Abstract
Neuroticism is a fundamental personality trait associated with proneness to feel negative affect. Here we ask how Neuroticism influences the neural response to positive and negative social interactions and how Neuroticism modulates the effect of intranasal oxytocin (OT) and vasopressin (AVP) on the neural response to social interactions. In a double-blind, placebo-controlled study, 153 male participants were randomized to receive 24 IU intranasal OT, 20 IU AVP or placebo. Afterwards, they were imaged with fMRI while playing an iterated Prisoner’s Dilemma Game. On a different day, subjects completed the NEO personality inventory to measure Neuroticism. Neuroticism was positively correlated with the neural response to negative social interactions in the anterior cingulate cortex/medial prefrontal cortex and with the neural response to positive social interactions in the insula, indicating that Neuroticism modulates neuropsychological processing of both negative and positive social interactions. Neuroticism did not modulate the effect of intranasal OT treatment on the neural response to either positive or negative social interactions. On the other hand, AVP treatment significantly interacted with Neuroticism to modulate the BOLD response to both positive and negative social interactions. Specifically, AVP increased anterior cingulate cortex/medial prefrontal cortex and lateral temporal lobe responses to negative social interactions to a greater extent in participants scoring high rather than low on Neuroticism. AVP also increased the insula response to positive social interactions to a greater extent in participants scoring high rather than low on Neuroticism. These results imply that AVP may increase emotion regulation in response to negative social interactions and the salience of positive social interactions to a greater extent in individuals high compared to low in Neuroticism. The current findings urge caution against uniform clinical application of nonapeptides and suggest that their efficacy may vary as a function of personality.
Keywords: Neuroticism, Oxytocin, Vasopressin, fMRI, Cooperation
Introduction
Neuroticism is a fundamental personality trait associated with proneness to feel negative affect (Costa & McCrae, 1992). Individuals high in Neuroticism are more prone to perceive rejection and feel less satisfaction and intimacy in romantic relationships (Downey & Feldman, 1996; White, Hendrick, & Hendrick, 2004). Furthermore, individuals high in Neuroticism report heightened grief after the loss of a known other (Bailley, 1999). These findings suggest Neuroticism is associated with greater perceived salience of social aversiveness (Eisenberger & Lieberman, 2005). Accordingly, a growing body of neuroimaging studies has demonstrated that Neuroticism shows a positive correlation with the neural response to negative stimuli in brain regions involved in salience and emotion processing, such as the insula, striatum and amygdala (Brühl, Viebke, Baumgartner, Kaffenberger, & Herwig, 2011; Harenski, Kim, & Hamann, 2009; Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003), as well as regions implicated in emotion regulation, such as dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC) and lateral temporal lobe (Canli, et al., 2001; Haas, Constable, & Canli, 2008; Harenski, et al., 2009; Jimura, Konishi, & Miyashita, 2009; Servaas, et al., 2013). Notably, Neuroticism is also positively correlated with the neural response to positive stimuli in the striatum (Brühl, et al., 2011; Schaefer, Knuth, & Rumpel, 2011), suggesting that Neuroticism is associated with enhanced salience of both negative and positive stimuli.
In addition to personality traits, there are known biological influences on human social-emotional functioning and related brain activity. For example, the neuropeptide oxytocin (OT) enhances trust behavior (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008), increases attention to and memory for positive stimuli (Domes, et al., 2013; Guastella, Mitchell, & Mathews, 2008), and augments the neural response to positive social events in brain regions associated with salience and reward processing (Groppe, et al., 2013; Rilling, et al., 2012; Scheele, et al., 2013). Further, OT decreases cortisol stress responses induced by negative social interactions (Ditzen, et al., 2009; Linnen, Ellenbogen, Cardoso, & Joober, 2012), and attenuates amygdala responses to negative stimuli (Kirsch, et al., 2005; Petrovic, Kalisch, Singer, & Dolan, 2008). On the other hand, AVP may play a role in inter-male aggressive communication such that AVP induces agonistic facial motor patterns of male participants in response to the faces of unknown men and attenuates perceptions of the friendliness of those faces (Thompson, George, Walton, Orr, & Benson, 2006; Thompson, Gupta, Miller, Mills, & Orr, 2004). Additionally, intranasal administration of AVP increased neural response to negative faces in brain areas important in salience processing (e.g., amygdala) (Brunnlieb, Münte, Tempelmann, & Heldmann, 2013) and emotional regulation (e.g., mPFC) (Zink, Stein, Kempf, Hakimi, & Meyer-Lindenberg, 2010). Nevertheless, AVP is not always anxiogenic, and has also been linked with affiliative, prosocial behavior in some contexts (Goodson & Thompson, 2010). For example, intranasal vasopressin increased empathic concern in response to emotional videos among individuals who received higher levels of paternal warmth (Tabak, et al., 2015). Furthermore, we previously showed that intranasal AVP treatment made men more likely to reciprocate cooperation from other men in an iterated PD game (Rilling, et al., 2012). Finally,. AVP treatment has been shown to enhance memory for not only angry, but also happy faces in humans (Guastella, Kenyon, Alvares, Carson, & Hickie, 2010). Therefore, previous studies suggest that AVP may facilitate the processing of both negative and positive events/stimuli.
Importantly, however, the effects of intranasal nonapeptide treatments on human social-emotional functioning are not ubiquitous, but are heterogeneous across individuals (Bartz, Zaki, Bolger, & Ochsner, 2011). For instance, OT decreased cortisol stress responses especially among participants low rather than high in emotional regulation abilities (Quirin, Kuhl, & Düsing, 2011). In addition, intranasal administration of OT normalizes hyperactivity of amygdala and mPFC to negative stimuli in individuals with generalized social anxiety disorder, whereas there were no effects of OT on the activity of amygdala and mPFC in the control group (Labuschagne, et al., 2010, 2012). These findings suggest that OT is better able to attenuate the salience of negative events among individuals low in social-emotional functioning. On the contrary, the effects of OT in enhancing salience of positive events might be blunted in individuals exhibiting low social-emotional abilities (Scheele, et al., 2014). There has been much less research on the effects of intranasal AVP in human social-emotional cognition and related brain functions, and to the best of our knowledge no study has yet investigated how effects of AVP are modulated by dispositional personality traits.
Here, we build on previous studies by investigating how Neuroticism modulates the neural response to real-time, experienced positive and negative social interactions in the context of an iterated Prisoner’s Dilemma (PD) game and how Neuroticism interacts with intranasal administration of OT and AVP. The iterated PD game is a model for relationships based on reciprocal altruism, or the reciprocal exchange of favors. In the game, two players chose to either cooperate with each other or not. Previous studies in our lab have demonstrated that reciprocated cooperation (CC) is associated with activation in brain regions that have been linked with reward processing such as striatum as well as high levels of positive affect (Rilling, et al., 2002); whereas unreciprocated cooperation (CD) is associated with activation in insula and amygdala as well as high levels of negative affect (Rilling, et al., 2007; Rilling, et al., 2008).
In light of previous findings, we expected that individuals scoring high on Neuroticism (as compared to those scoring low on Neuroticism) would show a) enhanced neural activation to negative social interactions in brain regions important in salience processing (e.g., amygdala, insula) and emotion regulation (e.g., dlPFC, ACC, mPFC) and b) enhanced neural activation to positive social interactions in areas involved in reward or salience processing (e.g., striatum, insula). Regarding the interaction between intranasal nonapeptide treatments and Neuroticism, we expected that OT would facilitate neural responses to positive social interactions among individuals low in Neuroticism (high social emotional functioning) more so than those high in Neuroticism (low social emotional functioning). We also expected that OT would attenuate the neural response to negative social interactions among individuals high in Neuroticism (low social emotional functioning) more so than those low in Neuroticism (high social emotional functioning). Finally, AVP might increase neural responses to both positive and negative social interactions among individuals low in Neuroticism more so than those high in Neuroticism, given that individuals scoring high in Neuroticism might show strong neural responses to positive and negative events even at baseline (i.e., the placebo group) and effects of AVP would be limited in those individuals. Alternatively, AVP treatment might have additive effects with Neuroticism such that the functions of AVP in enhancing neural response to positive and negative events would be stronger among individuals high in Neuroticism than those low in Neuroticism.
Material and Methods
Subjects
153 men from the Emory University community between the ages of 18 and 22 (mean age=20.7 years) were randomized to receive intranasal OT (n=50), intranasal AVP (n=49), or intranasal placebo (n=54). All subjects gave written informed consent, and the study was approved by the Emory University Institutional Review Board and the U.S. Food and Drug Administration. Fourteen men (OT n=5, AVP n=4, and placebo n=5) were excluded from the neuroimaging analysis due to excessive motion (>1.5 mm) (n=8), missing data (n=2), abnormal brain anatomy (n=1) or to not completing the NEO-PI-RI questionnaire (n=3).
Behavioral procedures
Administration of OT, AVP or placebo
Both experimenters and subjects were blind to the treatment subjects received. All solutions were administered intranasally. The OT group self-administered 24 IU oxytocin (Syntocinin-Spray, Novartis), and the AVP group self-administered 20 IU of AVP (American Reagent Laboratories, Shirley, NY, USA). In each case, this required 10 nasal puffs to administer 1 ml of solution. The placebo group self-administered 10 nasal puffs of either OT placebo or AVP placebo (both including all ingredients, i.e., preservatives, without the active pharmacological substance). Half of the placebo subjects received OT placebo and half received AVP placebo. Subjects were instructed to place the nasal applicator in one nostril and depress the lever until they felt a mist of spray in the nostril, to then breathe in deeply through the nose, and afterwards to place the applicator in the other nostril and repeat the process.
Prisoner’s Dilemma task
In the game, two players choose to either cooperate or defect and receive a payoff that depends upon the interaction of their respective choices. The game version used in the current study is a sequential-choice PD game, in which player 1 chooses and player 2 is then able to view player 1’s choice before making his own choice (Figure 1). Each of the four outcomes is associated with a different payoff. Player cooperation followed by partner cooperation (CC) pays $2 to both player and partner, player cooperation followed by partner defection (CD) pays $0 to the player and $3 to the partner, player defection followed by partner defection (DD) pays $1 to both player and partner, and player defection followed by partner cooperation (DC) pays $3 to the player and $0 to the partner. While being imaged with fMRI, subjects played 30 rounds of a sequential-choice, iterated Prisoner’s Dilemma game in each of four sessions. For two sessions, subjects were told they were playing with the human partners (two males) they were introduced to. For the other two sessions, subjects were told that they were playing with a computer partner. In reality, subjects were always playing with a pre-programmed computer algorithm for all four sessions. For both human and computer partners, in one of the two sessions, subjects played in the role of first mover (player 1) and their partner played in the role of second mover (player 2). In the second session, roles were reversed. Subjects were compensated with a total of approximately $120; the exact amount was obtained by multiplying the total earnings across all four runs of the PD Game by 2/3.
Figure 1.
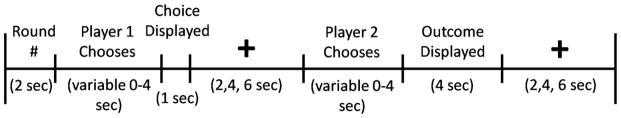
Timeline of PD task.
NEO Personality Inventory
Between one day and two weeks after the fMRI scan, participants returned to the lab to complete the NEO-PI-R (240 items answered on a 5-point scale) to measure the personality traits: Neuroticism, Extraversion, Agreeableness, Openness, and Conscientiousness (Costa & McCrae, 1992). In the current sample of subjects, the Cronbach’s alpha for the Neuroticism scale was 0.92.
Neuroimaging procedures
Behavioral analysis
In this manuscript, statistical analyses are limited to player 1 data with human partners. The frequencies of CC and CD outcomes and Neuroticism scores were compared across treatment groups (OT, AVP, placebo) using one-way ANOVA. Correlations between Neuroticism and the number of CC and CD outcomes were calculated across all three drug treatment groups combined while controlling for drug treatment, as well as separately for each drug group.
Anatomical image acquisition
Subjects were positioned head first in the supine position inside the scanner (Siemens Trio 3T), with padded head restraint to minimize head motion during scanning. Each scanning session began with a 15 s scout, followed by a 5 min T1-weighted MPRAGE 3d scan that was acquired in the sagittal plane and accelerated by generalized auto-calibrating partially parallel acquisitions (GRAPPA) with a factor of 2 (TR=2600 ms, TE=3.02 ms, matrix=256×256×176, FOV=256 mm×256 mm×176 mm, slice thickness=1.00 mm, gap=0 mm).
fMRI image acquisition
Subjects were imaged while playing the PD game. Functional scans used an EPI sequence with the following parameters: TR=2000 ms, TE=28 ms, matrix=64×64, FOV=224 mm, slice thickness=2.5 mm, 34 axial slices with a slice gap of 1.05 mm. TE was minimally decreased from the typical value (32 ms) in order to reduce magnetic susceptibility artifact in the orbitofrontal region. The duration of each EPI scan was about 12 min (30 PD round×~20 s per round, plus five null trials×14 s per trial). After each of the four sessions, while still in the scanner, subjects rated their emotional reaction to the four PD game outcomes (CC, CD, DC, and DD). Seven-point Likert scales were used to rate the following emotions or feelings: afraid, angry, happy, guilty, disappointed, and relieved.
MRI image analysis
Image processing was conducted with FEAT (FMRI Expert Analysis Tool) version 6.00, part of FSL (FMRIB’s Software Library, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Preprocessing involved motion correction using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002), slice timing correction using Fourier-space time-series phase-shifting, non-brain removal using BET (Smith, 2002), spatial smoothing using a Gaussian kernel of FWHM 5 mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with cut-off=100.0 s). Registration to MNI space via corresponding extracted T1 brain was carried out using Boundary-Based-Registration (Greve & Fischl, 2009). Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich, Ripley, Brady, & Smith, 2001).
For the player 1 runs, a separate general linear model (GLM) was defined for each subject that examined the neural response to both the epoch in which the choice to cooperate or defect was made, as well as to the epoch in which the trial outcome was revealed. More specifically, the following regressors were defined for each subject in the role of player 1: (1) the beginning epoch when round number and the partner’s face or a picture of computer was displayed, (2) the choice epoch when the subject chose to cooperate (choice C), (3) the choice epoch when the subject chose to defect (Choice D), (4) CC outcomes, (5) CD outcomes, (6) DC outcomes, and (7) DD outcomes. Parameter estimates for CC and CD outcomes were computed at every voxel within the brain for second (group) level analysis.
At the group level, random effect models were specified to investigate the influence of Neuroticism on the blood-oxygen-level dependent (BOLD) fMRI response to CC and CD outcomes and its interactions with nonapeptide (OT/AVP vs. placebo) treatments. First, we investigated correlations between the BOLD fMRI response and Neuroticism, including data from all three drug treatment groups, while controlling for drug effect by adding two dummy variables corresponding to drug treatments into the GLM model as nuisance regressors. The first dummy variable was coded as 1 if intranasal OT was administered and 0 otherwise. The second dummy variable was coded as 1 if intranasal AVP was administered and 0 otherwise. Furthermore, we assessed the potential interaction of drug treatments with Neuroticism by comparing correlations of Neuroticism with BOLD fMRI response between nonapeptide (OT/AVP) treatments and placebo treatment. Higher level (group level) analysis was carried out using Ordinary Least Square (OLS) model in FEAT. Unless noted otherwise, the Z statistic images were thresholded using clusters determined by Z>1.96 (voxel–wise 2-tailed p<0.05), and a family-wise error (FWE)–corrected cluster significance threshold of p<0.05 was applied to the supra-threshold clusters. To visualize results from whole-brain analyses, functional regions of interest (ROIs) were defined as a 10 mm cube centered on the voxel of peak activation. Average percent signal changes of each ROI were extracted via FSL’s Featquery (http://fsl.fmrib.ox.ac.uk/fsl/fsl4.0/feat5/featquery.html).
Other methodological details, such as exclusion criteria of subjects, the preparation of drugs, PD tutorial and practice trials, pre-programmed computer algorithm for the PD game, monitoring of vital signs, Positive and Negative Affect Schedule (PANAS) ratings, counterbalancing of human and computer sessions, and confederate introductions are described in our recent study (Rilling, et al., 2012).
Results
Behavioral Results
The number of CC and CD outcomes and Neuroticism scores in each drug group are illustrated in Table 1. There were no significant effects of drug treatments on these variables (all p>0.05). In addition, there were no significant correlations between Neuroticism and number of CC or CD outcomes (Table 2, all p>0.05).
Table 1.
The average (S.E.M) number of CC and CD outcomes and Neuroticism scores in each drug treatment group.
| placebo | oxytocin | vasopressin | df | F | p | |
|---|---|---|---|---|---|---|
| # of CC | 12.19 (0.80) | 10.84 (0.82) | 10.53 (0.79) | 2, 149 | 1.22 | 0.30 |
| # of CD outcomes | 5.49 (0.28) | 5.34 (0.31) | 5.18 (0.35) | 2, 149 | 0.24 | 0.79 |
| Neuroticism outcomes | 45.01 (1.43) | 46.42 (1.44) | 47.57 (1.66) | 2, 146 | 0.73 | 0.49 |
Table 2.
The correlation coefficient (p value) between Neuroticism and number of CC and CD outcomes in each drug treatment.
| Neuroticism correlates with | placebo | oxytocin | vasopressin | all groups |
|---|---|---|---|---|
| # of CC outcomes | 0.05 (0.71) | 0.04 (0.79) | 0.21 (0.16) | 0.10 (0.22) |
| # of CD outcomes | −0.06 (0.70) | 0.002 (0.99) | 0.08 (0.60) | 0.01 (0.87) |
Neuroimaging Results
CD outcomes
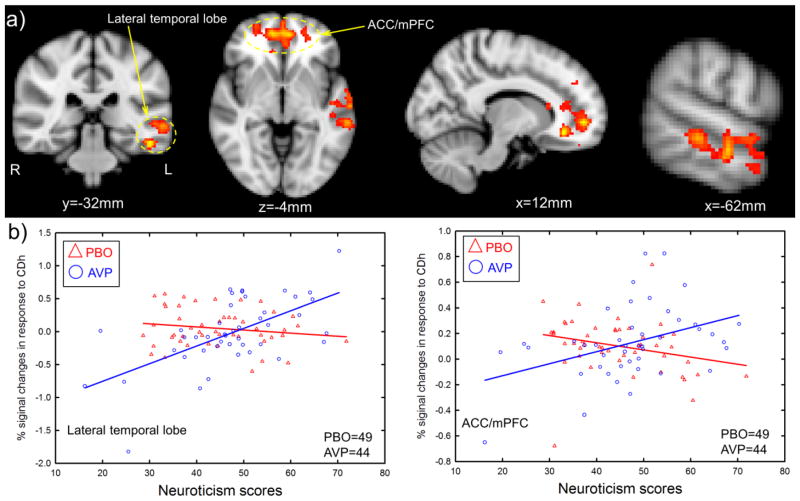
Neuroticism was positively correlated with BOLD responses to CD outcomes in several brain regions including ACC/mPFC, frontal pole, precuneus/cuneus, and middle temporal cortex (a more stringent voxel-wise threshold of p<0.01, one tailed along with the cluster threshold of p<0.05 FWE corrected was employed to better localize activations, Figure 2 & Table 3), while controlling for drug treatment (OT, AVP, placebo). Furthermore, there were significant interactions between AVP treatments and Neuroticism on activation in the ACC/mPFC and left lateral temporal lobe (Figure 3 & Table 4). These interactions revealed that AVP treatment (as compared to placebo treatment) increased ACC/mPFC and lateral temporal lobe response to negative social interactions to a greater extent among participants scoring high on Neuroticism compared to those scoring low on Neuroticism (Figure 3). There were no significant interactions of Neuroticism with OT treatment on the fMRI response to CD outcomes with the same threshold.
Figure 2.
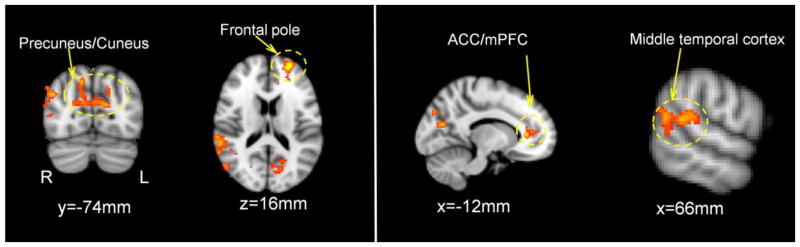
Brain regions where Neuroticism positively correlated with the neural response to CD outcomes, controlling for drug treatments (voxel-wise threshold of one-tailed p<0.01 in conjunction with cluster-wise threshold of p<0.05 FWE-corrected). ACC=anterior cingulate cortex, mPFC=medial prefrontal cortex.
Table 3.
Brain regions where Neuroticism was positively correlated with BOLD responses to CD outcomes, collapsing across data from all three drug treatment groups. Whole-brain random effect analysis; voxel-wise one-tailed p<0.01 in conjunction with cluster-wise p<0.05 FWE-corrected.
| Brain Regions | MNI Coordination of Local Maxima (mm) | Local Maxima | Cluster Size (voxel) | ||
|---|---|---|---|---|---|
|
|
|||||
| x | y | z | Z | ||
| Anterior Cingulate Cortex/Medial Prefrontal Cortex/Frontal Pole | −26 | 56 | 14 | 4.46 | 465 |
|
| |||||
| Anterior Cingulate Cortex/medial Prefrontal Cortex | −12 | 34 | 6 | 3.95 | |
| Frontal Pole | −30 | 68 | 22 | 3.50 | |
|
| |||||
| Precuneus/Cuneus | 14 | −72 | 42 | 3.65 | 740 |
|
| |||||
| R Cuneus | 22 | −72 | 20 | 3.15 | |
| L Cuneus | −12 | −72 | 18 | 3.56 | |
|
| |||||
| R Lateral Occipital Cortex/Middle Temporal Gyrus | 44 | −74 | 30 | 3.71 | 852 |
|
| |||||
| Middle Temporal Gyrus | 54 | −48 | 6 | 3.55 | |
Figure 3.
Interactions between AVP treatment and Neuroticism on the BOLD response to CD outcomes. a) Brain regions where AVP increased neural response to CD outcomes among participants high in Neuroticism more so than those low in Neuroticism (voxel-wise threshold of two-tailed p<0.05 in conjunction with cluster-wise threshold of p<0.05 FWE-corrected). b) Scatter plots from functionally defined region of interest (ROI) at lateral temporal lobe and ACC/mPFC, which confirmed whole-brain analysis illustrated in a). ACC=anterior cingulate cortex, mPFC=medial prefrontal cortex.
Table 4.
Brain regions where AVP treatment interacted with Neuroticism to modulate BOLD responses to CD outcomes. Whole-brain random effect analysis; voxel-wise two-tailed p<0.05 in conjunction with cluster-wise p<0.05 FWE-corrected.
| Brain Regions | MNI Coordination of Local Maxima (mm) | Local Maxima | Cluster Size (voxel) | ||
|---|---|---|---|---|---|
|
|
|||||
| x | y | z | Z | ||
| dorsal Anterior Cingulate Cortex/medial Prefrontal Cortex | 12 | 52 | −2 | 4.36 | 2678 |
|
| |||||
| R Paracingulate Gyrus | 12 | 52 | −2 | 4.36 | |
| L Frontal Pole | −8 | 62 | 24 | 4.34 | |
| L Superior Medial Prefrontal Gyrus | −6 | 46 | 32 | 3.88 | |
| L Paracingulate Gyrus | −6 | 50 | −4 | 3.78 | |
| ventromedial Prefrontal Cortex | −2 | 34 | −18 | 3.32 | |
|
| |||||
| L Lateral Temporal Lobe | −50 | −34 | −18 | 4.54 | 895 |
|
| |||||
| L Inferior Temporal Gyrus | −50 | −34 | −18 | 4.54 | |
| L Middle Temporal Gyrus | −64 | −18 | −8 | 3.83 | |
| L Temporal Pole | −56 | 8 | −10 | 3.38 | |
| L Superior Temporal Gyrus | −52 | −16 | −6 | 3.29 | |
CC outcomes
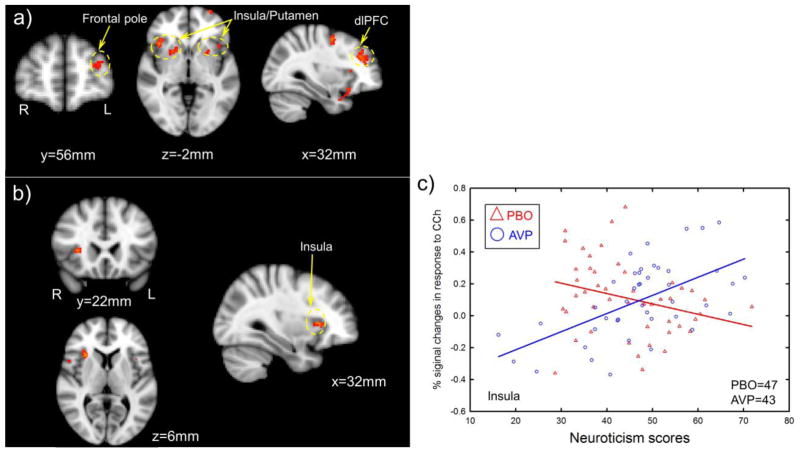
Neuroticism was positively correlated with BOLD responses to CC outcomes in several brain regions, including bilateral insula/putamen, left frontal pole, and right dlPFC (a more stringent voxel-wise threshold of p<0.005 along with the cluster threshold of p<0.05 FWE corrected was employed to better localize activations, Figure 4 & Table 5), while controlling for drug treatment (OT, AVP, placebo). Furthermore, there were significant interactions of Neuroticism with AVP treatment on the BOLD responses in the right insula (30/20/6 mm, cluster size=54, Z=4.28, voxel-wise threshold of p<0.001 along with the cluster size larger than 40 voxels, Figure 4), revealing that AVP treatment (as compared to placebo treatment) increased right insula response to positive social interactions to a greater extent among participants scoring high on Neuroticism compared to those scoring low on Neuroticism. Notably, this brain region was also identified using voxel-wise threshold of p<0.05 along with the cluster threshold of p<0.05 FWE corrected (30/20/6 mm, cluster size=2543, Z=4.28). There were no significant interactions of Neuroticism with OT treatment on the fMRI response to CC outcomes with the same threshold.
Figure 4.
Interactions between AVP treatment and Neuroticism on the BOLD response to CC outcomes. a) Brain regions where Neuroticism positively correlated with the neural response to CC outcomes, controlling for drug treatments (voxel-wise threshold of two-tailed p<0.005 in conjunction with cluster-wise threshold of p<0.05 FWE-corrected). b) Brain regions where AVP increased neural response to CC outcomes among participants high in Neuroticism more so than those low in Neuroticism (voxel-wise threshold of two-tailed p<0.001 along with the cluster size larger than 40 voxels). c) Scatter plots from functionally defined region of interest (ROI) at insula, which confirmed whole-brain analysis illustrated in b). dlPFC=dorsolateral prefrontal cortex.
Table 5.
Brain regions where Neuroticism was positively correlated with BOLD responses to CC outcomes, collapsing across data from all three drug treatment groups. Whole-brain random effect analysis; voxel-wise two-tailed p<0.005 in conjunction with cluster-wise p<0.05 FWE-corrected.
| Brain Regions | MNI Coordination of Local Maxima (mm) | Local Maxima | Cluster Size (voxel) | ||
|---|---|---|---|---|---|
|
|
|||||
| x | y | z | Z | ||
| R Insula/Putamen/Temporal Pole | 40 | 14 | −26 | 4.31 | 644 |
|
| |||||
| R Temporal Pole | 40 | 14 | −26 | 4.31 | |
| R Insula/Frontal Operculum | 42 | 22 | 6 | 4.31 | |
| R Putamen | 22 | 10 | −6 | 4.09 | |
|
| |||||
| L Insula/Putamen | −28 | 14 | 8 | 3.94 | 271 |
|
| |||||
| L Insula | −28 | 14 | 8 | 3.94 | |
| L Frontal Operculum | −32 | 16 | 10 | 3.89 | |
| L Putamen | −22 | 10 | −6 | 3.85 | |
|
| |||||
| L Frontal Pole | −22 | 70 | 20 | 4.54 | 245 |
|
| |||||
| L Frontal Pole | −22 | 70 | 20 | 4.54 | |
|
| |||||
| R Middle Frontal Gyrus/Precentral Gyrus/Superior Frontal Gyrus | 36 | 0 | 56 | 4.25 | 205 |
|
| |||||
| R Middle Frontal Gyrus | 36 | 0 | 56 | 4.25 | |
| R Precentral Gyrus | 40 | −6 | 56 | 3.64 | |
| R Superior Frontal Gyrus | 28 | 0 | 62 | 3.35 | |
|
| |||||
| R dorsolateral Prefrontal Cortex | 32 | 36 | 28 | 3.77 | 196 |
|
| |||||
| R Frontal Pole | 32 | 44 | 28 | 3.77 | |
Discussion
The goal of the current study was to examine how Neuroticism modulates the neural response to positive and negative social interactions and how Neuroticism interacts with intranasal OT and AVP administration to influence the neural response to social interactions.
Our first hypothesis was that individuals scoring high on Neuroticism (as compared to those scoring low on Neuroticism) would show enhanced neural activation to negative social interactions in brain regions important in salience processing (e.g., amygdala, insula) and emotion regulation (e.g., ACC, mPFC). In addition, Neuroticism was expected to show positive correlation with neural activation to positive social interactions in areas involved in reward or salience processing (e.g., striatum, insula). These were partially confirmed by our results insofar as there were positive correlations between Neuroticism and the neural response to negative social interactions in the ACC/mPFC and positive correlations between Neuroticism and the neural response to positive social interactions in the insula.
Our findings complement previous reports that Neuroticism is positively correlated with the neural response to positive pictures in brain regions associated with salience (Brühl, et al., 2011; Schaefer, et al., 2011) and with the neural response to negative pictures/words in brain areas related to emotion regulation (Canli, et al., 2001; Harenski, et al., 2009; Jimura, et al., 2009; Servaas, et al., 2013). These findings suggest that Neuroticism modulates neuropsychological processing of both negative and positive events. Notably, we did not find expected relations between Neuroticism and neural response to negative events in salience-related brain regions (e.g., insula and amygdala). Although several previous studies have reported that individual high in Neuroticism showed stronger neural response to negative stimuli in the insula or amygdala (Brühl, et al., 2011; Harenski, et al., 2009; Paulus, et al., 2003), a recent meta-analysis concluded that there is no consistent correlation between Neuroticism and amygdala/insula activity to emotional stimuli (Servaas, et al., 2013). We speculate that enhanced activation in emotion regulation areas among individuals high in Neuroticism might inhibit hyperactivity of salience-related areas to negative events. Alternatively, Neuroticism may interact with other factors to modulate emotional responses. For example, the association between Neuroticism and depressive symptomology is buffered by perceived social support (Dwyer, Murphy, O’Sullivan, & Di Blasi, 2014), and the association between Neuroticism and poor organizational performance is buffered by cognitive ability (Perkins & Corr, 2006).
Second, we expected that Neuroticism would interact with intranasal OT treatment to influence the neural response to both negative and positive social interactions. However, this hypothesis was not supported by our findings. This null finding is surprising since previous studies have frequently reported that the effects of OT on social-emotional functioning are modulated by characteristics of individuals (Bartz, et al., 2011). However, previous observations on how personality traits modulate the effects of OT are not conclusive. On the one hand, many studies have reported that the effects of OT in reducing stress or the salience of negative events are more effective in individuals exhibiting low rather than high social-emotional abilities (De Dreu, 2012; Labuschagne, et al., 2010, 2012; Quirin, et al., 2011; Simeon, et al., 2011). On the other hand, several other studies have shown that these OT functions might be blunted in individuals with unsupportive early parenting experiences (Bakermans-Kranenburg, van IJzendoorn, Riem, Tops, & Alink, 2011; Meinlschmidt & Heim, 2007). Accordingly, a recent meta-analysis showed that effectiveness of intranasal OT administration is diminished among clinical samples associated with untoward childhood experiences (e.g., social anxiety) (Bakermans-Kranenburg & van IJzendoorn, 2013). Therefore, exactly how characteristics of individuals modulate OT functions in social-emotional cognition awaits further investigation.
Finally, our findings confirmed our hypothesis that AVP treatment would interact with Neuroticism to modulate the BOLD response to both positive and negative social interactions, suggesting that AVP effects on brain function are modulated by personality. In particular, AVP increased ACC/mPFC and lateral temporal lobe response to negative social interactions to a greater extent in participants scoring high on Neuroticism compared to those scoring low on Neuroticism. ACC/mPFC is involved in controlled, top-down regulation of negative emotional processing (Etkin, Egner, & Kalisch, 2011) and is activated when emotional conflict needs to be overridden (Egner, Etkin, Gale, & Hirsch, 2008; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006) or when participants reappraise their emotional feelings (Kanske, Heissler, Schönfelder, Bongers, & Wessa, 2010; Urry, et al., 2006). Furthermore, lateral temporal lobe is consistently involved in reappraisal strategy of emotion regulation (Goldin, McRae, Ramel, & Gross, 2008; Ochsner, Bunge, Gross, & Gabrieli, 2002). This region is thought to represent semantic knowledge about emotion and may play an intermediary role between prefrontal control systems and emotional processing systems in emotion regulation (Ochsner, Silvers, & Buhle, 2012; Silvers, Buhle, Ochsner, & Silvers, 2013). Therefore, the current findings suggest that AVP may have increased emotion regulation in response to negative social interactions to a greater extent in participants high in Neuroticism as compared to those low in Neuroticism. Similarly, AVP increased the insula response to positive social interactions to a greater extent in participants high in Neuroticism relative to those low in Neuroticism. Given the crucial role of insula in salience processing (Menon & Uddin, 2010), our results suggest that AVP increases the salience of positive social interactions to a greater extent in participants high in Neuroticism than those low in Neuroticism. In short, the patterns of interaction between AVP treatment and Neuroticism suggest that AVP increases the neural response to both negative and positive events more so among individuals high rather than low in Neuroticism.
Taken together, our findings complement previous observations that Neuroticism influences neural responses to both positive and negative pictures or words by demonstrating modulation of neural responses to positive and negative social interactions by Neuroticism. More importantly, our observed interactions between AVP treatment and Neuroticism on the neural response to social interactions are generally consistent with recent ideas that individual characteristics modulate the efficacy of intranasally administered nonapeptides (Bartz, et al., 2011; Olff, et al., 2013). These findings urge caution against uniform application of nonapeptides and suggest that clinical efficacy of nonapeptides or their antagonists may vary as a function of personality, which might have implications in the potential use of nonapeptides or their antagonists to treat a variety of psychiatric disorders (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011).
HIGHLIGHTS.
Investigate Neuroticism and neuropeptides modulation of brain activity.
Over 150 male subjects are studied.
Prisoner’s Dilemma game is used to model everyday social interactions.
Neuroticism modulates the neural responses to positive and negative social events.
Modulatory effects of vasopressin depends on level of Neuroticism.
Acknowledgments
This study was supported by National Institute of Mental Health [grant number R01 MH084068-01A1] and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. We thank Susan Rogers, Jianguo Xu and Larry Young for assistance with various aspects of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailley SE. Personality and grieving in a university student population. 1999. [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Riem MM, Tops M, Alink LR. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Social cognitive and affective neuroscience. 2011:nsr067. doi: 10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in cognitive sciences. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Viebke MC, Baumgartner T, Kaffenberger T, Herwig U. Neural correlates of personality dimensions and affective measures during the anticipation of emotional stimuli. Brain Imaging and Behavior. 2011;5:86–96. doi: 10.1007/s11682-011-9114-7. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Münte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain research. 2013;1499:29–42. doi: 10.1016/j.brainres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral neuroscience. 2001;115:33. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Neo personality inventory–revised (neo-pi-r) and neo five-factor inventory (neo-ffi) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- De Dreu CK. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012;37:871–880. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz S, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychological medicine. 2013;43:1747–1753. doi: 10.1017/S0033291712002565. [DOI] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of personality and social psychology. 1996;70:1327. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Dwyer A, Murphy M, O’Sullivan D, Di Blasi Z. Perceived social support and neuroticism interact in predicting depression level among depressed university students. The Irish Journal of Psychology. 2014:1–9. [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why it hurts to be left out: The neurocognitive overlap between physical and social pain. The social outcast: Ostracism, social exclusion, rejection, and bullying. 2005:109–130. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Current opinion in neurobiology. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, Spreckelmeyer KN. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biological psychiatry. 2013;74:172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biological psychiatry. 2010;67:1220–1222. doi: 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biological psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Haas BW, Constable RT, Canli T. Stop the sadness: Neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. Neuroimage. 2008;42:385–392. doi: 10.1016/j.neuroimage.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Kim SH, Hamann S. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:1–15. doi: 10.3758/CABN.9.1.1. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jimura K, Konishi S, Miyashita Y. Temporal pole activity during perception of sad faces, but not happy faces, correlates with neuroticism trait. Neuroscience letters. 2009;453:45–48. doi: 10.1016/j.neulet.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2010:bhq216. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. International Journal of Neuropsychopharmacology. 2012;15:883–896. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- Linnen AM, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15:393–402. doi: 10.3109/10253890.2011.631154. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of cognitive neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Perkins AM, Corr PJ. Cognitive ability as a buffer to neuroticism: Churchill’s secret weapon? Personality and Individual Differences. 2006;40:39–51. [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of neuroscience. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Goldsmith DR, Glenn AL, Jairam MR, Elfenbein HA, Dagenais JE, Murdock CD, Pagnoni G. The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia. 2008;46:1256–1266. doi: 10.1016/j.neuropsychologia.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Knuth M, Rumpel F. Striatal response to favorite brands as a function of neuroticism and extraversion. Brain research. 2011;1425:83–89. doi: 10.1016/j.brainres.2011.09.055. [DOI] [PubMed] [Google Scholar]
- Scheele D, Kendrick KM, Khouri C, Kretzer E, Schläpfer TE, Stoffel-Wagner B, Güntürkün O, Maier W, Hurlemann R. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology. 2014;39:2078–2085. doi: 10.1038/npp.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, van der Velde J, Costafreda SG, Horton P, Ormel J, Riese H, Aleman A. Neuroticism and the brain: A quantitative meta-analysis of neuroimaging studies investigating emotion processing. Neuroscience & Biobehavioral Reviews. 2013;37:1518–1529. doi: 10.1016/j.neubiorev.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN, Silvers J. The neuroscience of emotion regulation: basic mechanisms and their role in development, aging, and psychopathology. The Handbook of Cognitive Neuroscience. 2013;1:52–78. [Google Scholar]
- Simeon D, Bartz J, Hamilton H, Crystal S, Braun A, Ketay S, Hollander E. Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology. 2011;36:1418–1421. doi: 10.1016/j.psyneuen.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, Lieberman MD, Eisenberger NI. Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: A randomized controlled trial. Psychoneuroendocrinology. 2015;51:253–261. doi: 10.1016/j.psyneuen.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, George K, Walton J, Orr S, Benson J. Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JK, Hendrick SS, Hendrick C. Big five personality variables and relationship constructs. Personality and Individual Differences. 2004;37:1519–1530. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex–amygdala circuitry during emotion processing in humans. The Journal of neuroscience. 2010;30:7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]