Abstract
Background and Aims
Visceral fat (VF) is a source of pro-inflammatory adipokines implicated in cardiac remodeling. We sought to determine the impact of visceral fat (VF) and subcutaneous fat (SQ) depots on left ventricular (LV) structure, function, and geometry in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods and Results
We performed a post-hoc analysis on 1,151 participants from MESA with cardiac magnetic resonance quantification of LV mass and LV mass-to-volume ratio (LVMV, an index of concentricity) and computed tomographic-derived SQ and VF area. Multivariable regression models to estimate association between height-indexed SQ and VF area (per cm2/m) with height-indexed LV mass (per height2.7) and LVMV were constructed, adjusted for clinical, biochemical, and demographic covariates. We found that both VF and SQ area were associated with height-indexed LV mass (ρ =0.36 and 0.12, P<0.0001, respectively), while only VF area was associated with LVMV (ρ =0.28, P<0.0001). Individuals with above-median VF had lower LV ejection fraction, greater indexed LV volumes and mass, and higher LVMV (all P < 0.001). In multivariable models adjusted for weight, VF (but not SQ) area was associated with LV concentricity and LV mass index, across both sexes.
Conclusion
Visceral adiposity is independently associated with LV concentricity, a precursor to heart failure. Further study into the role of VF in LV remodeling as a potential therapeutic target is warranted.
Keywords: Obesity, Cardiac magnetic resonance imaging, Visceral Adiposity, Remodeling
INTRODUCTION
Obesity is associated with increased lifetime risk of heart failure (HF), even after adjustment for hypertension, coronary artery disease and diabetes1, with a nearly 5% increase in HF risk for every 1 kg/m2 increase in body mass index (BMI). In both animal models and humans, the obese state is characterized by increased right and left ventricular (LV) mass, concentric LV remodeling, decreased systolic and diastolic performance, and increased myocardial fibrosis—all pathogenic features predisposing to HF2, 3. However, whether the degree of obesity itself—or its associated inflammation and insulin resistance—produces myocardial changes remains unclear. Indeed, using data from the Multi-Ethnic Study of Atherosclerosis (MESA), our group has reported a BMI-independent association between impaired fasting glucose and cardiac remodeling by cardiac magnetic resonance (CMR) imaging4, consistent with other large, community based cohorts5. These results suggest that cardiometabolic changes may be mediators of obesity-related cardiac remodeling and incident HF. In this context, increased visceral adiposity has emerged as a depot for abnormal pro-inflammatory signals that characterize obesity-related vascular dysfunction, LV hypertrophy, and HF6. Recent results from one community-based study suggest that visceral adipose burden may identify otherwise healthy individuals with greater LV hypertrophy and concentric remodeling regardless of BMI7. These findings suggest a role for visceral adiposity in reinforcing subclinical changes in myocardial structure more generally, and potentially in promoting future heart failure.
To address a potential role for adiposity and its distribution in cardiac remodeling, we investigated the association between both visceral and subcutaneous fat areas and indices of structure and function in MESA participants. We hypothesized that visceral adiposity would be associated with increased LV mass and a greater degree of concentric LV remodeling (as defined by LV mass-to-volume ratio). Through the diversity within MESA, we were able to further examine whether age, sex, race, and selected biomarkers of adiposity and ventricular remodeling might modify this association. Ultimately, these results might provide a basis for adiposity distribution as a robust imaging biomarker for identifying those individuals at high risk for cardiac remodeling and future heart failure.
MATERIALS AND METHODS
Participant population
The overall design of the MESA study has been described previously8. In brief, MESA consists of 6,814 men and women of different ethnicities (white, African American, Chinese American, and Hispanic) enrolled from six sites throughout the United States, all of whom were free of cardiovascular disease (prior myocardial infarction, angina pectoris, revascularization, HF, atrial fibrillation, stroke, or peripheral arterial disease) at the time of study enrollment. Baseline demographics, medical history, medications, body mass index (in kg/m2), resting systolic blood pressure, fasting blood glucose, and dysglycemia status were assessed at the index MESA clinic visit.9
At Exams 2 and 3, a subgroup of 1,970 MESA participants underwent abdominal computed tomography (CT) scans for aortic calcium that were analyzed for visceral and subcutaneous fat area (at exam 2, 756/577 and at exam 3, 1,172/1,114; respectively). We investigated the association between visceral and subcutaneous adipose tissue area by CT (at Exam 2/3) and CMR indices of cardiac structure and function at Exam 1 (median interval 3.1 years, IQR 1.7–3.4 years). We excluded patients with cancer, renal disease, or cirrhosis at Exam 1; all subjects were required to have complete CT data for adiposity (at Exams 2 or 3) and CMR data at Exam 1. Our complete dataset (CT adiposity and CMR data) consisted of 1,151 MESA participants.
Fasting blood samples collected at the third MESA Exam were used to quantify additional biomarkers of insulin resistance, systemic inflammation, and neurohormonal activation (interleukin-6/IL-6, high-sensitivity C-reactive protein, leptin, adiponectin, insulin, and tumor necrosis factor TNF-α) as previously described10–12. Plasma renin and aldosterone were measured at the time of CT scan. Protocols were approved by the Institutional Review Board at each participating institution. All MESA participants provided written informed consent.
Measurement of visceral and subcutaneous fat area
Electron-beam computed tomographic (CT) scanners were utilized at Northwestern University and University of California, Los Angeles (Imatron C-150), with the following settings: collimation 3 mm, slice thickness 6 mm, reconstruction using 25 6-mm slices with 35-cm field of view and normal kernel. Multi-detector CT scanners were utilized at Columbia University, Wake Forest University, and University of Minnesota field centers (Sensation 64, GE Lightspeed; Siemens S4 Volume Zoom; and Siemens Sensation 16). CT imaging was interpreted blinded to clinical information.
Visceral adiposity was defined as the fat enclosed by the visceral cavity. Fat tissue was defined as being between −190 and −30 Hounsfield units (HU). Within each area of interest (subcutaneous and visceral), we assigned the density value assigned to each pixel using the MIPAV 4.1.2 software (National Institutes of Health, Bethesda, MD) as fat or lean tissue, calculating the total visceral and abdominal fat area (in terms of centimeters2). Six transverse cross section slices of data were analyzed (2 at L2–3, 2 at L3–4 and 2 at L4–5). Visceral and subcutaneous fat area were calculated as the sum of visceral and subcutaneous fat area over all available slices, and indexed to height (meters). Inter- and intra-rater reliabilities for total abdominal, subcutaneous, and visceral cavity areas were 0.99 for all measures. In cases where parts of the abdominal cavity were outside the field of view of the CT, we used imputation methods (detailed in published work from MESA13) for subcutaneous fat (in 226 patients, 19.6%) and visceral fat (in 3 patients, 0.3%).
Cardiac magnetic resonance (CMR) assessment of LV structure and function
CMR imaging was performed at 1.5 Tesla at the index examination as previously described14–16. Assessment of ventricular function was performed using electrocardiographically-gated fast gradient echo cine images (repetition time 6 msec, minimal echo time, flip angle 20°, 8 mm slice thickness with 2 mm gap, matrix 256×160, field of view adjusted to body size, receiver bandwidth 32 kHz). LV volumes and mass were determined by short-axis volumetric coverage, normalized to body surface area. Papillary muscles were included in the LV volumes and excluded from LV mass. LV mass to LV end-diastolic volume was calculated as an index of concentric remodeling. LV mass was indexed to height2.7 (in meters2.7). MASS software (version 4.2, Medis, The Netherlands) at a single reading center by readers blinded to clinical data was used to quantify data.
Statistical analysis
Baseline clinical, demographic, biochemical and CMR indices were compared across above-and below-median strata of visceral and subcutaneous adipose area using standard methods (Wilcoxon rank-sum or Kruskal-Wallis tests for continuous covariates; chi-square test for categorical covariates). We calculated Spearman correlation coefficients between height-indexed LV mass or concentric LV remodeling (LV mass-to-volume ratio) and visceral or subcutaneous adipose area and selected biomarkers of adiposity and HF. Multivariable linear regression models for indexed LV mass and LV mass-to-volume ratio were constructed, adjusted for age, sex, race, systolic and diastolic blood pressure, lipid lowering medications, hypertension medications, diabetes, smoking, high-density lipoprotein, triglycerides (log-transformed), total intentional exercise, weight, and family history of myocardial infarction and excess alcohol intake (>2 drinks per day), all measured at Exam 1. These covariate adjustments were chosen to be consistent with prior work within MESA17. In addition, to explore the effects of weight and BMI on these relationships, we constructed models further adjusted for weight or BMI. We evaluated the presence of effect modification by age, sex, and race on the association between visceral or subcutaneous adipose area and indexed LV mass or LV mass-to-volume using interaction terms. Finally, we constructed linear regression models considering non-imputed data only, excluding imputed data for subcutaneous fat in models with subcutaneous fat, and excluding imputed data for visceral fat in models with visceral fat. Where appropriate, covariates were log-transformed to reduce skewness. Models were assessed for standard assumptions of linear regression, as appropriate. SAS version 9.4 (SAS Institute, Cary, NC) or R version 3.1.1 (R project) was used for all analysis, and a two-tailed p-value < 0.05 was considered significant.
RESULTS
Clinical and biochemical characteristics stratified by visceral and subcutaneous fat burden
Baseline clinical, demographic, and biochemical indices at Exam 1 (time of CMR) stratified by median visceral fat (VF at Exam 2 or 3; 485.6 cm2/m, interquartile range 329.7–707.2 cm2/m) are shown in Table 1. MESA participants with above-median VF were more likely to be slightly older and male, with more prevalent dysglycemia (by diabetes status, fasting glucose), lower high-density lipoprotein concentration, and more frequent hypertension. Individuals with above-median VF area had a higher serum aldosterone, plasma renin activity, high-sensitivity C-reactive protein (hsCRP) and IL-6, and fasting insulin, and a lower adiponectin relative to below-median VF area (all P<0.01). Subcutaneous (SQ) fat area and BMI were higher in individuals with greater VF. MESA participants with higher SQ fat (around median 637.2 cm2/m; Supplementary Table 1) were more likely to be female, with a higher BMI and visceral fat burden (by waist circumference and VF area), though there were no differences in dysglycemia status. Similar to VF, above-median SQ fat was associated with biomarkers of inflammation and insulin resistance (e.g., hsCRP, IL-6), though serum aldosterone and adiponectin concentrations were similar relative to below-median SQ fat.
Table 1.
Baseline clinical, demographic, and biochemical characteristics, stratified by above- and below-median visceral fat area.
| Variable (at Exam 1, unless otherwise noted) | Low Visceral Fat (N= 575) |
High Visceral Fat (N=576) |
P |
|---|---|---|---|
| Age (years) | 59 (52–68) | 62 (54–69) | 0.001 |
|
| |||
| Male Gender | 210 (37%) | 364 (63%) | <0.0001 |
|
| |||
| Race | <0.0001 | ||
| Caucasian | 209 (36%) | 231 (40%) | |
| Chinese American | 122 (21%) | 75 (13%) | |
| African American | 150 (26%) | 77 (13%) | |
| Hispanic | 94 (16%) | 193 (34%) | |
|
| |||
| Smoking Status | 0.12 | ||
| Never Smoker | 331 (58%) | 300 (52%) | |
| Former Smoker | 174 (30%) | 206 (36%) | |
| Current Smoker | 69 (12%) | 70 (12%) | |
|
| |||
| Metabolic Syndrome | 81 (14%) | 250 (44%) | <0.0001 |
|
| |||
| Glycemic Control | <0.0001 | ||
| Normal Fasting Glucose | 499 (87%) | 394 (69%) | |
| Impaired Fasting Glucose | 49 (9%) | 98 (17%) | |
| Untreated diabetes | 6 (1%) | 25 (4%) | |
| Treated diabetes | 20 (3%) | 57 (10%) | |
|
| |||
| Total Cholesterol (mg/dl) | 192 (173–217) | 193 (172–215) | 0.49 |
|
| |||
| High Density Lipoprotein (mg/dl) | 54 (45–66) | 44 (39–52) | <0.0001 |
|
| |||
| Triglycerides (mg/dl) | 93 (66–129) | 137 (94–187) | <0.0001 |
|
| |||
| Low Density Lipoprotein (mg/dl) | 115 (96–137) | 117 (101–135) | 0.19 |
|
| |||
| Body mass index (kg/m2) | 24.7 (22.6–26.8) | 28.4 (26.2–31.0) | <0.0001 |
|
| |||
| Systolic blood pressure (mmHg) | 118 (108–136) | 127 (113–143) | <0.0001 |
|
| |||
| Diastolic blood pressure (mmHg) | 71 (64–77) | 75 (68–81) | <0.0001 |
|
| |||
| Waist Circumference (cm) | 87.7 (81.0–94.5) | 100 (94–107) | <0.0001 |
|
| |||
| Weight (pounds) | 148.3 (130.8–166.5) | 178 (158–197) | <0.0001 |
|
| |||
| Fasting glucose, Exam 1 (mg/dl) | 86 (81–93) | 92 (86–101) | <0.0001 |
|
| |||
| Urine Albumin-Creatinine Ratio | 4.7 (3.1–8.1) | 6.0 (3.6–11.2) | <0.0001 |
|
| |||
| Coronary Artery Calcium Score | 0 (0–22.4) | 6.8 (0–91) | <0.0001 |
|
| |||
| Height-indexed visceral fat area (cm2/m) | 329.7 [242.6–412.6] | 707.2 [579.0–859.5] | <0.0001 |
|
| |||
| Height-indexed subcutaneous fat area (cm2/m) | 561.2 [394.4–791.0] | 695.1 [507.8–920.8] | <0.0001 |
|
| |||
| Serum biomarkers (at Exam 3) | |||
| Adiponectin (ng/ml)* | 21479.5 [14274.3–31646.9] | 15182.9 [10569.2–20815.6] | <0.0001 |
| Aldosterone (pg/ml)* | 127.2 [88.7–181.0] | 139.0 [100.2–188.0] | 0.003 |
| Plasma renin (ng/ml)* | 0.47 [0.24–0.91] | 0.65 [0.33–1.38] | <0.0001 |
| High-sensitivity CRP (mg/l)* | 1.1 [0.6–2.3] | 1.6 [0.8–3.5] | <0.0001 |
| Interleukin-6 (pg/ml)* | 1.4 [1.0–2.1] | 2.0 [1.4–3.0] | <0.0001 |
| Insulin (pg/ml)* | 168.0 [122.7–230.3] | 259.4 [193.5–382.0] | <0.0001 |
| Leptin (pg/ml)* | 8887.2 [3362.3–21432.2] | 13192.6 [6443.9–28681.1] | <0.0001 |
| Tumor necrosis factor-α (pg/ml)* | 4.2 [3.2–5.7] | 4.8 [3.7–6.4] | <0.0001 |
Abbreviations: CT, computed tomography; CRP = C-reactive protein. Values represent median (interquartile range) or number (percentage).
Missing observations for adiponectin (2/0), aldosterone (23/17), plasma renin (54/32), CRP (22/5), insulin (3/0), leptin (8/0), TNF-α (5/2) for (low/high) visceral fat subgroups.
Association of adiposity with indices of LV structure, function, and remodeling
CMR indices of LV structure, function, and remodeling stratified by SQ and VF area are shown in Table 2. Individuals with above-median VF had a slightly lower LV ejection fraction, with greater indexed LV volumes and mass, and a higher LV mass-to-volume ratio (all P < 0.001). On the other hand, individuals with above-median SQ fat had a similar LV ejection fraction as individuals with below-median SQ fat. In addition, while LV end-diastolic volume and mass were increased with higher SQ fat, LV concentricity (by LV mass-to-volume index) was similar regardless of SQ fat.
Table 2.
Indices of left ventricular structure, function, and remodeling, stratified by above- and below-median visceral and subcutaneous fat area.
| Variable | Height-indexed Visceral Fat Area | Height-indexed Subcutaneous Fat Area | ||||
|---|---|---|---|---|---|---|
| Below-median | Above-median | P | Below-median | Above-median | P | |
| LVEF | 70.8 [66.4–74.5] | 69.2 [64.2–73.8] | 0.0002 | 69.4 [64.6–74.0] | 70.5 [65.7–74.3] | 0.1 |
| LVEDVI | 30.7 [27.3–34.3] | 32.1 [28.5–36.2] | 0.0001 | 30.9 [27.1–34.8] | 32.0 [28.5–35.8] | 0.0003 |
| LVESVI | 8.9 [7.3–10.8] | 9.8 [7.8–12.1] | <0.0001 | 9.3 [7.2–11.6] | 9.4 [7.7–11.4] | 0.31 |
| LVMI | 33.2 [28.8–37.9] | 37.3 [33.0–42.9] | <0.0001 | 34.8 [30.2–40.1] | 35.7 [31.6–40.8] | 0.02 |
| LVMV | 1.1 [0.9–1.2] | 1.2 [1.0–1.3] | <0.0001 | 1.1 [1.0–1.3] | 1.1 [1.0–1.3] | 0.48 |
Abbreviations: LVEF, left ventricular ejection fraction; LVEDVI = height-indexed left ventricular end-diastolic volume; LVESVI = height-indexed left ventricular end-systolic volume; LVMI = height-indexed left ventricular mass; LVMV = left ventricular mass to volume ratio. Quantities represent median (interquartile range). P values represent Wilcoxon rank-sum testing between the above- and below-median fat strata.
VF area was more closely correlated to indexed LV mass than SQ area (ρ =0.36 and 0.12, both P<0.0001, respectively) and only VF area was significantly correlated with LV mass-to-volume ratio (ρ=0.28, P<0.0001; Supplementary Table 2). In addition, greater VF fat was correlated with poorer LV function (with LVEF: ρ=−0.10, P=0.0005), while SQ fat was weakly associated with modestly higher LV function (ρ=0.06, P=0.04).
The relationship between VF and SQ fat and LV mass and LV concentricity by age, sex, race, and obesity status is displayed in Figure 1. Despite heterogeneity in LV concentricity and LV mass by sex (males higher), race (African American higher), obesity status (obese higher), or age (older age higher), none of these demographic indices modified the relationship between VF and LV mass or geometry: VF was consistently associated with higher LV mass and concentricity regardless of age, obesity status, sex, and race. SQ fat was generally not associated with increased LV mass or concentricity.
Figure 1.
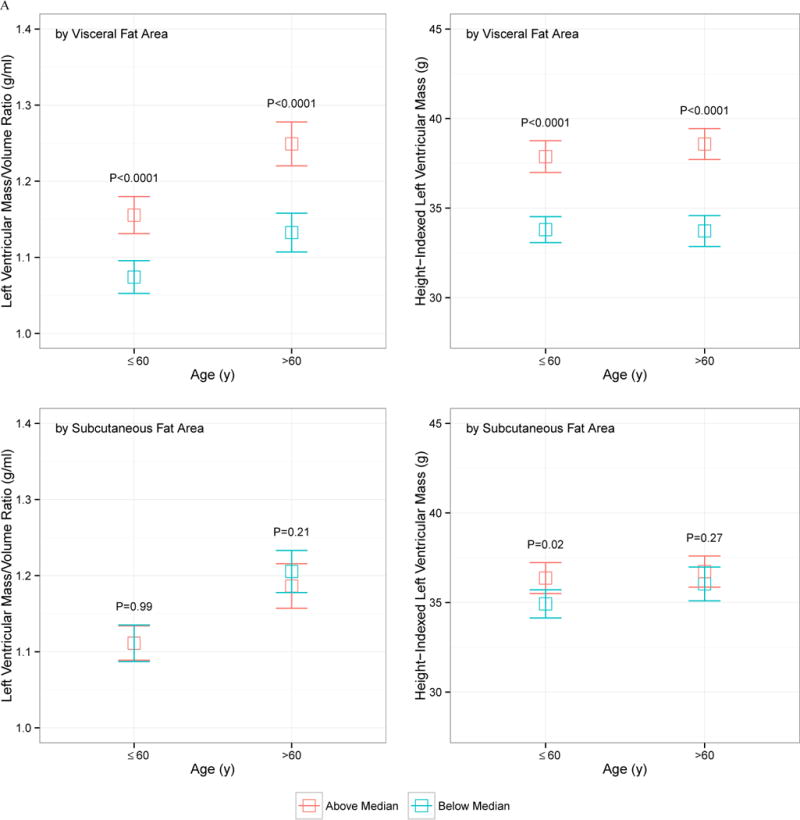
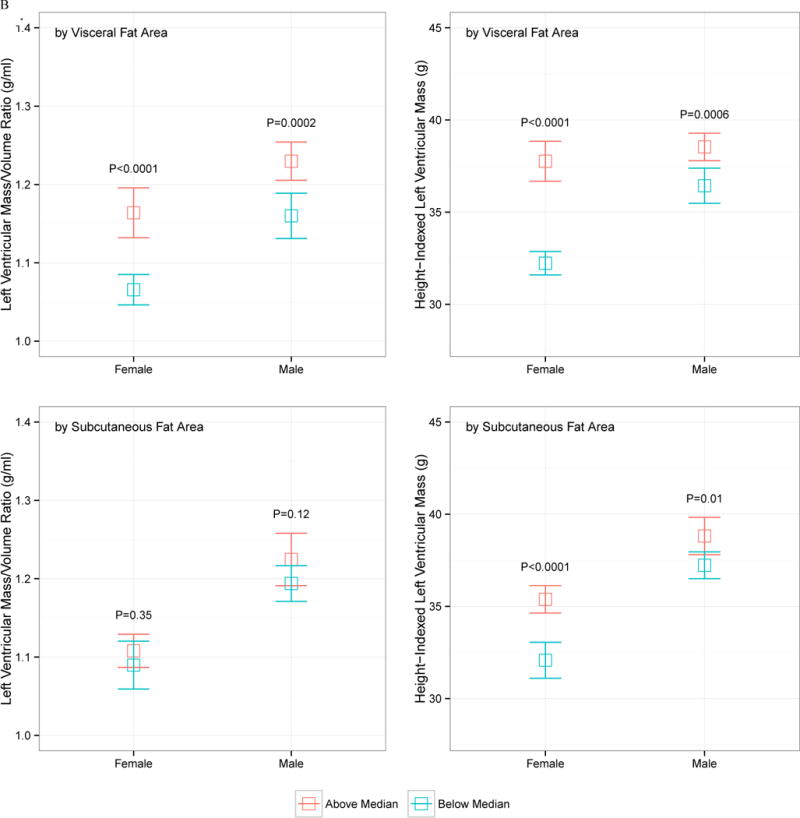
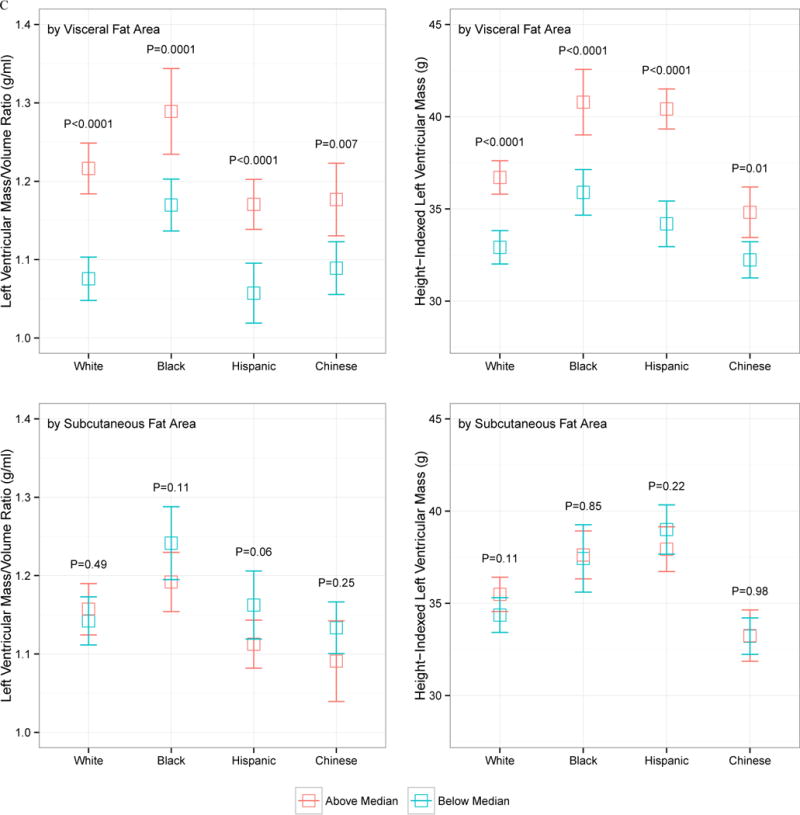
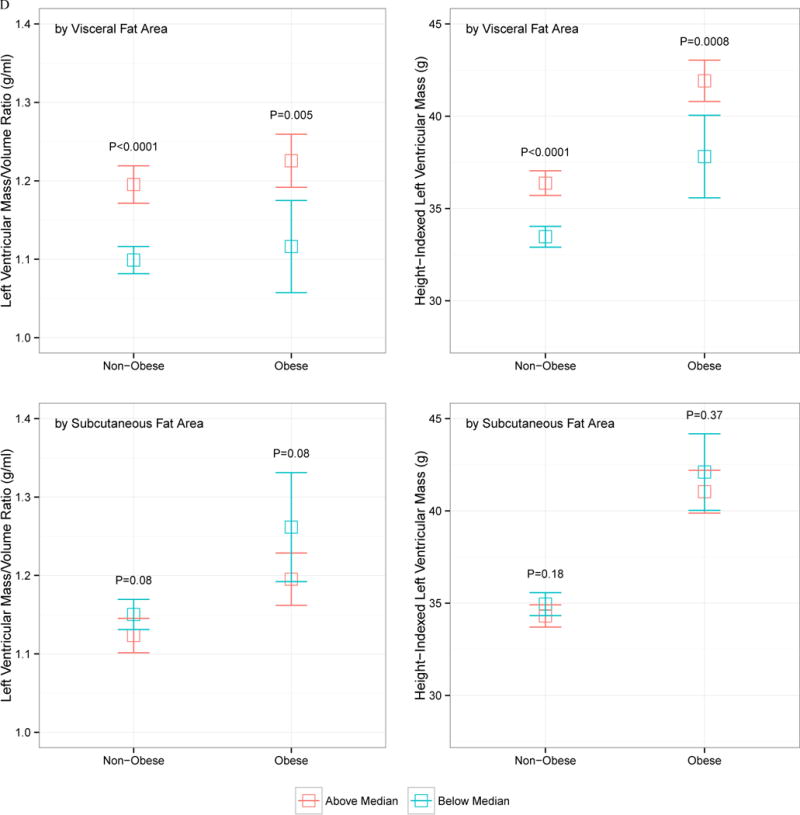
Indexed left ventricular mass and left ventricular mass-to-volume ratio, stratified by above and below median visceral or subcutaneous fat area, by age (A), sex (B), race (C), or obesity status (D; obese > 30 kg/m2). The error bars represent 95% confidence interval around the mean, and P values represent comparison of above- and below-median VF or SQ fat area value
Visceral fat is associated LV remodeling across age, sex, race, and BMI
In our model for LV mass-to-volume ratio, both visceral and subcutaneous fat were predictors of LV concentricity (Table 3). When both VF and SQ were added to this model, VF was associated with increased concentricity (β=0.016, P<0.0001) but SQ was not associated with any change in concentricity (Table 3A). These associations were directionally consistent with further adjustments by BMI or weight. When both VF and SQ were included in models, VF and SQ areas appeared to have opposite associations with concentricity. There was no evidence that age, sex, obesity status, or race modified the observed association between VF or SQ area and LV mass-to-volume ratio.
Table 3.
Multivariable linear regression models for left ventricular mass-to-volume ratio (panel A; LVMV) and left ventricular mass index (panel B; LVMI). All models were adjusted for age, gender, race, systolic and diastolic blood pressure, use of lipid lowering medications, hypertension medications, diabetes status, high density lipoprotein, triglycerides (log transformed), total intentional exercise, heavy alcohol use (> 2 drinks per day), stroke work, and family history of myocardial infarction. Models on the right panel were adjusted for left ventricular stroke work, as defined in text. Beta-coefficients are reported for every 100 cm2/m.
| (A) | ||||||
|---|---|---|---|---|---|---|
| No weight/BMI adjustment | BMI-adjusted | Weight-adjusted | ||||
| LVMV | P | LVMV | P | LVMV | P | |
| Model 1: Visceral Fat | 0.0168 | <0.0001 | 0.00609 | 0.06 | 0.00884 | 0.004 |
| Model 2: Subcutaneous Fat | 0.00685 | 0.0007 | −0.00723 | 0.006 | −0.00296 | 0.22 |
| Model 3: Visceral and Subcutaneous Fat | ||||||
| Visceral Fat | 0.0155 | <0.0001 | 0.00648 | 0.047 | 0.00979 | 0.002 |
| Subcutaneous Fat | 0.00228 | 0.29 | −0.00745 | 0.005 | −0.00429 | 0.08 |
| (B) No weight/BMI adjustment—LV Mass Index | |||||
|---|---|---|---|---|---|
| Covariate | Women | Men | Interaction P | ||
| β | P | β | P | ||
| Model 1: Visceral Fat only |
0.972 | <0.0001 | 0.198 | 0.09 | <0.0001 |
| Model 2: Subcutaneous Fat only |
0.369 | <0.0001 | 0.266 | 0.02 | 0.72 |
| Model 3: Visceral Fat Subcutaneous Fat |
0.839 0.160 |
<0.0001 0.07 |
0.113 0.223 |
0.38 0.08 |
0.0002 0.40 |
| BMI adjusted—LV Mass Index | |||||
|---|---|---|---|---|---|
| Covariate | Women | Men | Interaction | ||
| β | P | β | P | ||
| Model 1: Visceral Fat only |
0.217 | 0.20 | −0.506 | 0.0001 | 0.03 |
| Model 2: Subcutaneous Fat only |
−0.316 | 0.002 | −0.532 | 0.0001 | 0.78 |
| Model 3: Visceral Fat Subcutaneous Fat |
0.254 −0.326 |
0.13 0.002 |
−0.475 −0.499 |
0.0003 0.0003 |
0.004 0.27 |
| Weight-adjusted—LV Mass Index | |||||
|---|---|---|---|---|---|
| Covariate | Women | Men | Interaction | ||
| β | P | β | P | ||
| Model 1: Visceral Fat only |
0.906 | <0.0001 | 0.0321 | 0.81 | <0.0001 |
| Model 2: Subcutaneous Fat only |
0.274 | 0.007 | 0.0799 | 0.57 | 0.53 |
| Model 3: Visceral Fat Subcutaneous Fat |
0.848 0.169 |
<0.0001 0.10 |
0.0191 0.0762 |
0.89 0.59 |
0.0002 0.47 |
For LV mass index, we found significant associations between VF (β=0.589 for every 100 cm2/m VF, P<0.0001) and SQ (β=0.210 for every 100 cm2/m SQ, P=0.0008) in separate models. When included together with full adjustments, only VF was associated with greater LV mass (β=0.548 for every 100 cm2/m VF, P<0.0001). We further stratified our regression analyses by sex, which are displayed in Table 3B. In general, the associations between VF and LV mass was more prominent in women. After adjustments for BMI, we observed a reversal of the relationship between VF and LV mass in general, which were not observed after adjustment for weight.
Finally, in models adjusted for all covariates (except weight or BMI) excluding imputed data for visceral fat, we found an association between VF and LV mass (β=0.363 for every 100 cm2/m SQ, P=0.0004) and LV mass-to-volume ratio (β=0.0163 for every 100 cm2/m SQ, P<0.0001). In adjusted models excluding imputed data for subcutaneous fat, we found an association between SQ and LV mass (β=0.407 for every 100 cm2/m SQ, P<0.0001) and LV mass-to-volume ratio (β=0.00873 for every 100 cm2/m SQ, P=0.0002).
DISCUSSION
In this multi-racial, multi-ethnic, community-based cross-sectional study nested within MESA, we demonstrate that visceral adiposity is associated with indices of LV structure and function. Individuals with higher visceral fat or subcutaneous fat area had greater LV mass and volumes, and greater visceral fat burden (but not subcutaneous fat) was associated with poorer LV ejection fraction. Importantly, visceral fat was associated with LV mass-to-volume ratio (an index of concentric LV remodeling), incremental to clinical risk factors, but not LV mass. Moreover, the association between visceral fat and concentric LV remodeling was not modified by age, sex, race, or obesity status, suggesting a strong, consistent relationship between VF with concentric LV remodeling. Given the importance of both LV hypertrophy and geometry in the pathogenesis of HF19, these findings implicate visceral adiposity as an important, independent fat depot contributing to pathologic LV remodeling.
Prior work in both MESA19 and the Framingham Heart Study5 has demonstrated an association between BMI and LV mass, concentricity, and incident HF independent of hypertension, diabetes, age, race, and sex. These changes appear to be modifiable with weight loss20–22, suggesting the plasticity of body composition effects on the heart. More recent work has suggested the importance of visceral adiposity and consequent systemic inflammation in cardiovascular risk6, giving rise to a new paradigm that metabolic health may explain the heterogeneity in cardiovascular risk seen in the obese. Indeed, in both epidemiologic studies23 and small animal models of obesity, the presence and extent of visceral adiposity is linked to a unique profile of pro-inflammatory cytokine release, promoting cardiac and vascular dysfunction6,24,25, LV hypertrophy26, arterial stiffness27, abnormal LV strain28, and ultimately decreased cardiorespiratory fitness.29 Although anthropometric markers of visceral fat (e.g., waist circumference and waist-to-hip ratio30) have been associated with abnormal cardiac structure, few large studies have embarked on a direct measurement of each central adiposity compartment (subcutaneous and visceral) and its relationship to the heart.
In the largest study of adiposity distribution and cardiac remodeling to date, Neeland and colleagues determined cross-sectional associations of adipose tissue distribution with cardiac structure and function (both by MRI) in 2,710 participants of the Dallas Heart Study7. In this important report, both SQ and VF were associated with LV mass and LV mass-to-volume ratio, with a stronger association of VF (relative to SQ) with these outcomes (β=0.41, β=0.13 for LV mass and β=0.31 and β=0.06 for concentricity, VF vs. SQ respectively). Associations were significant for both SQ and VF fat after full multivariable adjustment for age, sex, Black race, hypertension, diabetes, and lean body mass (among other covariates). On the other hand, Neeland and colleagues found “lower body fat” (gluteal adiposity) was associated with decreased LV mass and more eccentric LV geometry; they propose LV concentricity as a function of the balance between visceral/subcutaneous adiposity and lower body fat—a favorable “metabolic sink” for inflammatory free fatty acids pathogenic in LV hypertrophy and remodeling. Our findings extend these results to a large group of multi-racial, multiethnic individuals across the United States, demonstrating that visceral (but not subcutaneous) adiposity is associated with concentric LV remodeling independent of clinical risk.
Relative to the Dallas Heart Study, our population was older and multi-racial with a lower frequency of obesity (only 21% obese, BMI 30+ kg/m2). The LV concentricity observed in MESA was lower than that reported by Neeland et al., suggesting that the relationships observed in the Dallas Heart Study may hold in an even less advanced state of cardiac remodeling. In a related analysis performed in the Strong Heart Study using echocardiography to quantify left ventricular structure and bioimpedance to measure differential adipose depots, de Simone and colleagues31,32 demonstrated that while BMI was not independently associated with indexed LV mass in either sex, adipose mass and waist-to-hip ratio accounted for significant variability of indexed LV mass in women but not in men. Our findings are coherent with those of de Simone and colleagues in that we observed an association between VF area and LV mass index primarily in women (Table 3).
One important issue is the BMI- or weight-independent association of fat distributions with LV structure. We observed a similar association between VF and LV mass index in weight-adjusted and unadjusted models. However, when adjustments were performed by BMI, we observed VF did not significantly predict LV mass in women (and was negatively associated in men), but SQ fat was associated with lower LV mass index in both sexes. While we recognize that the similar indexing of LV mass index in our study (indexed to 1/height2.7) and BMI (also proportional to 1/height2) may well account for some of the instability in regression coefficients in these regressions, these findings may also suggest that after accounting for “generalized” adiposity with BMI, distinct adipose tissue compartments (e.g., SQ vs. VF) may impact ventricular remodeling differently. Indeed, as noted in previous work, the neurohormonal profile of VF is more deleterious than SQ fat (a potentially more “inert” store of adipose tissue mass). These results call for additional work utilizing direct measures of total lean body mass and CT determined visceral and subcutaneous fat to dissect these associations. In addition, studies with serial measures of SQ and VF depots with weight change are necessary to evaluate these suppositions prospectively.
The results of this study must be viewed in light of its design. One important aspect of this study to mention is the frequency of imputed data. A total 226 individuals had imputed data for subcutaneous adiposity; only 3 patients had imputed visceral fat measures. As expected, the 226 individuals who had imputed subcutaneous data had a higher overall BMI, weight, and waist circumference, likely due to size or field of view constraints in the CT scanner (Supplemental Table 3). There was a higher visceral fat content in those individuals with imputation, which is consistent with their higher generalized adiposity (higher BMI). Therefore, systematic exclusion of these individuals (with higher visceral adiposity) would have introduced severe biases in our findings. Nevertheless, we still observed similar associations after exclusion of these subjects with either visceral or subcutaneous imputation in respective models (Supplemental Table 4). These findings highlight the importance of field of view and size constraints to imaging individuals on the obesity spectrum. Adiposity CT assessment was not performed at the same MESA examination as CMR, thereby introducing the possibility that adiposity and cardiac structure had changed during this interval. We attempted to minimize the influence of these changes by utilizing covariate data from Exam 1 (at the time of the MRI), such that interval changes in medical therapy or cardiovascular events during the course of MESA follow-up would not influence our results. In addition, we did not acquire DEXA measurements of lean body mass, as in previous work7, though our work utilized BMI, a clinically used measure of adiposity. Future work is necessary within MESA and other longitudinal cohorts with careful cardiometabolic phenotyping to determine associations between cardiovascular outcomes, LV remodeling, and adipose tissue distribution.
CONCLUSION
In a large, multi-ethnic, multi-racial sample of American adults free of cardiovascular disease, we demonstrated that visceral adiposity is associated with concentric LV remodeling. The absence of effect modification by age, race, sex, or obesity status underscores the importance of determination of fat distribution in our highest risk patients. Future work using imaging assessments of adipose tissue distribution to determine and modify risk by targeting visceral adiposity (and its attendant inflammation) for HF prevention is warranted.
Supplementary Material
Highlights.
We investigated the relationship between fat distribution and left ventricular remodeling
We studied 1,151 participants from a multi-ethnic community based cohort
We found that visceral adiposity was associated with concentric remodeling
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
FUNDING: This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. Dr. Shah is supported by an American Heart Association Fellow-to-Faculty Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No authors have any relevant disclosures.
References
- 1.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Garza CA, Pellikka PA, Somers VK, Sarr MG, Collazo-Clavell ML, Korenfeld Y, Lopez-Jimenez F. Structural and functional changes in left and right ventricles after major weight loss following bariatric surgery for morbid obesity. Am J Cardiol. 2010;105:550–6. doi: 10.1016/j.amjcard.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 3.Wong C, Marwick TH. Alterations in myocardial characteristics associated with obesity: detection, mechanisms, and implications. Trends Cardiovasc Med. 2007;17:1–5. doi: 10.1016/j.tcm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, Kwong RY, Bluemke DA, Lima JA, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1698–706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velagaleti RS, Gona P, Chuang ML, Salton CJ, Fox CS, Blease SJ, Yeon SB, Manning WJ, O’Donnell CJ. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:257–63. doi: 10.1161/CIRCIMAGING.109.911438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, Grundy SM, de Lemos JA, Drazner MH. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–7. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multiethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 9.Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (MultiEthnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:140–6. doi: 10.1016/j.jacc.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison MA, Bluemke DA, McClelland R, Cushman M, Criqui MH, Polak JF, Lima JA. Relation of leptin to left ventricular hypertrophy (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112:726–30. doi: 10.1016/j.amjcard.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah RV, Abbasi S, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, Kwong R, Bluemke DA, Lima JA, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.01.053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida AL, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA, Lima JA. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral Adiposity and the Risk of Metabolic Syndrome Across Body Mass Index: The MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett DK, McClelland RL, Bank A, Bluemke DA, Cushman M, Szalai AJ, Jain N, Gomes AS, Heckbert SR, Hundley WG, Lima JA. Biomarkers of inflammation and hemostasis associated with left ventricular mass: The Multiethnic Study of Atherosclerosis (MESA) International journal of molecular epidemiology and genetics. 2011;2:39–400. [PMC free article] [PubMed] [Google Scholar]
- 15.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–92. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 17.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Simone G, Devereux RB, Kimball TR, Mureddu GF, Roman MJ, Contaldo F, Daniels SR. Interaction between body size and cardiac workload: influence on left ventricular mass during body growth and adulthood. Hypertension. 1998;31:1077–82. doi: 10.1161/01.hyp.31.5.1077. [DOI] [PubMed] [Google Scholar]
- 19.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 20.Karason K, Wallentin I, Larsson B, Sjostrom L. Effects of obesity and weight loss on left ventricular mass and relative wall thickness: survey and intervention study. Bmj. 1997;315:912–6. doi: 10.1136/bmj.315.7113.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMahon SW, Wilcken DE, Macdonald GJ. The effect of weight reduction on left ventricular mass. A randomized controlled trial in young, overweight hypertensive patients. N Engl J Med. 1986;314:334–9. doi: 10.1056/NEJM198602063140602. [DOI] [PubMed] [Google Scholar]
- 22.Alpert MA, Lambert CR, Terry BE, Kelly DL, Panayiotou H, Mukerji V, Massey CV, Cohen MV. Effect of weight loss on left ventricular mass in nonhypertensive morbidly obese patients. Am J Cardiol. 1994;73:918–21. doi: 10.1016/0002-9149(94)90829-x. [DOI] [PubMed] [Google Scholar]
- 23.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, Gokce N. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canas JA, Sweeten S, Balagopal PB. Biomarkers for cardiovascular risk in children. Curr Opin Cardiol. 2013;28:103–14. doi: 10.1097/HCO.0b013e32835dd0ce. [DOI] [PubMed] [Google Scholar]
- 25.Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, Tracy R, Jacobs DR., Jr Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–91. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 26.Di Bonito P, Moio N, Scilla C, Cavuto L, Sibilio G, Forziato C, Sanguigno E, Saitta F, Iardino MR, Capaldo B. Preclinical manifestations of organ damage associated with the metabolic syndrome and its factors in outpatient children. Atherosclerosis. 2010;213:611–5. doi: 10.1016/j.atherosclerosis.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 28.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T, Manlhiot C, Jaeggi ET, Bradley TJ, Mertens L. Interaction between myocardial and vascular changes in obese children: a pilot study. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2012;25:401–410. doi: 10.1016/j.echo.2011.12.018. e1. [DOI] [PubMed] [Google Scholar]
- 29.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94:3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammar KA, Redfield MM, Mahoney DW, Johnson M, Jacobsen SJ, Rodeheffer RJ. Central obesity: association with left ventricular dysfunction and mortality in the community. Am Heart J. 2008;156:975–81. doi: 10.1016/j.ahj.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Simone G, Pasanisi F, Ferrara AL, Roman MJ, Lee ET, Contaldo F, Howard BV, Devereux RB. Relative fat-free mass deficiency and left ventricular adaptation to obesity: the Strong Heart Study. International journal of cardiology. 2013;168:729–33. doi: 10.1016/j.ijcard.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Simone G, Izzo R, De Luca N, Gerdts E. Left ventricular geometry in obesity: Is it what we expect? Nutrition, metabolism, and cardiovascular diseases: NMCD. 2013;23:905–12. doi: 10.1016/j.numecd.2013.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


