Abstract
Purpose
To increase intra-procedural efficiency in the use of clinical resources and to decrease planning time for cervical-cancer brachytherapy treatments through redesign of the procedure’s process map.
Methods and Materials
A multi-disciplinary team identified all tasks and associated resources involved in cervical-cancer brachytherapy in our institution, and arranged them in a process map. A redesign of the treatment planning component of the process map was conducted with the goal of minimizing planning time. Planning time was measured on 20 consecutive insertions, of which 10 were performed with standard procedures and 10 with the redesigned process map, and results compared. Statistical significance (p <0.05) was measured with a 2-tailed T-test.
Results
Twelve tasks involved in cervical-cancer brachytherapy treatments were identified. The process map showed that in standard procedures, the treatment planning tasks were performed sequentially. The process map was redesigned to specify that contouring and some planning tasks are performed concomitantly. Some quality assurance (QA) tasks were reorganized to minimize adverse effects of a possible error on procedure time. Test “dry runs” followed by live implementation confirmed the applicability of the new process map to clinical conditions. A 29% reduction in planning time (p <0.01) was observed with the introduction of the redesigned process map.
Conclusions
A process map for cervical-cancer brachytherapy was generated. The treatment planning component of the process map was redesigned, resulting in a 29% decrease in planning time and a streamlining of the QA process.
Keywords: Brachytherapy, efficiency, process maps, workflow
INTRODUCTION
Intracavitary brachytherapy after external beam radiotherapy is the standard of care for dose escalation of locally advanced cervical-cancer patients (1, 2). The treatment usually involves multiple insertions of an applicator composed of an intrauterine component (a tandem) and a vaginal component (ring or ovoids) (1–6). Historically, treatment planning was performed using a standard plan with a pear-shaped distribution and dose specified at point A. Imaging was limited to 2D plain x-ray films. Since the 1990s, 3D imaging with computed tomography (CT) and magnetic resonance imaging (MR) has become more common (7–10), allowing image-guided insertion and planning. Insertion-specific plan optimization has become feasible with the introduction of high-dose-rate (HDR) brachytherapy. The increased use of imaging for the insertion and the customized planning has been linked to improved outcomes and low toxicity (11, 12). Nevertheless, the added complexity involved in 3D planning with dose optimization, an attempt to summate external beam dose, and quality assurance (QA) of HDR plans and treatments may result in an increase in planning time and plan verification time (13). These increased times result in increased demands on clinic resources, which may deter some centers from fully adopting image-based brachytherapy. Moreover, several QA practices have been developed and are required by the Nuclear Regulatory Commission to ensure the safe administration of brachytherapy treatments. Depending on the specific implementation of the QA practices in intracavitary brachytherapy, the timing of detection and correction of possible errors may result in significant delays in the administration of treatment after applicator insertion.
Multiple guidelines are available to establish a safe and effective cervical-cancer brachytherapy practice (1, 2, 14, 15). Recently, particular attention has been dedicated to failure mode and effect analysis (FMEA) (16, 17), which can be used to improve safety for brachytherapy procedures (18, 19). One of the strengths of FMEA is its focus on potential pathways of failure. FMEA analysis is performed through the delineation of a process map (20–22), which is a description of the various components of a process and their relations to each other. FMEA analysis is generally performed by a multidisciplinary team (23). In industry and the public sector, process-map analysis has been used to improve the utilization of resources (24, 25). While multiple publications have reported process maps in radiotherapy (16, 22, 26), and also in particular in cervical-cancer brachytherapy (13), the analysis and redesign of the process map to increase efficiency is an approach that has not yet been used in radiotherapy applications. In this work, we describe the standard process map for cervical-cancer brachytherapy and provide results showing how this map was redesigned to reduce treatment planning time. We also sought to implement in the process map an efficient and safe QA practice which, without loss of quality, would reduce the delay in patient treatment due to possible errors.
METHODS AND MATERIALS
The analysis performed in this study was conducted by a medical physicist in consultation with a multidisciplinary team composed of an attending radiation oncologist (authorized user) specializing in gynecologic brachytherapy; a nurse routinely participating in gynecologic brachytherapy procedures; an operating room technical assistant responsible for maintenance and preparation of the equipment; and a radiation therapist responsible for patient set-up and ultrasound imaging.
Identification of process components (tasks)
The process under investigation is CT-guided brachytherapy for cervical cancer in our department. Standard fractionation for cervical-cancer patients in our institution is 25 fractions of 1.8 Gy of external beam radiation to the whole pelvis, followed by 5 fractions of 5.5 Gy as a boost to the remaining clinical target volume (CTV) with brachytherapy. Procedures are performed in a dedicated brachytherapy suite equipped with a CT scanner and with ultrasound (27). Tandem-and-ring or tandem- and-ovoid applicators are used (Nucletron, an Elekta company, Stockholm, Sweden), with the option of inserting interstitial needles through the ring and ovoids when necessary. The procedure is ambulatory. The overall process is defined as all tasks occurring between the patient check-in in the Department of Radiation Oncology on the day of the procedure to the exit of the patient from the brachytherapy suite after treatment. Tasks associated with exams and patient imaging occurring before the day of the procedure are not considered in this analysis.
Process map and redesign
A process map of the intracavitary insertion workflow was generated by analyzing the dependencies between the tasks and organizing the information in a visual map (standard process map). After completion of the process map, a redesign of the treatment planning sub-process (defined as all tasks between end of implantation and treatment) was conducted, with the goal of minimizing anesthesia time and redistributing QA tasks to reduce the effect of potential errors on procedure time, without loss of quality. The redesigned process map was put into clinical practice after validation through simulations and dry-runs.
Measure of effect of process map redesign
The effect of process-map redesign on planning time was measured in an IRB-approved retrospective analysis by calculating the time between acquisition of the planning CT and completion of the planning process (using the timestamp associated with the planning physicist signature, which occurs at the end of planning, after final physician review, but before independent check). Twenty consecutive insertions were considered in this study with IRB approval: 10 consecutive insertions performed prior to the implementation of the redesigned process map, and 10 consecutive insertions performed after the implementation of the optimized process map. Statistical significance (p <0.05) of the difference in planning time pre- and post-process map redesign was measured with a 2-tailed Student T-test.
The planning time analysis was performed retrospectively and at the time of the procedures the personnel was not aware that such a study would be conducted. Four physicists, 2 attending physicians, and 2 radiation therapists were involved in the planning, QA and treatment of the 20 insertions considered in this study.
RESULTS
Identification of process components (tasks)
Twelve tasks were identified. The personnel and resources needed for each task and the pre- requisite(s) needed for each task are listed in Table 1.
Table 1.
List of tasks in a cervical-cancer brachytherapy treatment. For each task, the personnel, resources and pre-requisite tasks needed to perform that task are listed. Anesthesia personnel remain with the patient through all the tasks.
| Task # | Task | Personnel | Resources | Pre-requisite task # |
|---|---|---|---|---|
| 1 | Pre-procedure evaluation | AU, RN, Anesthesia | Lab work, patient chart | None |
| 2 | Pre-insertion preparations | AU, RN, RT, TA | Brachy suite | #1 |
| 3 | Applicator insertion | AU, RN, RT, TA | Brachy suite, applicator, ultrasound | #2 |
| 4 | Imaging | AU, RT, AMP | Brachy suite, CT scanner | #3 |
| 5 | Contouring | AU | TPS | #3, #4 |
| 6 | Standard plan | AMP | TPS | #3 |
| 7 | Prior radiation EQD2 | AMP, AU | EQD2 Spreadsheet, prior dose information | None |
| 8 | Plan optimization | AU, AMP | TPS, EQD2 spreadsheet | #5, #6, #7 |
| 9 | QA preparation | AMP | TPS, R&V | #8 |
| 10 | Independent Check | AMP (not same of tasks #6 to #9) | Secondary calculation software, TPS, R&V, TPS | #9 |
| 11 | Treatment | AU, AMP, RT | Brachy suite, TCS, plan printout | #10 |
| 12 | Post-treatment | AU, RN, TA | Brachy suite | #11 |
Key: AU = Authorized User; RN = Registered Nurse; RT = Radiation Therapist; TA = Technical Assistant; Brachy = brachytherapy; AMP = Authorized Medical Physicist; CT = Computed Tomography; TPS = Treatment Planning System; EQD2 = Equivalent Dose in 2-Gy fractions; R&V = Record & Verify; TCS = Treatment Console System.
Pre-procedure evaluation
Upon arrival in the department, the patient undergoes an evaluation to verify that there are no medical contraindications to anesthesia or to the procedure. A technical assistant confirms availability of brachytherapy applicators and sterilization of the equipment.
Pre-insertion preparations
The patient is transferred on a stretcher to the brachytherapy suite and general anesthesia is inducted. The patient is transferred to the CT table, put in the lithotomy position, and a sterile field is prepared for the insertion. A surgical safety pause is conducted. A medical examination under anesthesia is conducted for preliminary applicator selection.
Applicator insertion
Ultrasound is used as needed during tandem insertion. Vaginal balloon packing (Radiadyne, Houston, TX) is used. The patient remains under anesthesia in the brachytherapy suite on the CT table until treatment and applicator removal.
Imaging
A CT scan is acquired (slice thickness of 1.25 mm, axial field-of-view of 20 cm, and superior-inferior field-of-view ranging from >2 cm inferior to the ring or ovoids to 1 cm above the uterine fundus). Adjustment to the insertion followed by additional imaging may be performed, or the insertion may be considered completed.
Contouring
The final CT scan is transferred to the Oncentra Brachytherapy1 treatment planning system (Nucletron, an Elekta Company). The CTV, the rectum, the bladder, the sigmoid, and the bowel (if needed) are contoured (28, 29). Prior planning CTs and diagnostic MR and PET images are available.
Standard plan
The applicator is digitized and a standard plan is generated. The standard plan consists of a standardized loading of the applicator providing a “pear-shaped” dose distribution with normalization to point A. Standard plans are applicator-specific, with no customization for patient anatomy. The generation of a standard plan as a starting point for further image-based optimization has been reported by other authors (9, 12, 30).
Prior radiation EQD2
A calculation of the equivalent biologic dose in 2-Gy fractions (EQD2) for the dose to 90% of the CTV (D90; α/β = 10 Gy) and dose to any 2-cm3 volume of the organs at risk (D2 cm3; α/β = 3 Gy) for all prior irradiation is performed. Prescription dose from the conformal external beam irradiation and D2cm3 values from prior brachytherapy treatments are summed (1, 2). If intensity modulated plans and/or boost external beam treatments have been delivered, a customized assessment based on dose metrics and isodose lines is performed. This customized assessment is aimed at identifying potential differences between the prior dose received by the tissue targeted by brachytherapy and the prescription dose of the external beam treatments. The EQD2 calculations are contained in an Excel spreadsheet (Microsoft Corporation, Redmond, WA) allowing the dynamic calculation of final EQD2 values once the dose metrics of the current fraction are inserted.
Plan optimization
Customized optimization is performed by manually modifying the dwell times. Optimization goals are a CTV D90 of 75–80 Gy and D2cm3 doses of <70–75 Gy for rectum and sigmoid and <90 Gy for bladder. It is critical not to plan by DVH metrics alone; DVH values cannot replace direct physician evaluation of the optimized dose distribution. The EQD2 spreadsheet is used for dose metrics evaluation. If the fraction under consideration is not the last fraction in the treatment course, it is assumed for the purpose of dose-metric evaluation that the patient will receive the same dose associated with the current fraction for all subsequent fractions.
QA preparation
After approval by the authorized user, an electronic printout of the plan is generated and the record-and-verify (R&V) treatment information is updated. A secondary dose calculation is performed and the plan is exported to the treatment console.
Independent check
An independent check by Physics checks that the correct image set, digitization, catheter lengths and channel assignments are noted on the treatment plan.
Treatment
The treatment printout is reviewed to confirm that it agrees with the treatment plan. The treatment plan must agree with the written directive. Stability of applicator positioning2, correct transfer tube attachment, and safety of treatment conditions are verified. A safety pause is conducted to confirm that all checks have been performed and that the treatment documentation is complete.
Post-treatment
The applicator is removed. The integrity of the applicator is confirmed and all equipment is inventoried and prepared for cleaning/sterilization. The patient is extubated and awakened before transfer out of the brachytherapy suite.
Process map and redesign
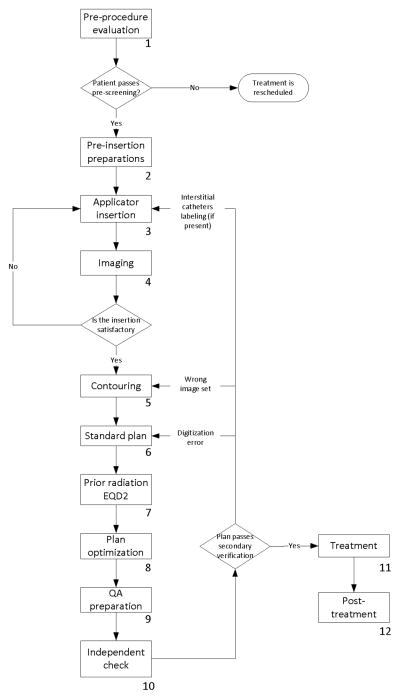
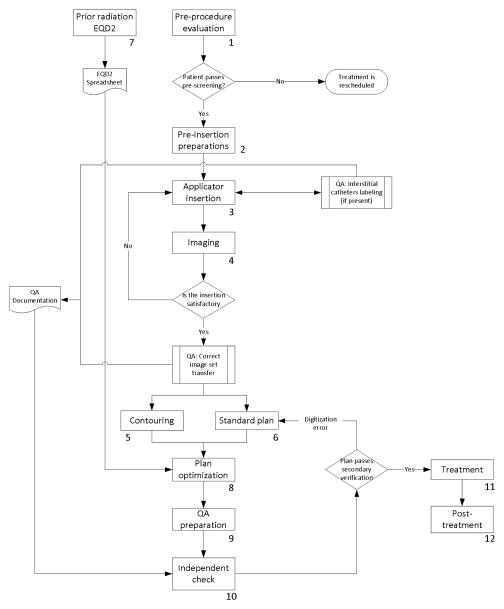
The standard process map is shown in Figure 1. The redesigned process map is presented in Figure 2. The main inefficiency in the pre-optimized process map lies in the sequential nature of the contouring and standard plan tasks, dictated by the shared need by the planning physicist and the authorized user of the treatment planning system. The alternative use of other contouring software already available in the department was considered. In the post-optimized workflow, standard plan is performed in Oncentra Brachy and contouring is performed in Eclipse (Varian Medical Systems, Palo Alto, CA), with DICOM RT structure files exported to Oncentra Brachy. The prior-irradiation EQD2 task, which does not have any pre-requisite, was moved to 1–3 days before the procedure.
Figure 1.
A process map of cervical-cancer brachytherapy treatments in our institution before 2013. The numbers associated with the components of the process map indicate the task number described in Table 1.
Figure 2.
A redesigned process map of cervical-cancer brachytherapy treatments in our institution. This new process map was put into clinical practice in 2013. The numbers associated with the components of the process map indicate the task number described in Table 1.
In the streamlining of the QA components, the independent check has been separated into sub-tasks to be performed and documented at different phases of the process. Verification of the correct image set was moved to occur when images are imported into the planning and contouring systems. A check of correct channel assignment was added at the time of implantation, as correct labeling of the catheters must be verified before the ring/ovoid is inserted into the patient in cases requiring an interstitial component attached to the ring/ovoid.
Measure of effect of process-map redesign
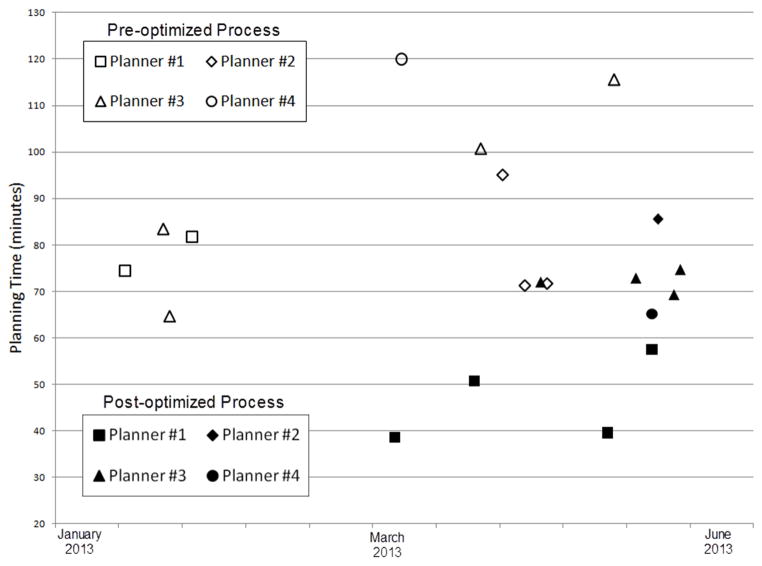
The 20 consecutive insertions considered in this study (of which 10 were performed using the standard process map and 10 using the redesigned process map) occurred between January 2013 and June 2013 and each used a tandem-and-ring applicator and did not require the use of interstitial needles. All physicists and physicians participated in planning with both the pre- and post-optimized process. The mean ±1 standard deviation of the planning time was 88±19 minutes using the pre- optimized process, and 63±16 minutes using the post-optimized process. The difference of 25 minutes corresponds to a 29% reduction in planning time and was statistically significant (p <0.01). Distribution of planning times by physicists and by date is represented in Figure 3.
Figure 3.
Distribution of planning time by planner and by date for the pre- and post-optimized process.
DISCUSSION
We established a process map for intracavitary brachytherapy for cervical cancer in our department. The inter-disciplinary process of establishing a process map has been shown to be useful to assess QA programs (16, 20, 23, 31). In this work, we used the process map to maximize the efficient use of clinical resources during the procedure and minimize anesthesia time associated with treatment planning. Through an analysis of the resources and pre-requisites associated with each task in the process map, we redesigned the treatment planning portion of the process map to introduce parallel components. We rearranged some QA tasks to enable detection of possible errors as early as possible in the process map, so as to minimize possible disruptions to a procedure resulting from tasks that would otherwise be performed after an error and before the QA task. These changes to the treatment planning process significantly decreased planning time by 29%. The redesigned process map was gradually introduced in our clinical practice between January and June 2013 and has become our standard of care.
The usefulness of creating a process map to increase efficiency has been established in industry, manufacturing and the public sector (20, 25). The application of similar techniques in medicine to improve safety and quality of treatments was suggested by Thomadsen et al. (19) in 2003. Caldwell (23) pointed out that with the increase in complexity in the medical process, and the need to coordinate multiple resources, improving operations “require[s] enhanced analysis of how, when and where information and resources are used in a time-critical task environment.” Chiozza et al. (17), in their discussion of the application of FMEA to medicine, point out the importance of choosing a process to analyze, of assembling a multidisciplinary team to collect relevant information on the process and of making a process-flow. Similarly, reports of application of FMEA in external beam radiation therapy (22), stereotactic radiotherapy (26) and brachytherapy (18, 19) describe the detailing of the process map as the first step of the process. The importance of establishing a process map as a step to increasing safety has also been discussed by the International Commission on Radiological Protection (20).
In addition to safety and QA, a process map can also be used as a tool to design efficient processes. While efficiency and resource management have been a focus of the industry studies (24), these objectives have not found broad application in radiotherapy. Mayadev et al. (13), in their report on procedure time for 217 tandem-and-ring insertions, discuss the possibility of redesigning targeted workflows to increase efficiency. However, we could not find an example of such a redesign in the literature. As Mayadev (13) explains, an increase in efficiency may result in an increased adoption of enhanced medical techniques. In a recent investigation of practice patterns in the treatment of cervical cancer in North America, Europe, Australia/New Zealand and Japan/Korea, Viswanathan et al. (7) noted that while 3D image guidance for insertion had been adopted by 66% of the 72 respondents to the study, a majority of centers do not perform customized 3D treatment planning, but rather use the same plan for all fractions. This practice has been shown to be sub-optimal (32–34). While the reasons behind the lack of adoption of 3D treatment planning vary among centers, it is possible that the resource-intensive nature of 3D treatment planning may be a contributing factor. In our work, we show that an optimization of the workflow can lead to a decrease in treatment planning time and can potentially broaden the adoption of 3D treatment planning at each fraction.
In a 2010 study (35) of planning time using our previous planning system (Plato, Nucletron, an Elekta company), we calculated that, over 26 plans, a mean of 107±20 min were spent in the planning process, of which 30±17 min were spent in the contouring task, 34±10 min in the standard-plan task, 9±12 min in the plan-optimization task, 21±8 min in the QA preparation task and 12±4 min in the independent check task. The further reduction in planning time of 25 minutes found in the current study is consistent with the assumption that this decrease is mostly due to the parallel performance of contouring and standard plan. Our analysis is potentially affected by confounding factors affecting planning speed unrelated to the change in process map. Three possible factors are: (i) a change in teamwork capability due to increased experience; (ii) uneven distribution of skill level among personnel in the plans considered in this study with the pre- and post-optimized process; and (iii) differing familiarity by personnel with the pre- and post-optimized process map. Factor (i) may result in a bias towards an overstatement of the gains obtained by the process map optimization process. All the personnel involved in this study already had considerable experience working together and were all expert in brachytherapy. Moreover, the time span considered was relatively short (6 months), and the pre- and post- optimization branches partially overlap temporally. For these reasons, we think that the effect of this bias is unlikely to meaningfully alter our results. Factor (ii) may result in a bias towards either an overstatement or an understatement of the gains obtained by the process map optimization process. All personnel contributed to both branches of our study. While we cannot rule out that a possible bias exists from different skill levels of physicists and physicians who might have contributed unevenly to the two branches, this effect appears unlikely to meaningfully alter our results. Lastly, factor (iii) may result in a bias towards an understatement of the gains obtained by the process map optimization process. We are unable to quantify this possible bias, but we note that this bias would not alter our conclusions regarding the utility of the efficiency analysis. No change in planning system or in optimization practice has occurred in the time span considered in this study, and all participating physicists and physicians were expert with the use of the treatment planning system and in general with gynecologic brachytherapy.
Further gains in the independent check task efficiency were recently reported (36) and occurred after the time period considered in this study. While the improvements in the process map described in this work are unique to our clinical process, the analysis performed can be applied to other clinical processes. In particular, given the interest in delineating a process map for safety analysis, clinics should consider concurrently performing an efficiency optimization along the lines of the one described in this work. In our experience, efficiency can be improved by explicitly identifying the resources involved in each task of the process map, the logical relation between tasks, and the availability within the department of unused or under-used resources. For instance, it is likely that the main contributor to the observed decrease in planning time is the parallel nature of the re-optimized process map. In order to identify this improvement, we observed that different personnel were involved in the contouring and standard-plan tasks, that the two tasks did not have a logical relation requiring them to be performed sequentially and that the only resource shared by the task was the planning system. An analysis of resources available in the clinic but not utilized for this process identified a different planning system that could be used for contouring. If such an additional resource were not found, a cost-analysis would be performed to assess the tradeoff between the cost of the acquisition of an additional resource and the expected gain in procedure time.
While parallel processes are the main contributor to an increase in efficiency, simply rearranging some tasks may also contribute to reducing the procedure time. While this rearrangement is not in itself an increase in efficiency for the department, the flexibility of performing the task in the moment that is the most advantageous for the personnel involved (e.g., in downtime between cases) may result in an increase in efficiency. Performing a quick task (such as EQD2 preparation) ahead of time will likely produce a modest reduction in total procedure time, but reduces the likelihood of possible missing information about prior dose at the time of the procedure. Finally, we performed an analysis of the QA tasks to minimize the amount of work occurring between a possible error and its detection. Such a rearrangement will not impact procedure time in most cases, but would avoid lengthy replication of tasks already performed if an error were to occur. While analyzing the process map for this type of improvement, it is important to assess possible wait times that are introduced due to the need for additional resources to perform QA before the next task is started in the process map. Moreover, all changes in QA procedures need to be evaluated to identify possible impacts on failure modes. No planning errors were observed in any of the 20 plans considered in this study, and no incident related to those treatments was reported. While this indicates that the change in process maps did not result in a loss of safety, the rate of errors in radiotherapy is small and this study was not designed to quantify this aspect.
Process maps of multiple processes in radiation therapy are available in the literature: Ford et al. (22) detailed one for the use of digitally reconstructed radiograph in external beam radiation therapy; Huq et al. (16) presented an IMRT process tree. More recently, process maps have been published for lung stereotactic radiotherapy (26) and for brachytherapy for cervical cancer (13). While some differences exist due to the different underlying clinical practice, the pre-optimized cervical-cancer brachytherapy process map described in this work resembles the one described in Mayadev et al. (13). In general, the nature of the radiotherapy process maps in the literature is linear. While this may reflect a desire to keep the logic behind the process as simple as possible to limit the potential for mistakes, other reasons may explain the linearity of the process. For instance, a possible explanation is that at some point in time multiple tasks shared one single, limited resource (e.g., a planning system used for planning and for contouring), forcing the two tasks to be executed consecutively instead of concomitantly. This technological barrier may have disappeared due to the wide distribution of the DICOM RT standard and the increased availability of additional commercial software that provides contouring capabilities. Nevertheless, these technological developments may not have in themselves triggered a re-thinking of the process map in most departments. Similarly, the advent of 3D treatment planning may have triggered a more thorough QA process of the image set used for planning, but the organization of the QA process within the process map may not have been revisited.
While there is no evidence that a linear process map is intrinsically safer than a more complex one, it appears to be a reasonable assumption that a complex system may be more susceptible to unintended propagation of errors resulting in possible safety concerns. For this reason, it is important that any change in an established process map be validated by a multidisciplinary analysis of the map, that any new workflow be tried in practice ‘dry runs’ and that the risk analysis involved in the process be revisited. In optimizing a workflow for efficiency, the focus is not speed of the procedure in itself, but a logical and efficient use of clinical resources. A re-optimized workflow may reduce the number of tasks to be performed under time pressure; it may remove wait time of personnel, which may reduce pressure on the rest of the team to rush to complete tasks; and it may reorganize information gathering and QA steps so as to decrease the effect that an unexpected event (such as the use of the wrong image set, or the unexpected unavailability of prior radiation data) may have on patient care. In this context, a redesign for efficiency may have a beneficial impact on safety and QA.
This work focused on CT-based, image-guided, cervical-cancer brachytherapy. This imaging modality is the most commonly used in our clinic for intracavitary brachytherapy. The possibility of performing magnetic resonance (MR)-guided insertion exists in our practice, and planning workflows associated with it have been previously described (37). Given the interest in MR guidance for cervical- cancer brachytherapy, optimization of MR-based workflows is an active area of future research. In particular, the needs associated with patient transport from MR units potentially located outside Radiation Oncology, with the optimization of the MR sequence and with the MR-based planning, will likely require the addition to the multi-disciplinary team of an MR physicist, an MR technician and possibly nursing staff from the department where the MR is located. The broad variability in the practice of MR-based cervical-cancer brachytherapy (e.g., concomitant use of other imaging modalities, the location of the MR unit, sequences used) suggests that the while the methodology of the efficiency analysis described in this work will be broadly applicable, the specific solutions will likely be of less general applicability.
CONCLUSION
A multidisciplinary team from physics, physicians, nursing, therapy and technical assistance designed a process map describing cervical-cancer brachytherapy in our institution. The treatment planning component of the process map has been redesigned, resulting in a significant decrease in planning time and streamlining of the QA aspect of treatment.
Acknowledgments
Thank you to Barbara Silver for reviewing this manuscript.
Funding:
This work was partially funded by the NIH R21 CA167800 (PI: Viswanathan)
Glossary
- CT
Computer Tomography
- CTV
Clinical Target Volume
- DICOM RT
Digitial Imaging and Communications in Medicine (Radiation Therapy extension)
- DVH
Dose Volume Histogram
- EQD2
Equivalent Biologic Dose in 2-Gy Fractions
- FMEA
Failure Mode and Effect Analysis
- HDR
High-dose-rate
- IMRT
Intensity Modulated Radiation Therapy
- MR
Magnetic Resonance Imaging
- NIH
National Institutes of Health
- QA
Quality Assurance
- R&V
Record and Verify
Footnotes
For the plans considered in this study, Version 4.0 of the software was used.
This can be performed by checking the attachment of the applicator to a securing board, by consulting clinical photography, and by clinical judgment by the attending physicians. If doubts persist on the correct positioning of the applicator, rescanning of the patient is possible. In our workflow, the patient does not leave the CT table between the planning scan and the treatment, and a shift of the applicator was never observed.
Conflict of Interest Statement:
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan AN, Beriwal S, De Los Santos JF, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012;11:47–52. doi: 10.1016/j.brachy.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffney DK, Du Bois A, Narayan K, et al. Practice patterns of radiotherapy in cervical cancer among member groups of the Gynecologic Cancer Intergroup (GCIG) Int J Radiat Oncol Biol Phys. 2007;68:485–490. doi: 10.1016/j.ijrobp.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Eifel PJ, Moughan J, Erickson B, et al. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: a patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:1144–1153. doi: 10.1016/j.ijrobp.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Erickson B, Eifel P, Moughan J, et al. Patterns of brachytherapy practice for patients with carcinoma of the cervix (1996–1999): a patterns of care study. Int J Radiat Oncol Biol Phys. 2005;63:1083–1092. doi: 10.1016/j.ijrobp.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Komaki R, Brickner TJ, Hanlon AL, et al. Long-term results of treatment of cervical carcinoma in the United States in 1973, 1978, and 1983: Patterns of Care Study (PCS) Int J Radiat Oncol Biol Phys. 1995;31:973–982. doi: 10.1016/0360-3016(94)00489-7. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan AN, Creutzberg CL, Craighead P, et al. International Brachytherapy Practice Patterns: A Survey of the Gynecologic Cancer Intergroup (GCIG) Int J Radiat Oncol Biol Phys. 2012;82:250–255. doi: 10.1016/j.ijrobp.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104–109. doi: 10.1016/j.ijrobp.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Kirisits C, Potter R, Lang S, et al. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62:901–911. doi: 10.1016/j.ijrobp.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 10.Kharofa J, Morrow N, Kelly T, et al. 3-T MRI-based adaptive brachytherapy for cervix cancer: Treatment technique and initial clinical outcomes. Brachytherapy. 2014;13:319–325. doi: 10.1016/j.brachy.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Potter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chargari C, Magne N, Dumas I, et al. Physics contributions and clinical outcome with 3D-MRI-based pulsed-dose-rate intracavitary brachytherapy in cervical cancer patients. Int J Radiat Oncol Biol Phys. 2009;74:133–139. doi: 10.1016/j.ijrobp.2008.06.1912. [DOI] [PubMed] [Google Scholar]
- 13.Mayadev J, Qi L, Lentz S, et al. Implant time and process efficiency for CT-guided high-dose-rate brachytherapy for cervical cancer. Brachytherapy. 2014;13:233–239. doi: 10.1016/j.brachy.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan AN, Thomadsen B. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles Brachytherapy. 2012;11:33–46. doi: 10.1016/j.brachy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Nath R, Anderson LL, Meli JA, et al. Code of practice for brachytherapy physics: report of the AAPM Radiation Therapy Committee Task Group No. 56. American Association of Physicists in Medicine. Med Phys. 1997;24:1557–1598. doi: 10.1118/1.597966. [DOI] [PubMed] [Google Scholar]
- 16.Huq MS, Fraass BA, Dunscombe PB, et al. A method for evaluating quality assurance needs in radiation therapy. Int J Radiat Oncol Biol Phys. 2008;71:S170–173. doi: 10.1016/j.ijrobp.2007.06.081. [DOI] [PubMed] [Google Scholar]
- 17.Chiozza ML, Ponzetti C. FMEA: a model for reducing medical errors. Clin Chim Acta. 2009;404:75–78. doi: 10.1016/j.cca.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson DA, Kolar MD. Failure modes and effects analysis applied to high-dose-rate brachytherapy treatment planning. Brachytherapy. 2013;12:382–386. doi: 10.1016/j.brachy.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Thomadsen B, Lin SW, Laemmrich P, et al. Analysis of treatment delivery errors in brachytherapy using formal risk analysis techniques. Int J Radiat Oncol Biol Phys. 2003;57:1492–1508. doi: 10.1016/s0360-3016(03)01622-5. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz Lopez P, Cosset JM, Dunscombe P, et al. ICRP publication 112. A report of preventing accidental exposures from new external beam radiation therapy technologies. Ann ICRP. 2009;39:1–86. doi: 10.1016/j.icrp.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Ciocca M, Cantone MC, Veronese I, et al. Application of failure mode and effects analysis to intraoperative radiation therapy using mobile electron linear accelerators. Int J Radiat Oncol Biol Phys. 2012;82:e305–311. doi: 10.1016/j.ijrobp.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Ford EC, Gaudette R, Myers L, et al. Evaluation of safety in a radiation oncology setting using failure mode and effects analysis. Int J Radiat Oncol Biol Phys. 2009;74:852–858. doi: 10.1016/j.ijrobp.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldwell BS. Tools for developing a quality management program: human factors and systems engineering tools. Int J Radiat Oncol Biol Phys. 2008;71:S191–194. doi: 10.1016/j.ijrobp.2007.06.083. [DOI] [PubMed] [Google Scholar]
- 24.Hicks B. Lean information management: Understanding and eliminating waste. International journal of information management. 2007;27:233–249. [Google Scholar]
- 25.Greasley A. Using process mapping and business process simulation to support a process-based approach to change in a public sector organisation. Technovation. 2006;26:95–103. [Google Scholar]
- 26.Perks JR, Stanic S, Stern RL, et al. Failure mode and effect analysis for delivery of lung stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83:1324–1329. doi: 10.1016/j.ijrobp.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Lee LJ, Damato AL, Viswanathan AN. Clinical outcomes of high-dose-rate interstitial gynecologic brachytherapy using real-time CT guidance. Brachytherapy. 2013;12:303–310. doi: 10.1016/j.brachy.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Viswanathan AN, Dimopoulos J, Kirisits C, et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Damato AL, Townamchai K, Albert M, et al. Dosimetric consequences of interobserver variability in delineating the organs at risk in gynecologic interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2014;89:674–681. doi: 10.1016/j.ijrobp.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindegaard JC, Tanderup K, Nielsen SK, et al. MRI-guided 3D optimization significantly improves DVH parameters of pulsed-dose-rate brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:756–764. doi: 10.1016/j.ijrobp.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Rath F. Tools for developing a quality management program: proactive tools (process mapping, value stream mapping, fault tree analysis, and failure mode and effects analysis) Int J Radiat Oncol Biol Phys. 2008;71:S187–190. doi: 10.1016/j.ijrobp.2007.07.2385. [DOI] [PubMed] [Google Scholar]
- 32.Davidson MT, Yuen J, D’Souza DP, et al. Image-guided cervix high-dose-rate brachytherapy treatment planning: does custom computed tomography planning for each insertion provide better conformal avoidance of organs at risk? Brachytherapy. 2008;7:37–42. doi: 10.1016/j.brachy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Pinnaduwage DS, Cunha JA, Weinberg V, et al. A dosimetric evaluation of using a single treatment plan for multiple treatment fractions within a given applicator insertion in gynecologic brachytherapy. Brachytherapy. 2013;12:487–494. doi: 10.1016/j.brachy.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Chi A, Gao M, Sinacore J, et al. Single versus customized treatment planning for image-guided high-dose-rate brachytherapy for cervical cancer: dosimetric comparison and predicting factor for organs at risk overdose with single plan approach. Int J Radiat Oncol Biol Phys. 2009;75:309–314. doi: 10.1016/j.ijrobp.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Hansen JL, Viswanathan A. Comparing Oncentra® Gyn and Plato™: A Time Study. Brachytherapy. 2010;9:S25–S26. [Google Scholar]
- 36.Damato A, et al. Independent Brachytherapy Plan Verification Software: Improving Efficacy and Efficiency. Radiother Oncol. 2014 doi: 10.1016/j.radonc.2014.09.015. http://dx.doi.org/10.1016/j.radonc.2014.09.015. [DOI] [PubMed]
- 37.Kapur T, Egger J, Damato A, et al. 3-T MR-guided brachytherapy for gynecologic malignancies. Magn Reson Imaging. 2012;30:1279–1290. doi: 10.1016/j.mri.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]