Abstract
Over the last 15 years, many studies have established an association of sleep apnea with inflammation and metabolic aberrations. However, no controlled studies have examined potential gender effects in this association. We recruited 120 middle-aged, predominantly non-obese mild-to-moderate sleep apneics and controls (62 males, 58 females). All participants underwent a clinical history, physical examination, and 1-night 8-hour polysomnography recording and provided a single fasting blood sample for assessment of interleukin-6 (IL-6), tumor necrosis factor receptor 1 (TNFR1), C-reactive protein (CRP), leptin, and adiponectin levels. Among non-sleep apneics, females had higher levels of TNFR1 (p = 0.01), CRP (p = 0.005), leptin (p < 0.001), and adiponectin (p < 0.001) compared to males, independent of age and body mass index. When analyzed separately by gender, sleep apneic men had elevated TNFR1 (p = 0.04), CRP (p = 0.06) and IL-6 (p = 0.11) relative to control men; in sleep apneic females, only CRP was elevated (p = 0.04). Furthermore, CRP was associated with apnea severity dose-response manner (p-linear = 0.04 in both genders) and was an independently associated with comorbid hypertension in apnea (p-linear = 0.005 for women; p-linear = 0.09 for men). In conclusion, although women have naturally higher levels of inflammatory and metabolic markers than men, sleep apneic men appear to have a more severe inflammatory profile compared to women. Our findings suggest that these markers should be analyzed and interpreted separately in men and women, and that a single measure of plasma CRP appears to be a clinically-useful marker of apnea severity and comorbid cardiovascular morbidity.
Keywords: sleep, apnea, inflammation, CRP, hypertension, gender
Introduction
Obstructive sleep apnea is a prevalent sleep disorder characterized by obstruction of the upper airway during sleep despite breathing effort, as well as an associated reduction in blood oxygen saturation. Seventeen to 24% of men and 5 to 9% of women in general population samples demonstrate an apnea-hypopnea index (AHI) of five or more events per hour of sleep, while 4% of men and 2% of women meet the current clinical and polysomnographic criteria for the diagnosis of sleep apnea warranting immediate therapeutic intervention (Bixler et al., 1998; Bixler et al., 2001; Young et al., 1993). Sleep apnea has been associated with the elevation of several pro-inflammatory cytokines, independent of obesity (Kritikou et al., 2014; Sahlman et al., 2010; Shamsuzzaman et al., 2000; Vgontzas et al., 1997; Vgontzas et al., 2000; Vgontzas et al., 2008) Among a number of inflammatory pathways, sleep apnea has been especially linked to activation of tumor necrosis factor (TNF)-α receptors, which stimulates secretion of interleukin (IL)-6, in turn triggering the synthesis of C-reactive protein (CRP) in the liver (Akira et al., 1990; Hirano et al., 1990). It is hypothesized that this inflammatory cascade, in addition to insulin resistance, mediates the link between sleep apnea and cardiometabolic complications (Vgontzas et al., 2005a).
Although sleep apnea was traditionally recognized in middle-aged, obese men, its occurrence in women as well as lean individuals is increasingly recognized. The prevalence of sleep apnea increases markedly after menopause, with post-menopausal women having a doubled rate of apnea compared to pre-menopausal women, even after accounting for neck circumference and body mass index (Bixler et al., 2001; Dancey et al., 2001). Also, while the maximum prevalence for obstructive sleep apnea peaks between ages 50–59 in men (Bixler et al., 1998), this peak is not seen in females until after age 65 (Bixler et al., 2001). Furthermore, men tend to have a higher AHI than women when matched for body mass index (Kapsimalis and Kryger, 2002), are more likely to exhibit the classical symptoms of excessive daytime sleepiness and snoring (Phillips et al., 2008), and the severity of their daytime sleepiness is more likely to be related to lack of regular exercise, depression, and minimum oxygen desaturation than AHI per se (Basta et al., 2008).
In healthy individuals, it has been demonstrated that peripheral levels of CRP (Cartier et al., 2009; Khera et al., 2005; Lakoski et al., 2006; McConnell et al., 2002, Wener et al., 2000), leptin (Couillard et al., 1997, Hellström et al., 2000; Hickey et al., 1996; Kennedy et al., 1997; Ostlund et al., 1996), and adiponectin (Böttner et al., 2004; Saltevo et al., 2009; Song et al., 2014) are naturally higher in women compared to men, independent of age, race, and body mass index. Despite this, although most studies of sleep apneics statistically control for gender in their analyses, very few have expressly investigated possible gender differences in inflammation.
A number of studies in large general population samples, both prospective and cross-sectional, have demonstrated a clear association between sleep apnea and hypertension (Bixler et al., 2000; Nieto et al., 2000; Peppard et al., 2000). Subsequent studies in large community-based cohorts have further demonstrated that men with sleep apnea have a significantly increased risk for hypertension (Hedner et al., 2006; Mohsenin et al., 2009) and stroke (Redline et al., 2010) compared to women. Additionally, a recent large study reported that men with serum CRP > 3.0 mg/L have significantly higher odds of both cardiovascular and all-cause mortality compared to women with the same CRP cut-off (Doran et al., 2013). However, no study to date has explored the link between gender and apnea-associated cardiovascular outcomes, such as hypertension, in the context of the inflammatory response.
The aim of our study was to examine potential gender differences in the association of sleep apnea with inflammation and metabolic markers, as well as the synergistic effect of apnea and hypertension on these outcomes in a sample of middle-aged, predominantly non-obese sleep apneics and controls. We hypothesized that the association of these markers with sleep apnea would be stronger in men than in women.
Methods
Participants
The study sample consisted of 120 middle-aged, predominantly non-obese mild-to-moderate sleep apneics and controls (62 males, 58 females; mean age = 54.67 ± 0.54 years). Participants were recruited through advertisements in the local community and screened according to research protocols by the Sleep Research and Treatment Center at Penn State Milton S. Hershey Medical Center (Hershey, PA, USA). All women in the study were post- menopausal (self-reported absence of menses for at least 12 months or total hysterectomy). Exclusion criteria included a history of diabetes mellitus, use of antiglycemic agents and/or fasting blood glucose levels > 126 mg/dL, ongoing infections, rheumatoid arthritis, insomnia, narcolepsy, and use of certain medications (psychotropics, steroids, sympathomimetics, sympatholytics, or hormone therapy for females). The study was approved by the Institutional Review Board at Penn State University College of Medicine and all participants provided written informed consent.
Sleep laboratory protocol
During their visit in the laboratory, all participants underwent a clinical history and physical examination, during which height and weight were recorded and body mass index (BMI) calculated (in kg/m2). Blood pressure was also assessed, with hypertension defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, or as the use of antihypertensive medication.
Sleep laboratory recordings were conducted in a sound-attenuated, light- and temperature-controlled room with a comfortable, bedroom-like atmosphere. Each subject was monitored continuously for one night for 8 h (22:30–23:00 until 6:30–7:00) using 16-channel polygraph recordings of EEG, electrooculogram (EOG) and electromyogram (EMG). Polysomnography (PSG), respiration (via thermocouple and thoracic strain gauges), and oximeter data were collected using Grass-Telefactor Gamma Sleep Recording software (Middleton, WI, USA). Visual sleep stage scoring was conducted by a registered polysomnography technologist blind to participant characteristics based on Rechtschaffen and Kales criteria (1968). Apnea-hypopnea index (AHI; number of apneas and hypopneas summed per hour) was also ascertained. An apnea was defined as cessation of airflow for ≥ 10 seconds and an out-of-phase strain gauge movement; a hypopnea was defined as a 50% airflow reduction and associated decrease in SaO2 of at least 4%. In stratifying our study sample, “the presence of sleep apnea” was defined as an AHI ≥ 5 events/hour of sleep.
Blood sampling
A single fasting blood draw (via venipuncture) was performed at 7:00 immediately after the end of the PSG recording. Blood was collected in EDTA-containing tubes and refrigerated until centrifugation (within 3 h). Blood was stored at −80 °C until assay.
Assays
Plasma interleukin-6 (IL-6), tumor necrosis factor receptor 1 (TNFR1), and high-sensitivity C-reactive protein (hsCRP) were measured via enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). The intra- and inter-assay coefficients of variation (CVs) were 7.4% and 7.8% for IL-6, 4.4% and 6.1% for TNFR1, and 5.5% and 11.6% for hsCRP. The lower detection limits were 0.094 pg/mL, 0.043 pg/mL, and 0.124 ng/mL for IL-6, TNFR1, and hsCRP, respectively. Leptin and adiponectin were assessed by commercially-available radioimmunoassays with CVs below 10%.
Statistical analysis
Two-tailed independent-samples t-tests were used to compare demographic and PSG variables between males and females (between-gender), or between controls and sleep apneics (within-gender). To examine differences in inflammatory and metabolic characteristics between more than two groups (e.g. increasing apnea severity), analyses of covariance (ANCOVA) with Bonferroni correction were conducted. Polynomial linear analysis was also performed to examine the association between increasing apnea severity (i.e. AHI < 5, 5 ≤ AHI < 15, and AHI ≥ 15) and inflammatory markers. Finally, to assess the association of sleep apnea associated with the comorbid cardiovascular outcome (i.e., hypertension) and inflammation, we examined differences between three groups: controls without hypertension, sleep apneics without hypertension, and sleep apneics with hypertension. Effect size was also assessed by calculating Cohen’s d statistic. Statistical significance was determined using the criterion p < 0.05. All analyses were adjusted for the confounders age and BMI. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM Corp., Armonk, NY).
Results
Demographic and PSG parameters of sleep apneic and control males and females are presented in Table 1. Within gender, the control and sleep apneic groups did not differ in age (all p > 0.05), but sleep apneics tended to have higher BMI (27.79 kg/m2 vs. 26.50 kg/m2 respectively, p = 0.08 for males; 31.09 kg/m2 vs. 28.37 kg/m2, p = 0.03 for females) and waist circumference (100.54 vs. 96.03 cm respectively, p = 0.03 for males; 99.10 vs. 92.33 cm respectively, p = 0.04 for females) than controls A larger neck circumference was also observed in males with sleep apnea compared to controls (39.87 cm vs. 38.22 cm, p = 0.04), but not in females (p = 0.60). Furthermore, systolic blood pressure was significantly elevated in females with sleep apnea (134.52 mmHg vs. 120.24 mmHg, p = 0.002), but this difference was not observed for diastolic blood pressure, nor any blood pressure measures in men (all p > 0.15). In this sample, controls and sleep apneics did not differ significantly in any sleep efficiency or architecture parameters (Table 1).
Table 1.
Demographic and sleep characteristics of study sample, stratified by gender.
| Male | Female | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (AHI < 5) (n=15) | Sleep Apnea (AHI ≥ 5) (n=47) | p | Control (AHI < 5) (n=23) | Sleep Apnea (AHI ≥ 5) (n=35) | p | |
| Age, years | 52.70 (1.52) | 53.46 (0.86) | 0.67 | 55.12 (1.19) | 56.86 (0.97) | 0.26 |
| BMI, kg/m2 | 26.50 (0.63) | 27.79 (0.35) | 0.08 | 28.37 (0.94) | 31.09 (0.76) | 0.03* |
| Waist circumference (cm) | 96.03 (1.78) | 100.54 (1.02) | 0.03* | 92.33 (2.47) | 99.10 (2.00) | 0.04* |
| Hip circumference (cm) | 101.67 (1.66) | 105.04 (0.95) | 0.08 | 108.26 (2.33) | 112.50 (1.90) | 0.17 |
| Neck circumference (cm) | 38.32 (0.65) | 39.87 (0.37) | 0.04* | 34.45 (1.12) | 35.21 (0.91) | 0.60 |
| AHI | 2.26 (4.37) | 22.94 (2.47) | <0.001*** | 1.76 (2.88) | 22.25 (2.33) | <0.001*** |
| Systolic blood pressure (mmHg) | 127.53 (4.15) | 132.33 (2.37) | 0.32 | 120.24 (3.42) | 134.52 (2.73) | 0.002** |
| Diastolic blood pressure (mmHg) | 77.27 (2.23) | 79.11 (1.27) | 0.48 | 73.52 (1.97) | 77.24 (1.57) | 0.15 |
| Total sleep time, min. | 368.77 (13.19) | 351.54 (7.11) | 0.80 | 368.24 (12.22) | 363.87 (8.87) | 0.68 |
| Sleep efficiency, % | 76.65 (2.79) | 73.05 (1.48) | 0.77 | 76.57 (2.52) | 75.51 (1.82) | 0.61 |
| Sleep onset latency, min. | 21.23 (3.75) | 20.00 (2.17) | 0.64 | 25.47 (4.58) | 30.90 (3.77) | 0.93 |
| Wake after sleep onset, min. | 95.90 (12.20) | 114.14 (7.40) | 0.83 | 90.43 (9.48) | 92.00 (7.35) | 0.60 |
| Total wake time, min. | 112.50 (13.52) | 129.73 (7.16) | 0.77 | 112.43 (12.17) | 117.97 (8.78) | 0.62 |
| Stage 1, % | 27.02 (2.90) | 30.75 (1.91) | 0.51 | 22.43 (1.87) | 24.97 (1.95) | 0.80 |
| Stage 2, % | 58.26 (2.63) | 52.61 (1.78) | 0.47 | 53.18 (1.75) | 50.78 (1.54) | 0.39 |
| Slow-wave sleep, % | 4.06 (1.46) | 4.81 (0.83) | 0.65 | 12.75 (1.86) | 12.88 (1.27) | 0.44 |
| Rapid eye movement sleep, % | 10.67 (1.83) | 11.83 (0.92) | 0.73 | 11.63 (1.45) | 11.37 (1.10) | 0.83 |
Data are means (standard error of the mean). AHI = apnea hypopnea index. BMI = body mass index.
Inflammatory and metabolic characteristics of controls and sleep apneics stratified by gender are presented in Table 2. Control females had significantly higher levels of TNFR1 (1.23 ng/mL vs. 0.97 ng/mL respectively; p = 0.01), CRP (1.83 ng/mL vs. 0.90 ng/mL, p=0.005), leptin (28.52 ng/mL vs. 6.83 ng/mL; p < 0.001) and adiponectin (13.38 ng/mL vs. 5.70 ng/mL; p < 0.001) compared to control males. These gender differences were seen in sleep apnea as well; female apneics had significantly higher levels of CRP (2.81 ng/mL vs. 1.56 ng/mL, p = 0.002), leptin (32.96 ng/mL vs. 5.54 ng/mL, p < 0.001), adiponectin (11.53 ng/mL vs. 5.13 ng/mL, p < 0.001), and a trend toward higher TNFR1 (1.26 ng/mL vs. 1.13 ng/mL, p = 0.08) compared to apneic males.
Table 2.
Inflammatory and metabolic markers in participants with and without sleep apnea, stratified by gender.
| Male | Female | |||
|---|---|---|---|---|
|
| ||||
| Control (AHI < 5) (n=15) | Sleep Apnea (AHI ≥ 5) (n=47) | Control (AHI < 5) (n=23) | Sleep Apnea (AHI ≥ 5) (n=35) | |
| IL-6 (pg/mL) | 0.82 (0.16) | 1.14 (0.09) | 1.57 (0.28) | 1.38 (0.22) |
| TNFR1 (ng/mL) | 0.97 (0.06) | 1.13 (0.04)# | 1.23 (0.06)* | 1.26 (0.05) |
| CRP (ng/mL) | 0.90 (0.30) | 1.56 (0.16) | 1.83 (0.36)** | 2.81 (0.28)** # |
| Leptin (ng/mL) | 6.83 (0.91) | 5.54 (0.50) | 28.52 (3.31)*** | 32.96 (2.65)*** |
| Adiponectin (ng/mL) | 5.70 (0.76) | 5.13 (0.42) | 13.38 (1.49)*** | 11.53 (1.19)*** |
Data are means (standard error of the mean). Inflammatory and metabolic variables are adjusted for age and BMI.
p < 0.05,
p < 0.01,
p < 0.001 for males vs. females
p < 0.05 for controls vs. sleep apnea
Because of these basal gender differences, the remaining analyses were conducted separately in males and females (within-gender). Sleep apneic males had a significantly higher concentration of plasma TNFR1 than controls (1.13 ng/mL vs. 0.97 ng/mL; p = 0.04) and a trend towards higher CRP (1.56 ng/mL vs. 0.90 ng/mL; p = 0.06) and IL-6 (1.14 pg/mL vs. 0.82 pg/mL; p = 0.11). In females, only CRP was elevated in sleep apneics compared to controls (2.81 ng/mL vs. 1.83 ng/mL; p = 0.04) (Table 2).
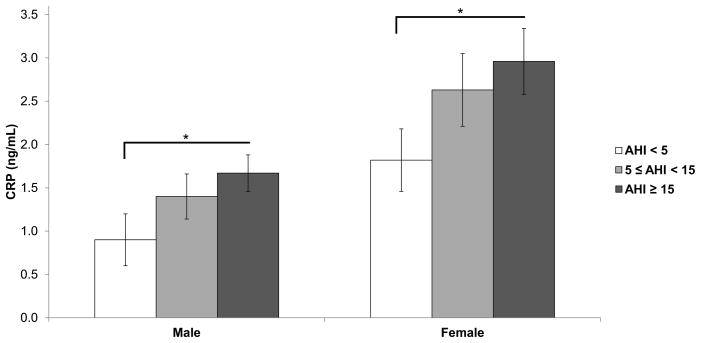
We then examined the association of increasing apnea severity (AHI < 5, 5 ≤ AHI < 15, and AHI ≥ 15) with inflammatory markers in men and women. A dose-response was observed with CRP in both genders (p-linear = 0.04; Figure 1). Men with AHI ≥ 15 (n = 27) had significantly higher CRP than control men (n = 15; 1.67 ng/mL vs. 0.90 ng/mL respectively; p = 0.04; Cohen’s d = 0.69). Similarly, women with AHI ≥ 15 (n = 20) had significantly higher CRP than control women (n = 23; 2.96 ng/mL vs. 1.82 ng/mL; p = 0.04; Cohen’s d = 0.67). No other differences were observed between the three sleep apnea severity groups in terms of plasma IL-6, TNFR1, leptin, or adiponectin in males or females.
Figure 1. C-reactive protein levels are elevated with increasing sleep apnea severity.
Mean C-reactive protein concentrations in males and females, stratified by sleep apnea severity. Data adjusted for age and BMI. Error bars represent standard error of the mean.
AHI = apnea/hypopnea index. CRP = C-reactive protein. * p < 0.05
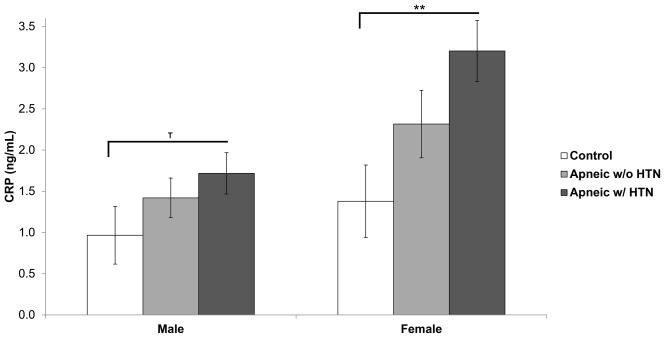
Finally, we assessed the association of sleep apnea with a comorbid cardiovascular outcome (i.e. hypertension) by examining differences in inflammatory markers between three groups (non-sleep apneic/non-hypertensive controls, sleep apneics without hypertension, and sleep apneics with hypertension). Importantly, AHI did not differ between sleep apneics with or without hypertension in both men (AHI = 20.87 vs. 24.92 respectively; p = 0.43) and women (AHI = 20.80 vs. 24.44; p = 0.49). A dose-response was observed with CRP in both genders, although this association was stronger in women (p-linear = 0.005) than in men (p = 0.09) (Figure 2). Sleep apneic women with hypertension (n = 20) had the highest CRP levels (3.20 ng/mL vs. 1.38 ng/mL in controls; p = 0.005; Cohen’s d = 1.09). In men, sleep apneics with hypertension (n = 23) also had the highest CRP levels (1.72 ng/mL vs. 0.97 ng/mL in controls; p = 0.09; Cohen’s d = 0.64). No other associations were observed between hypertension and any of the inflammatory or metabolic variables in either men or women.
Figure 2. Association of C-reactive protein with sleep apnea and comorbid hypertension in males and females.
Mean C-reactive protein concentrations of controls, sleep apneics without hypertension, and sleep apneics with hypertension. Data adjusted for age and BMI. Error bars represent standard error of the mean.
HTN = hypertension. ** p < 0.01, T < 0.1
Discussion
This is the first large study to examine gender differences in the profiles of inflammatory and metabolic markers in middle-aged, predominantly non-obese sleep apneics and controls. Although healthy women tend to have significantly higher values of TNFR1, CRP, leptin, and adiponectin than men, men with apnea appear to have a more severe inflammatory response, independent of age and body mass index. CRP is consistently elevated in both genders, and appears to be a significant predictor of apnea severity as well as comorbid cardiovascular problems in both men and women. Our findings suggest that the inflammatory and metabolic abnormalities in sleep apnea should be examined separately in men and women, and CRP may be a clinically useful and reliable marker of sleep apnea severity and comorbid cardiovascular problems in both genders.
Previous work in the cardiovascular field supports our finding that peripheral CRP (Cartier et al., 2009; Khera et al., 2005; Lakoski et al., 2006; McConnell et al., 2002; Wener et al., 2000;), leptin (Couillard et al., 1997; Hellström et al., 2000; Hickey et al., 1996; Kennedy et al., 1997; Ostlund et al., 1996), and adiponectin levels (Böttner et al., 2004; Saltevo et al., 2009; Song et al., 2014) are naturally higher in women compared to men, independent of age, race, and body mass index. Our study expands these findings in demonstrating that other inflammatory markers, such as TNF, are also higher in women than in men. Although gender differences in inflammatory and metabolic markers among healthy individuals have mostly been observed within pre-menopausal women and age-matched males, our findings indicate that these differences persist at least several years beyond menopause. All women in our study were post-menopausal, and the mean age of our control females was five years older than the widely cited median age (50 years) of menopause onset in American women (Kato et al., 1998; National Institute on Aging, 2008). These higher levels of inflammatory and metabolic markers in women compared to men are likely related to the fact that women, independent of BMI, tend to have more body fat than men (Ley et al., 1992). Furthermore, previous work has demonstrated that in males and females matched for body fat content, women tend to have higher plasma concentrations of CRP (Khera et al., 2005) and leptin (Couillard et al., 1997; Kennedy et al., 1997) per unit fat mass. Interestingly, a recent study by Doran et al. (2013) in a large, representative U.S. population sample demonstrated that men with serum CRP > 3.0 mg/L have significantly higher odds of both cardiovascular and all-cause mortality, whereas neither outcome was observed in women with the same CRP cut-off. For these reasons, inflammatory and metabolic markers should be examined separately in men and women, and different cut-off points should be applied for the two genders.
In the present study, we found that men with sleep apnea showed significantly elevated TNFR1 levels and a trend toward higher CRP and IL-6 levels as compared to control men. In females, however, only CRP was significantly elevated in sleep apneics (p < 0.04). Together, these data suggest that, in middle-aged individuals, the inflammatory and metabolic aberrations in males with mild-to-moderate sleep apnea appear to be more severe than that of females. This can be explained by previous work showing that even within a relatively non-obese sample of males, visceral fat is the strongest predictor of sleep apnea, whereas total fat in females is most strongly associated with sleep apnea (Vgontzas et al., 2000; Kritikou et al., 2013). Due to the nature of visceral fat and its propensity to accumulate more in males (Lemieux et al., 1993), this may explain why men are more susceptible to a poorer metabolic and pro-inflammatory profile. Compared to subcutaneous fat, visceral fat has reduced sensitivity to insulin (Saltiel and Kahn, 2001) and is more vascularized, innervated, and contains a larger number of inflammatory and immune cells, making it more vulnerable to metabolic risks (Ibrahim, 2010). Although we did not examine specific fat types in the present study, it is likely that the sleep apneic men exhibited a greater proportion of this more harmful fat type than their female counterparts, accounting for the different inflammatory profiles between men and women. Interestingly, we did not observe significantly elevated leptin or reduced adiponectin in sleep apneics compared to controls in either males or females. Previous work by our group has demonstrated that obese and non-obese male sleep apneics have significantly higher plasma leptin levels (Kritikou et al., 2013; Kritikou et al., 2014; Vgontzas et al., 2000). However, in the present study, we examined only mild-to-moderate sleep apneics in contrast to more severe apnea, and only a single morning sample was used compared to twice a day or 24-hour blood sampling in prior studies.
When we examined the association between inflammatory and metabolic markers with sleep apnea severity, only CRP emerged with a significant dose-response trend in both men and women (Figure 1). In order to further assess the relationship of inflammatory markers and sleep apnea with clinically-meaningful adverse outcomes, we examined the association of the various inflammatory and metabolic markers with hypertension, controlling for severity of apnea. Once again, a significant dose-response association was observed most strongly with CRP relative to other inflammatory and metabolic markers. In females, independent of age and BMI, sleep apneics with hypertension had significantly higher levels of plasma CRP compared to controls and sleep apneics without hypertension. The same linear trend was observed in men, but was not as strong, due most likely to the higher variability of the values in the male control group (Figure 2). Finally, the stronger and consistent association of CRP with severity of sleep apnea and associated cardiovascular morbidity, compared to the other markers, may reflect that CRP levels do not exhibit a circadian influence (Meier-Ewert et al., 2001).
No studies to date have explored the synergistic effect of apnea and hypertension on inflammatory and metabolic outcomes in men and women. Three of the larger, population-based studies in the field, in fact, reported no effect of gender on sleep apnea-associated hypertension prevalence in middle-aged participants (Bixler et al., 2000; Nieto et al., 2000; Peppard et al., 2000). In contrast, a population-based study has shown that men in the highest AHI tertile have a 3.7-fold risk of hypertension, whereas women in the highest tertile only have a 1.6-fold increased risk (Hedner et al., 2006). Furthermore, men within the highest BMI quartile have been shown to have a 2-fold higher risk for hypertension than BMI-matched women, independent of sleep apnea (Mohsenin et al., 2009). Another large prospective study reported a significantly increased stroke risk in the highest AHI quartile in men, but not women (Redline et al., 2010). Interestingly, there is also evidence of a gender difference in hypertension risk in response to intermittent hypoxia in an animal model; Hinojosa-Laborde and Mifflin (2005) demonstrated that female rats subjected to intermittent hypoxia were at a lower risk for developing elevated blood pressure than males. Interestingly, a recent study reported an association between blunted nocturnal blood pressure decline (“non-dipping”) and sleep apnea with elevated serum CRP, but this association was not observed in sleep apneics with a normal dipping pattern; however, this study did not analyze the genders separately (Ishikawa et al., 2008). Our results, showing a more severe inflammatory profile in men than women, suggest that future work should examine carefully the differential association of sleep apnea with cardiovascular complications between the two genders.
There are several limitations to our study that may impact its generalizability. First, the study was conducted in middle aged, mild-to-moderate sleep apneics who were relatively non-obese. Additional studies should explore the profiles of inflammatory and metabolic markers in individuals comprising a larger age range, within a larger sample of obese individuals, and in those with more severe sleep apnea. Second, we only performed a blood draw in the morning after awakening (7:00). We have previously demonstrated that plasma IL-6 has a pulsatile 24-hour secretion (Vgontzas et al., 2005b), and TNFα (Uthgenannt et al., 1995), leptin (Langendonk et al., 1998), and adiponectin (Gavrila et al., 2003) also display circadian rhythmicity. Because this phenomenon is not detected in CRP (Meier-Ewert et al., 2001), combined with our consistent observation of elevated CRP with increasing apnea severity and cardiovascular problems, this suggests that CRP may be the best preclinical marker to assess the degree of inflammation in sleep apnea when only a single blood draw is possible. Finally, within some of our groups (e.g. control males), we have a small sample size, resulting in larger variability. While, overall, we examined a large sample of 120 participants, breaking them into discrete categories limited our statistical power. Despite this, Cohen’s d calculations suggest that the effect size of both apnea severity (Figure 1) and hypertension (Figure 2) on plasma CRP levels are moderate to large in both genders (Cohen’s d range = 0.64 – 1.09). Future studies should aim to recruit equal numbers of sleep apneics and controls.
In summary, our findings suggest that the inflammatory and metabolic abnormalities in sleep apnea should be analyzed and interpreted separately in men and women. Not only are there contrasting profiles between genders in controls vs. sleep apneics, but the impact of inflammation on cardiovascular risk also differs between men and women. Although healthy women have higher levels of plasma inflammatory and metabolic markers than their male counterparts, the inflammatory response to obstructive sleep apnea does not appear to be as severe in females as it is in males. While the women of our sample are post-menopausal; it is likely that the protective effects of female hormones may still be in effect within these early stages. This is supported by work in a large population cohort suggesting that apnea in women peaks at age 65, roughly 15 years after the onset of menopause (Bixler et al., 2001). Finally, CRP, compared to other inflammatory markers, may be a clinically useful and reliable marker of apnea severity and comorbid cardiovascular problems in both men and women because its secretion is not influenced by circadian factors.
Highlights.
Plasma inflammatory and metabolic markers are higher in non-apneic women than men.
Sleep apneic men, but not women, have significantly elevated TNFR1, CRP, and IL-6.
In both genders, CRP is associated with apnea severity in a dose-response manner.
CRP is independently associated with apnea-associated hypertension in both genders.
Acknowledgments
The authors thank the sleep technicians and staff of the General Clinical Research Center at the Pennsylvania State University College of Medicine for their support with this project. This research was supported by NIH grant R01 HL 64415.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–7. [PubMed] [Google Scholar]
- Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler EO, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A, Kales A. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–95. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, Blüher M, Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–61. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- Cartier A, Côte M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, Després J. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89:1307–14. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- Couillard C, Mauriège P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia. 1997;40:1178–84. doi: 10.1007/s001250050804. [DOI] [PubMed] [Google Scholar]
- Dancey DR, Hanly PJ, Soong C, Lee B, Hoffstein V. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120:151–5. doi: 10.1378/chest.120.1.151. [DOI] [PubMed] [Google Scholar]
- Doran B, Zhu W, Muennig P. Gender differences in cardiovascular mortality by C- reactive protein level in the United States. Am Heart J. 2013;166:45–51. doi: 10.1016/j.ahj.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Linblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case- control study. Eur Respir J. 2006;27:564–70. doi: 10.1183/09031936.06.00042105. [DOI] [PubMed] [Google Scholar]
- Hellström L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. 2000;247:457–62. doi: 10.1046/j.1365-2796.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, Houmard JA, Marks RH, Caro JF. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59:1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46:1016–21. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa I, Hoshide S, Eguchi K, Ishikawa S, Pickering TG, Shimada K, Kario K. Increased low-grad inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndrome. J Hypertens. 2008;26:1181–7. doi: 10.1097/HJH.0b013e3282fd9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part I: Clinical features. Sleep. 2002;25:409–16. [PubMed] [Google Scholar]
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Endocrinol Metab. 1998;51:1271–6. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatansin W, Wians FH, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–9. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Kritikou I, Basta M, Tappouni R, Pejovic S, Fernandez-Mendoza J, Nazir R, Shaffer M, Liao D, Bixler EO, Chrousos GP, Vgontzas AN. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J. 2013;41:601–9. doi: 10.1183/09031936.00183411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritikou I, Basta M, Vgontzas AN, Pejovic S, Liao D, Tsaoussoglou M, Bixler EO, Stefanakis Z, Chrousos GP. Sleep apnoea, sleepiness, inflammation and insulin resistance in middle-aged males and females. Eur Respir J. 2014;43:145–55. doi: 10.1183/09031936.00126712. [DOI] [PubMed] [Google Scholar]
- Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’Agostino RB, Herrington DM. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–8. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Langendonk JG, Pijl H, Toornvliet AC, Burggraaf J, Frölich M, Schoemaker RC, Doornbos J, Cohen AF, Meinders AE. Circadian rhythm of plasma leptin levels in upper and lower body obese women: influence of body fat distribution and weight loss. J Clin Endocrinol Metab. 1998;83:1706–12. doi: 10.1210/jcem.83.5.4717. [DOI] [PubMed] [Google Scholar]
- Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després J. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–4. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- McConnell JP, Branum EL, Ballman KV, Lagerstedt SA, Katzmann JA, Jaffe AS. Gender differences in C-reactive protein concentrations-confirmation with two sensitive methods. Clin Chem Lab Med. 2002;40:56–9. doi: 10.1515/CCLM.2002.011. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47:426–30. [PubMed] [Google Scholar]
- Mohsenin V, Yaggi HK, Shah N, Dziura J. The effect of gender on the prevalence of hypertension in obstructive sleep apnea. Sleep Med. 2009;10:759–62. doi: 10.1016/j.sleep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging. Menopause: Time for a Change. U.S. Department of Health and Human Services, Publication #08-6143; 2008. p. 4. [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Ostlund RE, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–13. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Phillips B, Collop NA, Drake C, Consens F, Vgontzas AN, Weaver TE. Sleep disorders and medical conditions in women. J Womens Health. 2008;17:1191–9. doi: 10.1089/jwh.2007.0561. [DOI] [PubMed] [Google Scholar]
- Rechtshaffen A, Kales A. Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, D.C: 1968. NIH Publication 204. [DOI] [PubMed] [Google Scholar]
- Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlman J, Miettinen K, Peuhkurinen K, Seppä J, Peltonen M, Herder C, Punnonen K, Vanninen E, Gylling H, Partinen M, Uusitupa M, Tuomilehto H Kuopio Sleep Aponoea Group. J Sleep Res. 2010;19:341–8. doi: 10.1111/j.1365-2869.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- Saltevo J, Kautiainen H, Vanhala M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend Med. 2009;6:463–70. doi: 10.1016/j.genm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- Song HJ, Oh S, Quan S, Ryu OH, Jeong JY, Hong KS, Kim DH. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatrics. 2014:14. doi: 10.1186/1471-2318-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005a;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005b;12:131–40. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Collins B, Basta M, Pejovic S, Chrousos GP. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–95. doi: 10.1111/j.1365-2362.2008.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2002;27:2351–9. [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]