| Other | 1 (2.7) | 1 (1.4) | |
| Total body weight (kg), mean (SD) | 106.2 (20.8) | 71.9 (13.3) | < 0.001 |
| Body mass index (kg/m2), mean (SD) | 36.7 (6.4) | 23.9 (3.1) | < 0.001 |
| Comorbidities, n (%) | |||
| Diabetes | 16 (43.2) | 18 (25.4) | 0.057 |
| Hypertension | 24 (64.9) | 41 (57.7) | 0.47 |
| Heart failure | 2 (5.4) | 1 (1.4) | 0.27 |
| Ascites | 0 (0) | 1 (1.4) | 1.00 |
| Concurrent nephrotoxins, mean (SD) | 1.0 (1.1) | 1.1 (1.1) | 0.65 |
| Type of Infection, n (%) | |||
| Bloodstream | 3 (8.1) | 7 (9.9) | 1.00 |
| Lungs | 15 (40.5) | 30 (42.3) | 0.86 |
| Cerebrospinal fluid | 1 (2.7) | 4 (5.6) | 0.66 |
| Urinary tract | 1 (2.7) | 5 (7.0) | 0.66 |
| Bone | 8 (21.6) | 12 (16.9) | 0.55 |
| Skin | 1 (2.7) | 1 (1.4) | 1.00 |
| Neutropenic fever | 0 (0) | 3 (4.2) | 0.55 |
| Sepsis | 21 (56.8) | 42 (59.2) | 0.63 |
| Renal function, mean (SD) | |||
| Baseline serum creatinine (mg/dL) | 0.99 (0.31) | 0.94 (0.21) | 0.52 |
| End serum creatinine (mg/dL) | 0.94 (0.22) | 0.92 (0.22) | 0.31 |
| Baseline creatinine clearance (mL/min) | 86.2 (24.6) | 82.0 (25.6) | 0.34 |
| End creatinine clearance (mL/min) | 89.2 (24.4) | 84.3 (26.3) | 0.35 |
| APACHE II* Score, mean (SD) | 12.7 (8.4) | 13.4 (7.5) | 0.39 |
| SOFA*Score, mean (SD) | 3.0 (2.8) | 3.3 (1.9) | 0.07 |
Abbreviations: APACHE II = Acute Physiology And Chronic Health Evaluation II; SOFA = Sequential Organ Failure Assessment
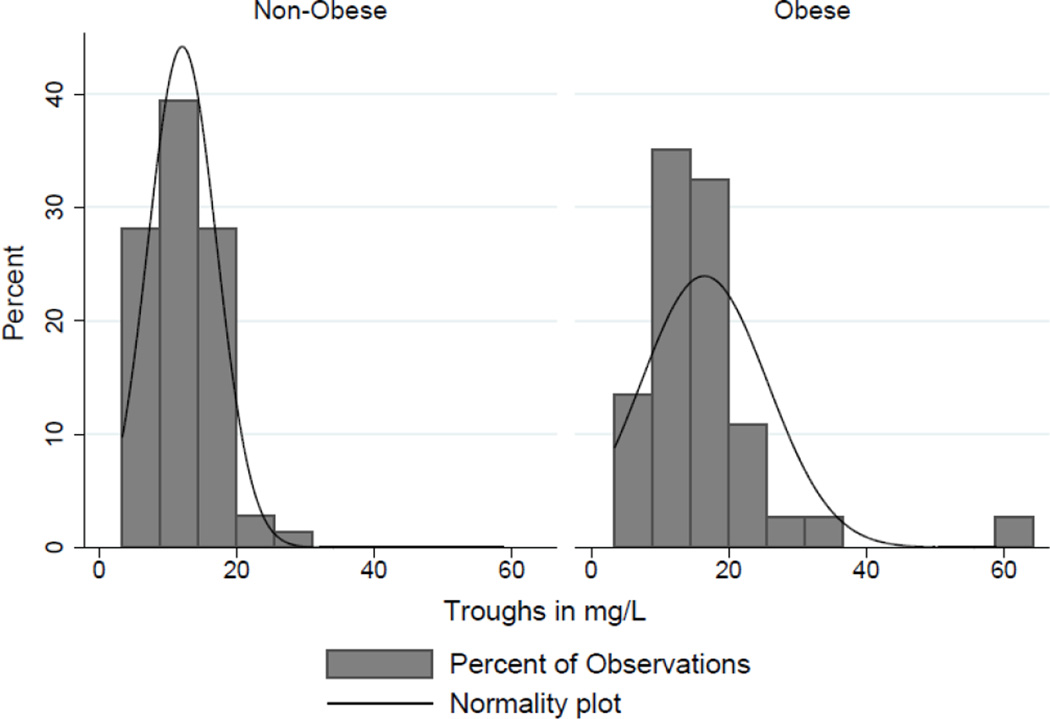
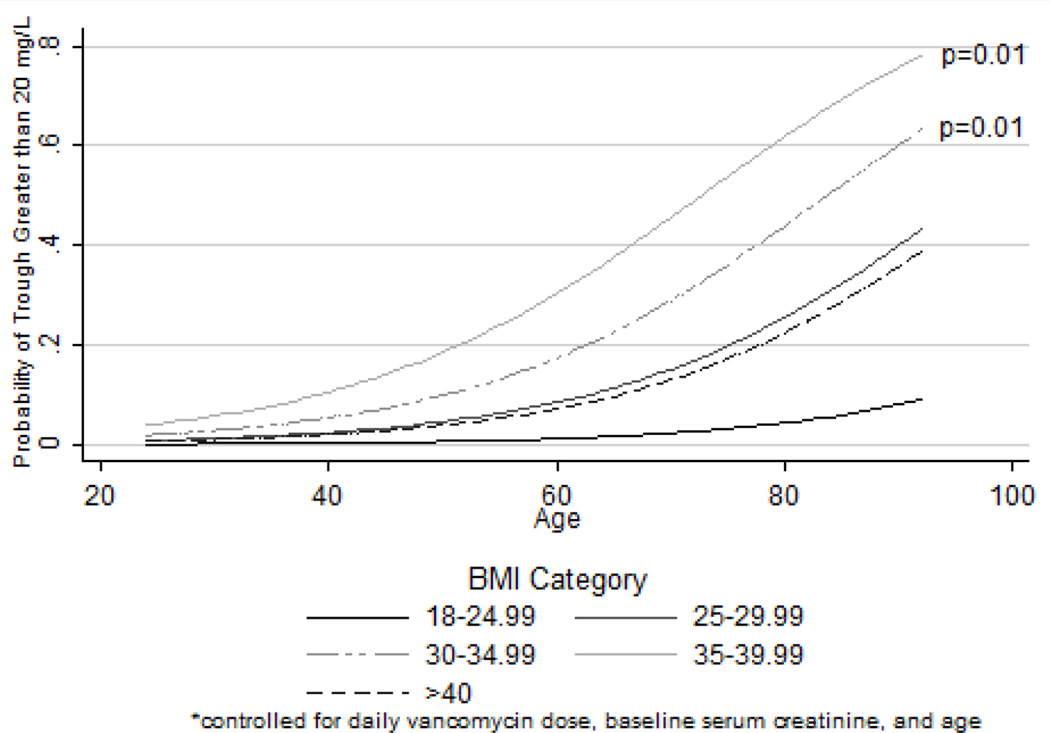
Despite being further from steady-state concentrations and receiving similar mg/kg based dosing, obese patients had significantly higher mean trough SVCs compared to non-obese patients (16.5 mg/L vs 12.1 mg/L, p=0.004) (Figure 1). A significantly higher proportion of obese patients had trough SVCs > 20 mg/L (18.9% vs 4.2%, p=0.03). This association remained significant in multivariate models; increasing levels of obesity were associated with increasing risk of trough SVCs > 20 mg/L relative to non-obese patients (see table 3). Relative to a BMI of 18–24.99 kg/m2, those with BMIs in the range of 30–34.99 kg/m2, 35–39.99 kg/m2 and > 40 kg/m2 were 23.41, 65.89 times and 15.15 times more likely to have trough SVCs greater than 20 mg/L, respectively. Increasing age and daily vancomycin dose were also significantly associated with increased risk of trough SVCs > 20 mg/L. Figure 2 depicts the probability of trough SVCs > 20 mg/L in each BMI category while controlling for daily vancomycin dose, baseline serum creatinine, and age. Estimated pharmacokinetic parameters for vancomycin in this cohort are presented in table 4. Obese patients on average had significantly larger estimated VDs and longer half-lives but similar vancomycin clearance (CLVAN). There were no statistically significant differences noted in any of the secondary clinical or safety outcomes.
Figure 1.
Histogram of trough distributions stratified by Obesity
Table 3.
Logistic regression model predicting risk factors for trough serum vancomycin concentrations > 20 mg/L (N=108)
| OR | 95% CI | P-value | |
|---|---|---|---|
| BMI* (kg/m2) category | |||
| < 24.99 | 1.00 | -- | -- |
| 25–29.99 | 10.70 | 0.74–155.47 | 0.08 |
| 30–34.99 | 23.41 | 1.99–275.66 | 0.01 |
| 35–39.99 | 65.89 | 2.75–1576.26 | 0.01 |
| > 40 | 15.15 | 0.63–362.45 | 0.09 |
| Vancomycin dose (mg/kg/day) | 1.15 | 1.01–1.31 | 0.03 |
| Age (years) | 1.13 | 1.03–1.24 | 0.01 |
| Baseline serum creatinine (mg/dL) | 3.48 | 0.11–109.54 | 0.48 |
BMI = Body Mass Index
Figure 2.
Logistic regression model depicting the probability of trough serum vancomycin concentrations > 20 mg/L in different BMI and age categories
Table 4.
Vancomycin dosing and pharmacokinetic characteristics of obese vs non-obese patients (N=108)
| Obese (n=37) |
Non-Obese (n=71) |
P-value | |
|---|---|---|---|
| Vancomycin trough (mg/L), mean (SD) | 16.5 (9.2) | 12.1 (5.0) | 0.004 |
| Vancomycin trough < 15mg/L, n (%) | 21 (56.8) | 49 (69.0) | 0.21 |
| Vancomycin trough > 20 mg/L, n (%) | 7 (18.9) | 3 (4.2) | 0.03 |
| Vancomycin dose (mg/kg/day), mean (SD) | 23.9 (7.3) | 26.0 (8.7) | 0.18 |
| Estimated vancomycin pharmacokinetic parameters, mean (SD) | |||
| CLVAN (L/hr) | 4.7 (1.3) | 4.4 (1.4) | 0.34 |
| VD (L) | 74.4 (14.5) | 50.4 (9.3) | < 0.001 |
| T1/2 (hr) | 11.8 (3.7) | 8.5 (2.5) | < 0.001 |
| Estimated half-lives elapsed prior to trough, mean (SD) | 5.1 (2.1) | 6.6 (2.8) | < 0.001 |
| Percent of steady state achieved, mean (SD) | 94.6 (4.4) | 97.5 (2.6) | < 0.001 |
4. Discussion
In this cohort, obesity and increasing levels of obesity were associated with more trough SVCs > 20 mg/L. Importantly, BMI categorization was a predictor of these supra-therapeutic concentrations. This is consistent with previous studies which indicated that vancomycin disposition is altered in obese patients[2–8] Similar to our results, Reynolds and colleagues observed that obese patients were at increased risk of elevated trough SVCs when vancomycin is given in the same mg/kg doses and intervals as non-obese patients.[11] Taken together, these data lend further support to their conclusion that obese patients may require different dosing strategies to achieve desired SVCs. Likely, a loading dose followed by reduced mg/kg doses is more appropriate for obese patients.
There were significantly more obese females than obese males. Females tend to have smaller volumes of distribution than men which could result in higher SVCs [23] and could partially account for the observation that obese patients on average had significantly higher troughs than non-obese patients. This is also consistent with other studies of vancomycin dosing and pharmacokinetics which have observed an association between female sex and higher SVCs [24]. De Waele and colleagues recently observed that a vancomycin dosing protocol utilizing continuous infusions was significantly more likely to achieve adequate early vancomycin levels in female patients (OR 4.22, 95% confidence interval 1.64–10.88). A similar study found that male sex was an independent predictor of inadequate vancomycin levels [25].
While this investigation focused on supra-therapeutic vancomycin concentrations for their role in toxicity, we also observed that a large percentage of patients (65% of the entire cohort) had trough SVCs < 15 mg/L, below the desired range for serious infections. Specifically, the following percentages were observed in the respective BMI categories, < 24.99, 25–29.99, 30–34.99, 35–39.99, > 40: 75.0%, 56.5%, 55.0%, 57.1%, 60.0%, respectively. It is also reasonable to conclude that obese patients require more frequent dosing until their larger peripheral compartment is filled. Other studies employing traditional vancomycin dosing strategies in obese patients have observed similar low rates of target SVC attainment.[7, 11]
There are several limitations to this study that need to be considered when evaluating our results. First, this was a retrospective study and data are subject to inherent biases. Our study describes a modest number of patients resulting in wide confidence intervals for standard World Health Organization categories. However, this work clearly shows a signal that patients with increasing obesity categorization score are at increased risk of supra-therapeutic concentrations. Future work will be necessary to narrow the estimate of effect size. Second, extrapolated clearance and half-lives were the only pharmacokinetic parameters calculated which were based on a single-compartment model and estimated based on formulas derived from previous population pharmacokinetic studies. However, almost all of our patients would be classified as having achieved “clinical steady-state”. Therefore, analysis of trough concentrations is valid and functioned as our primary endpoint.
In conclusion, in this cohort of patients treated empirically with weight-based dosing schemes of vancomycin, increasing BMI category and classification of obesity were associated with trough SVCs above the desired range (> 20 mg/L). Consistent with other studies, factors such as sex and renal function also appear to contribute significantly to the probability of attaining therapeutic vancomycin troughs. Development of alternative vancomycin dosing strategies and individualized therapeutic drug monitoring with dosing intervention are needed to optimize goal concentrations for obese individuals with serious infections.
Acknowledgements
Dr. Scheetz’s effort on this manuscript was supported in part by 1R15AI105742-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Transparency declarations
Preliminary results of this study were presented in abstract and poster form at the 46th American Society of Health-System Pharmacists Midyear Clinical Meeting in New Orleans, Louisiana on December 4th–8th, 2011
Dr. Scheetz has served on a consulting board for Trius Pharmaceuticals with all proceeds being donated to Midwestern University. He is a grant-funded investigator for Cubist Pharmaceutical for an antimicrobial stewardship study.
Contributor Information
Janice Richardson, Email: Janice.Richardson2@va.gov.
Marc Scheetz, Email: mschee@midwestern.edu.
References
- 1.WHO. Obesity and Overweight Fact Sheet. 2013
- 2.Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO., Jr Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54:621–625. doi: 10.1007/s002280050524. [DOI] [PubMed] [Google Scholar]
- 4.Vance-Bryan K, Guay DR, Gilliland SS, Rodvold KA, Rotschafer JC. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37:436–440. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leong JV, Boro MS, Winter M. Determining vancomycin clearance in an overweight and obese population. Am J Health Syst Pharm. 2011;68:599–603. doi: 10.2146/ajhp100410. [DOI] [PubMed] [Google Scholar]
- 6.Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit. 1994;16:513–518. doi: 10.1097/00007691-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hall G, 2nd, Payne KD, Bain AM, Rahman AP, Nguyen ST, Eaton SA, et al. Multicenter evaluation of vancomycin dosing: emphasis on obesity. Am J Med. 2008;121:515–518. doi: 10.1016/j.amjmed.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grace E. Altered vancomycin pharmacokinetics in obese and morbidly obese patients: what we have learned over the past 30 years. J Antimicrob Chemother. 2012;67:1305–1310. doi: 10.1093/jac/dks066. [DOI] [PubMed] [Google Scholar]
- 9.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 10.Shargel L, Yu ABC, Wu-Pong S. Applied biopharmaceutics & pharmacokinetics. 6th ed. New York: McGraw-Hill; 2012. [Google Scholar]
- 11.Reynolds DC, Waite LH, Alexander DP, DeRyke CA. Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm. 2012;69:944–950. doi: 10.2146/ajhp110324. [DOI] [PubMed] [Google Scholar]
- 12.Kullar R, Davis SL, Taylor TN, Kaye KS, Rybak MJ. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy. 2012;32:195–201. doi: 10.1002/j.1875-9114.2011.01017.x. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Obesity : preventing and managing the global epidemic : report of a WHO consultation. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 17.Winter MA, Guhr KN, Berg GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy. 2012;32:604–612. doi: 10.1002/j.1875-9114.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell MB, Dwinell AM, Bannick-Mohrland SD. Predictive performance of equations to estimate creatinine clearance in hospitalized elderly patients. Ann Pharmacother. 1992;26:627–635. doi: 10.1177/106002809202600503. [DOI] [PubMed] [Google Scholar]
- 19.Workgroup KDIGOKAKI. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2:1–138. [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.Leonard AE, Boro MS. Vancomycin pharmacokinetics in middle-aged and elderly men. Am J Hosp Pharm. 1994;51:798–800. [PubMed] [Google Scholar]
- 23.Chen ML, Lee SC, Ng MJ, Schuirmann DJ, Lesko LJ, Williams RL. Pharmacokinetic analysis of bioequivalence trials: implications for sex-related issues in clinical pharmacology and biopharmaceutics. Clin Pharmacol Ther. 2000;68:510–521. doi: 10.1067/mcp.2000.111184. [DOI] [PubMed] [Google Scholar]
- 24.De Waele JJ, Danneels I, Depuydt P, Decruyenaere J, Bourgeois M, Hoste E. Factors associated with inadequate early vancomycin levels in critically ill patients treated with continuous infusion. Int J Antimicrob Agents. 2013;41:434–438. doi: 10.1016/j.ijantimicag.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Ocampos-Martinez E, Penaccini L, Scolletta S, Abdelhadii A, Devigili A, Cianferoni S, et al. Determinants of early inadequate vancomycin concentrations during continuous infusion in septic patients. Int J Antimicrob Agents. 2012;39:332–337. doi: 10.1016/j.ijantimicag.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–437. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]