Abstract
Cardiomyocytes, the individual contractile units of heart muscle, are long-lived and robust. Given the longevity of these cells, it can be easy to overlook their dynamic intracellular environment that contain rapid protein movements and frequent protein turnover. Critical gene transcription and protein translation occur continuously, as well as trafficking and localization of proteins to specific functional zones of cell membrane. As heart failure becomes an increasingly important clinical entity, growing numbers of investigative teams are examining the cell biology of healthy and diseased cardiomyocytes. In this review, we introduce the major architectural structures and types of protein movements within cardiac cells, and then review recent studies that explore the regulation of such movements. We conclude by introducing current translational directions of the basic studies with a focus on novel areas of therapeutic development.
Introduction
Clinical therapies for failing cardiac muscle are dominated by striving to limit the consequences of chronic external stress stimuli. For instance, blocking the renin-angiotensin-axis and adrenergic stimuli has resulted in therapeutic mainstays such as beta-blockers, ace-inhibitors, and aldosterone blockers. At the same time, thanks to pioneers in cardiac biology, there has been a wealth of information accumulated on basic biology of cardiac muscle cells that is now coinciding with a surge in the incidence and morbidity of patients with advanced heart failure. Future medical therapies for heart failure will leverage new understandings about how myocytes pathologically remodel during stress. These additional therapies will not so much block external stimuli but rather they will likely intervene on intracellular pathways to help restore normal myocyte function.
Each individual cardiomyocyte is a highly organized, robust, and dynamic system, not unlike a bustling walled-in city with portals to the outside world. The nuclei of cardiomyocytes continuously transcribe genes that are then translated into proteins. These proteins are shuttled throughout the cytoplasm to specific organelles and functional subdomains of surrounding cell membrane. Just as a historic city wall would have guard towers, drawbridges, and defensive structures that each consist of task-specific elements, the cardiomyocyte cell membrane has particular regions such as those designated for cell-cell communication (intercalated discs) and initiating contractile signaling (T-tubules). Proteins and channels that function at intercalated disc regions are different from proteins and channels that function at T-tubules. A question for all cells, and in particular cardiomyocytes, is how in normal homeostatic conditions particular regions of membrane are populated with the appropriate proteins.
When a walled city is attacked, it immediately marshals resources for immediate defense, even if to cause negative long term consequence. During a long defense, however, cities can alter their production facilities and internal transportation networks to accommodate the changing needs. Like attacked cities, diseased cardiomyocytes subjected to less energy and more stress undergo pathologic remodeling of membrane proteins and even membrane structures. As we learn about normal movements of cardiac intracellular proteins, and disease related changes of these movements, interventions can be designed to target positive intracellular remodeling. The long-term objective of cell biology laboratories is to treat heart failure by not just blocking external adrenergic and renin-angiotensin signals, but improving how cardiomyocytes respond to such stressors.
In this review article, we briefly introduce the basics of cardiomyocyte organization and then focus on trafficking, which is the ordered movement of cardiomyocyte proteins. Special emphasis is placed on ion channels and, in particular Connexin 43 (Cx43) gap junctions and L-type calcium channels (LTCCs). Cx43 channels are important for cell-cell electrical communication and LTCCs are important for contraction. Cx43 channels need to localize to intercalated discs at the longitudinal ends of cardiomyocytes and LTCCs need to localize to T-tubules that are at the transverse borders of cardiomyocytes. An analysis of Cx43 and L-type calcium channels therefore covers different regions and many fundamental concepts of cardiomyocyte organization. The final section of this review is a brief summary of state-of-the-art therapies that are being developed based on what has been learned to date of cardiomyocyte cell biology.
Cardiomyocyte Organization
Human hearts consist of several billion cells that need to contract in synchrony for an effective heartbeat. To achieve synchronous contraction, before each heartbeat action potentials must rapidly spread throughout the myocardium. As an action potential initiates in each cardiomyocyte, intracellular calcium release occurs, resulting in cellular contraction. Thus rapid spatial transmission of action potentials is need for coordinated myocyte activity. Both rapid propagation of action potentials and intracellular calcium release require, for each cell, a sophisticated cascade of ion channel activation that is dependent on time as well as on the localization of particular ion channels at particular subdomains of the plasma membrane. The proper formation and correct delivery of these channels to their membrane subdomain is referred to as protein trafficking.
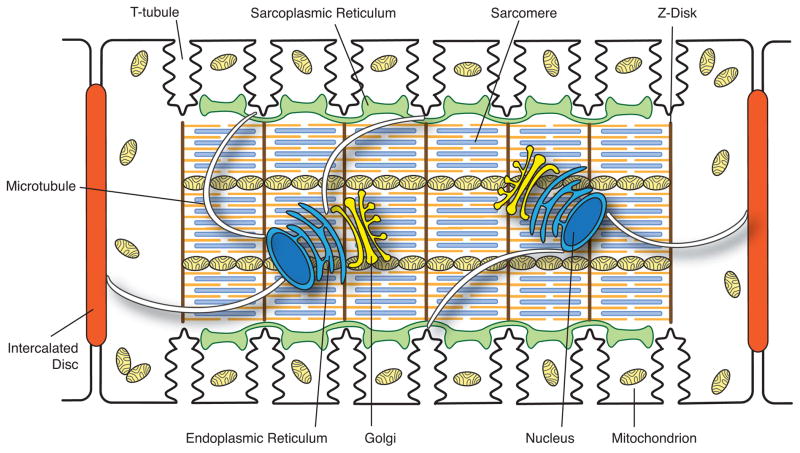
A central component in the sorting and delivery of membrane proteins to their subcellular destination is the Golgi apparatus (Figure 1). The Golgi complex is usually found adjacent to the lateral side of the two nuclei in mammalian ventricular cardiomyocytes. Co-localized with each Golgi is the centrosome at which microtubules are nucleated and extend throughout the cell [1]. Sorting of proteins mainly takes place at the trans Golgi network (TGN) [2]. Cargo proteins are sorted into post-Golgi carriers, which are docked onto molecular motors and delivered to cell periphery along microtubules [3]. In the context of trafficking, one can consider the Golgi to be the “loading dock” and microtubules the “highways” along which packets of channels are delivered to the plasma membrane.
Figure 1. Schematic illustration of the internal structures of an adult ventricular cardiomyocyte.
Intercalated discs located at the longitudinal sides of each ventricular cardiomyocyte mediate the cell-to-cell propagation of action potentials. T-tubules, which are rich in voltage-gated L-type calcium channels, are positioned closely to the sarcoplasmic reticulum, the internal calcium store. Sarcomeres form myofibrils, which are responsible for cardiomyocyte contraction upon intracellular calcium release. The Golgi apparatus and microtubules serve as the “loading dock” and “highways”, respectively, to deliver ion channels to specific subdomains on the plasma membrane. Mitochondria provide the energy needed for the contraction of cardiomyocytes.
Cardiac muscle contracts more than 3 billion times during the average lifetime of human beings. Cardiomyocytes have a large number of mitochondria whose primary role is to produce ATP through oxidative phosphorylation to support the high-energy demand of the beating heart. Mitochondria occupy ~30% of the volume of a cardiomyocyte and are positioned beneath the sarcolemma, between myofibrils, and near the nucleus [4] (Figure 1). These different subgroups of mitochondria, identified by spatial location in the myocyte, appear to have different morphologies and properties [4, 5]. In addition to providing energy, mitochondria play an important role in regulating cell death, propagating Ca2+ signaling, and mediating cardiac protection to ischemia/reperfusion and other stresses [6, 7].
Each heartbeat, the normal sequence of events leading to contraction is 1) initiation of an action potential at the sinoatrial node, 2) myocyte-to-myocyte spread of action potentials through the atria, across the AV node, and through the His-Purkinje systems, 3) myocyte-to-myocyte spread of action potentials in ventricular myocardium in apex to base order, 4) individual cellular action potentials allowing calcium-entry through calcium channels at T-tubules, triggering larger calcium release from the corresponding internal sarcoplasmic reticulum, 5) calcium release causing myofibril contraction. The myocyte-to-myocyte spread of action potentials occur across intercalated disc regions at the longitudinal ends of each ventricular myocyte. Calcium-induced calcium release is triggered at cardiac T-tubules. Thus, in general, the intercalated disc regions are responsible for myocyte-to-myocyte electrical communication and the T-tubule regions are responsible for triggering and regulating the strength of each contraction (Figure 1).
Heart contraction is accomplished, at the subcellular level, by contraction of individual subcellular contractile units named sarcomeres. A sarcomere occupies the region between two Z-lines (Figure 1), and consists of thick filaments of myosin and thin filaments of actin that are anchored to Z-discs. Myofibrils are connected to the sarcolemma along the longitudinal axis at the adherens junctions (fascia adherens) of the intercalated discs and across the lateral axis at the costameres connecting Z-discs to T-tubules [8, 9]. The outer cell membrane is linked to cortical cytoskeletal desmosomes and actin microfilaments (F-actin), which provide mechanical support to maintain cell shape and specialized sarcolemmal subdomains such as intercalated discs, caveolae and T-tubules [10, 11].
Trafficking in Healthy and Diseased Hearts
Introduction
The internal organization of cardiomyocytes is complex, with thousands of individual proteins each contributing to an overall equilibrium. For inherited (genetic) disease, a mutation in a single protein disrupts the equilibrium, which can manifest later in life as a generalized myopathy. Multiple instances exist for mutations negatively affecting trafficking of ion channels. Anderson et al., for instance, have found that of 28 clinically relevant mutations in Kv11.1, most reduce hERG current not by altering Kv11.1 expression or kinetics, but by negatively affecting Kv11.1 trafficking to the membrane [12]. Mohler et al. have identified that mutations in Nav1.5 which limit Nav1.5’s binding to ankyrin-G cause aberrant Nav1.5 trafficking and result in human Brugada syndrome [13]. Patel and colleagues have found that mutations in desmosomal desmoplakin affects Cx43 trafficking to intercalated discs, contributing to arrhythmogenesis in Arrhythmogenic Cardiomyopathy [14, 15]. For acquired muscle disease, such as ischemic heart disease, cellular myopathy and syndromic heart failure also can take weeks to months or years to develop. In acquired disease, trafficking is also highly relevant, as discussed below.
We have divided the section on trafficking into three main types which are forward (antegrade) trafficking to the membrane, channel behavior once in the membrane, and reverse (retrograde) trafficking from the membrane. Each subsection is divided by healthy physiology and what is known of the diseased response.
Forward Trafficking
Healthy Physiology
A remarkable aspect of cardiac ion channel biology is that individual ion channels have half-lives on the order of hours. Connexin43 (Cx43) gap junction proteins, which form the gap junctions that occur at intercalated discs of ventricular cardiomyocytes and are responsible for cell-cell communication, have a half-life of 1–3 hours [16, 17]. Supplemental Video 1, from reference [18] illustrates the rapid time course of Cx43 trafficking. Interested readers are referred to all supplemental videos in [19, 20], for high resolution visualization of Cx43 trafficking. Potassium and calcium channels, and sodium-calcium exchanger have half-lives that are reported in the 2–8 hour range [21–24]. It is not yet clear whether channel half-lives on the plasma membrane are longer or shorter than overall protein half-life, and it is already being found that different pools of channels exist within the cardiomyocyte [25]. Also, channels such as the sodium channel may have a slightly extended half-life of about 35 hours [26], however these times likely vary based on cell type and specific membrane partners. Half-life is likely not just a function of intrinsic protein susceptibility to degradation molecules, but is heavily influenced by location of the channel in a particular membrane subdomain or region in the cell. Proteins that are spatially protected from being in contact with degradation processes will experience slower turnover. However despite small variances in turnover, the overall implication for a turnover on the scale of several to tens of hours is that, in the course of any day, the bulk of the ion channels in each cardiomyocyte are regenerated and have to be newly positioned. Thus, the trafficking of channels is a dynamic ongoing occurrence, and has a critical impact on channel function.
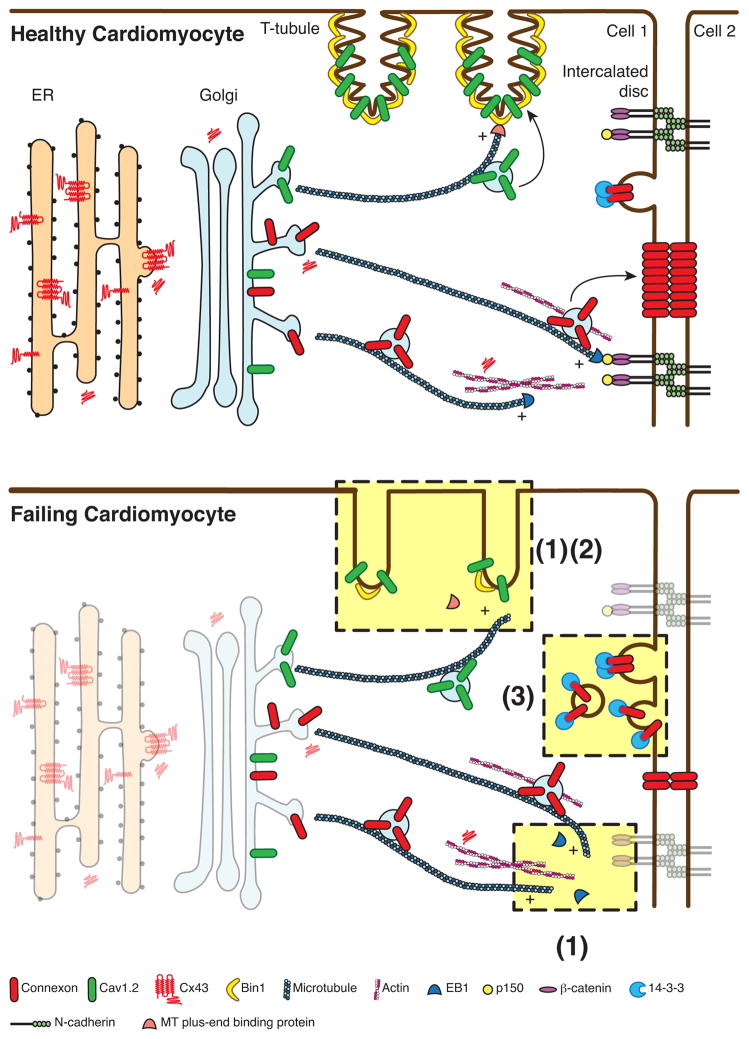
The short life span of ion channels suggests there needs to be efficiency in their life-cycle and movements which are, in order: formation, delivery to the correct subdomain on plasma membrane, behavior once in membrane, and internalization back into the cell. In 2007, we found that Cx43 hemichannels could be trafficked by microtubules directly to adherens junctions at the intercalated disc [19]. Specificity of delivery consisted of the 1) hemichannels, and 2) plus-end-binding proteins (EB1) at the end of microtubules which attach to 3) membrane anchors (adherens junction complex), allowing delivery of the hemichannels from the microtubule to adherens junction containing membrane [19]. This finding, and subsequent studies [18, 27, 28] have led to our Targeted Delivery model of channel delivery. Targeted Delivery is the understanding that channels, once formed and exiting the Golgi, can be rapidly directed across the cytoplasm to their respective specific membrane subdomains. The highways for transport are microtubules whose negative ends originate at Golgi oriented microtubule-organizing centers and whose positive ends are growing outward and can be captured at the plasma membrane by membrane anchor proteins and complexes. Specificity of delivery is a combination of the individual channel, the plus-end-tracking proteins at the positive ends of microtubules which guide microtubule growth and capture, and the membrane bound anchor complex which captures the microtubule, completing the highway for channel delivery (Figure 2).
Figure 2. Schematic representation of vesicular trafficking in healthy and failing hearts.
Ion channel proteins are synthesized by ribosomes. In the case of connexin 43 (Cx43) multiple isoforms are produced as a result of alternative translation. Nascent transmembrane proteins are translocated to the membrane of the rough endoplasmic reticulum, transported through the Golgi apparatus and to the trans-Golgi network (TGN). Channel proteins are sorted into vesicular carriers and docked onto microtubules at the TGN, and subsequently delivered to their subcellular destinations in cooperation with actin “way stations” along the route. Microtubule plus-end binding proteins interact with anchor proteins of specific membrane subdomains, allowing targeted delivery of cargo proteins. The interaction between EB1, a microtubule plus-end binding protein, and the adherens junction complex ensures the targeted delivery of Cx43 hemichannels to the intercalated discs, whereas the association of microtubules and bridging integrator 1 (BIN1), a membrane scaffolding protein, warrants the delivery of Cav1.2, a voltage-gated L-type calcium channel protein, to T-tubules. Channel proteins on the plasma membrane undergo internalization for degradation.
In failing cardiomyocytes, expression of ion channels on the cell surface and the morphology of T-tubules are altered. As highlighted in light yellow, possible mechanisms underlying these changes include (1) dissociation of microtubule plus-end binding proteins from microtubules; (2) reduced expression of membrane scaffolding proteins; and (3) increased internalization. Under oxidative stress EB1 dissociates from the tip of microtubules, impairing the attachment of microtubules to the adherens junction and the delivery of Cx43 to the intercalated discs. During acute cardiac ischemia 14-3-3 mediated internalization of Cx43 is increased, diminishing the amount of Cx43 channels on the plasma membrane. In failing hearts the expression of BIN1 is significantly reduced, resulting in detachment of microtubules from sarcolemma and reduction in Cav1.2 delivery to the T-tubules. The dense membrane folds in T-tubules are also lost as a result of low expression of BIN1.
The Targeted Delivery model has been supported by multiple subsequent reports [28–32]. At the same time, important details of this delivery paradigm continue to be elucidated. For instance Patel et al. recently reported that the desmosome associated linker protein desmoplakin binds to EB1, and is involved in targeted delivery of Cx43 to intercalated discs [14]. Also, non-sarcomeric actin appears to be involved in channel delivery. Actin is necessary for Cx43 forward delivery [33, 34]. It remains to be determined how actin interacts with channels and the microtubule apparatus. At any given point in time, the majority of intracellular Cx43 channels are not moving rapidly on microtubules, but rather are stationary and associated with non-sarcomeric actin [33].
Involvement of non-sarcomeric actin in channel trafficking raises several interesting questions, the most fundamental of course being why is actin involved. If vesicles containing channels can depart the Golgi and ride a microtubule highway straight to its proper subdomain, is there a need for actin filaments that appear to slow down vesicle transport? We believe actin has at least two important roles in forward delivery. The first is to help contribute specificity to delivery. Vesicles transported along microtubules on kinesin motors move rapidly, at a rate of about 1 micron per second [19]. Thus delivery to most locations at a cell membrane can occur within a minute. Association with important accessory proteins and post-translational modification of channels, both of which can affect delivery destination, probably also happen en route between the Golgi and membrane. Hopping off the microtubule highway on an actin “way station”, which is analogous to a highway rest stop with convenience stores, could allow the channel and vesicle containing it to pick up accessory proteins and allow for needed post-translational modification at Z-disc, subcortical locations, or other important regions in the cytoplasm. The way stations could also serve as a channel reservoir. For instance the cardiac Z-disc and costamere are actin rich structures. If L-type calcium channels are needed acutely in T-tubule membrane, there could be a signal for rapid delivery to occur from the Z-disk and costamere reservoir to the nearby T-tubule membrane. Finally, an actin way station could allow the channel containing vesicles to use multiple microtubule highways in their delivery path. The Golgi exit microtubule could be destined for an actin way station, allowing for a different membrane domain specific microtubule to finish the delivery to the right membrane subdomain.
The second potential role for actin in microtubule based forward delivery pertains to the microtubules themselves. In non-myocyte systems, actin can help stabilize and guide microtubules [35, 36]. Actin could be the blueprint along and across which microtubule highways are patterned. In this respect, actin involvement could be an upstream to microtubules in determining location of channel delivery.
With regard to accessory proteins, Cx43 hemichannels are notable for, despite extensive examination, not being associated with their own unique beta-subunits that assist in their trafficking. Recently, it was identified that these hemichannels do have important subunits that are N-terminal truncated isoforms of the same full length protein [37]. The smaller proteins are created through the phenomenon of alternative translation [37]. In order to understand alternative translation, it should be recognized that traditional translation of mRNA begins with the first coding triplet, which is always an AUG (Methionine). Most transcribed genes (mRNA strands) have other AUG sites downstream of the first one. The Cx43 gene GJAI has six in frame AUG triplets beyond the first one. Alternative translation occurs when ribosomal translation initiates not at the first triplet, but at a downstream triplet. By initiating translation at downstream sites, alternative translation creates N-terminal truncated proteins that lack the respective non-translated upstream (N-terminal) portions of the proteins.
In the case of Cx43, GJA1 mRNA produces the expected full-length 43 kDa protein as well as proteins that are approximately 32 kDa, 29 kDa, 26 kDa, 20 kDa, 11 kDa and 7 kDa in size [37]. Cx43 is the first mammalian ion channel shown to be subjected to alternative translation, which has already been confirmed by two separate reports [38, 39]. It has also been found that the 20 kDa isoform (GJA1-20K) assists with Cx43 forward trafficking. We currently understand the smaller Cx43 isoforms to effectively be the ‘beta-subunits’ that promote and thus autoregulate full length Cx43 trafficking to the plasma membrane [37].
A critical aspect of Targeted Delivery is for microtubules to be captured by the appropriate membrane anchor, allowing channel delivery directly to regions of membrane that happen to contain the particular anchor. For Cx43 delivery to the intercalated disc, EB1-tipped microtubules bind to N-Cadherin associated beta-catenin and also p150(Glued) [19]. Desmoplakin may also be involved in capturing the EB1-tiped microtubule for Cx43 delivery [14], although the transmembrane domain still appears to be N-Cadherin rather than desmosomal desmoglein [15]. For Cav1.2 channel delivery to T-tubules, a membrane anchor is the lipophilic membrane scaffolding protein BIN1 [28] (Figure 2). We discuss below how microtubule anchor complexes may be affected in disease. Also, other anchor complexes likely exist. For instance, ankyrin-G binds to and regulates Nav1.5 localization [40, 41] and ankyrin-B regulates the membrane targeting and subsequent regulation of the Na+/Ca2+ exchanger, Na+/K+ ATPase, and IP3 receptor [42]. In addition, fibroblast growth factor homologous factors are potent regulators of Nav1.5 and Cav1.2 localization to the sarcolemmal membrane [43, 44].
Pathophysiology
Recent studies have explored forward trafficking of cardiac ion channels in different disease states. A difficulty with such studies is that the short life spans of individual channels (hours) are dwarfed by the chronicity (months, years and decades) of most failing hearts. A further complication is that dynamic channel behavior is best studied with in vitro cellular studies, well removed from intact animals and humans. A simple knockout mouse model establishes the importance of a channel or trafficking partner, but does not reveal the signaling interplay that occurs with other proteins during protein movements.
Trafficking related channel studies need to occur with proteins intact, yet in environmental conditions that mimic those of failing heart. One important such condition is oxidative stress that occurs in ischemia-reperfusion injury as well as ischemic and non-ischemic heart failure. When isolated cardiomyocytes are subjected to oxidative stress, Cx43 gap junction delivery to intercalated discs is impaired due to disruption of the forward trafficking machinery [18]. Specifically, the oxidative stress causes the microtubule plus-end proteins to disassociate from the tips of microtubules, impairing microtubule attachment to adherens junction structures and subsequent delivery of Cx43 hemichannels to plasma membrane [18] (Figure 2). Such studies provide evidence that forward trafficking and likely other ion channels are impaired in acquired heart failure. At present we do not know how oxidative stress causes EB1 displacement and disassembly of the forward trafficking apparatus. We have preliminary investigations on the role of actin in maintaining EB1 based microtubule integrity, and the response of these cytoskeletal fibers to stress conditions. This remains an active area of investigation.
In failing heart, forward trafficking of Cav1.2 channels to T-tubules is also impaired [27]. Biochemical assessment of Cav1.2 channel content in failing heart indicates no difference in total channel content compared to healthy muscle, yet channel localization to T-tubules is impaired [27]. A difference between impaired forward delivery of Cx43 channels and Cav1.2 channels in failing hearts exists with their respective anchor proteins. Even in diseased heart muscle, the adherens junction structures for Cx43 delivery to intercalated discs remain intact [18], whereas transcription of BIN1 protein, needed to anchor microtubules for Cav1.2 delivery to T-tubules, is reduced by half [27] (Figure 2). In animal models, successful treatment of heart failure and recovery of function correlates with recovery of muscle BIN1 levels [45, 46].
In the preceding section, we introduced recent reports that the actin cytoskeleton could be an important regulator of forward trafficking that is potentially upstream of microtubule based trafficking [33, 47]. In acute ischemic injury, Cx43 dissociates from actin and forward trafficking is impaired [33]. Despite these data, it remains to be determined whether actin filaments can serve as Cx43 sorting centers and microtubule organizers, or whether actin can actively bring Cx43 and other channels to the membrane surface. In another recent study, it was identified the actin motor myosin 5B assists with forward trafficking of Kv1.5 and Cx43 [48], suggesting actin could do more than sorting proteins and organizing microtubules in the delivery of channels to the membrane surface.
Membrane Organization
Healthy Physiology
Once channels are inserted into the plasma membrane, it is possible that they undergo lateral diffusion to other regions of membrane. However multiplexing with scaffolding proteins and cytoskeleton elements will limit subsequent diffusion, and the overall extent and rate of lateral diffusion are unclear, with reports that vary significantly. For Cx43 hemichannels, it was previously understood that the channels are randomly placed in plasma membrane to subsequently diffuse laterally to plaque regions [49, 50]. This model of lateral diffusion does not preclude, but has generally been supplanted by the model of Targeted Delivery [19] by which channels are delivered to the membrane subregions in which they are to function. It is likely that most channels still move laterally within the plasma membrane, but in confined local zones rather than widely traversing regions of the cell surface. In 2011 Rhett et al. identified and named the “perinexus” as a region adjoining gap junction plaques in which hemichannels can collect and diffuse with movement into the plaque regulated by ZO-1 [51]. Such local movements of Cx43 and other channels are highly likely and areas such as the perinexus could be transitional zones in which post translational modification and protein clustering occur.
A recent development in cardiac membrane biology is the finding that T-tubule invaginations are not simply straight and planar, but instead contain complex folds tight and narrow enough to limit the free flow of extracellular ions [9]. High resolution imaging of intercalated disc regions reveal that intercalated disc associated membrane is also non-linear, with finger-like and low frequency undulations [52, 53]. We speculate that membrane morphology such as location of critical curvature and inflection points may compartmentalize trafficking domains as well as support structures such as the perinexus, thereby affecting channel activity.
Pathophysiology
The mechanisms of pathologic remodeling of gap junctions during disease states remain poorly understood. One possibility is that membrane signals which permit directed targeting of connexons to intercalated discs may themselves be relocated to lateral membrane during disease, thus attracting hemichannel delivery [32]. Another possibility is that connexons can become untethered from plaques during disease, and diffuse within the membrane to lateral regions [54]. The high rate of connexin43 turnover, rapid rate of connexon delivery to plasma membrane, and lack of direct visualization of connexon movements once in the plasma membrane, together severely limit the ability for us to understand the mechanisms by which remodeled and poorly localized connexons arrive at their new destination. It will probably be necessary to label connexons in live cells, and record their real-time lateral movements to determine their fate once in the plasma membrane of health or stressed myocardial cells.
We discussed above that L-type calcium channels have diminished forward trafficking in failing heart [27]. There already exists significant experimental evidence that T-tubule membrane remodels in failing heart [55–57]. The mechanisms of T-tubule remodeling in failing hearts is an area of active research. Microtubule trafficking of junctophilin-2 has been implicated in impaired T-tubule maintenance during heart failure [58]. However the role of junctophilin in T-tubule maintenance during heart failure has been questioned due to a lack of decrease with heart failure as T-tubule structures are diminished [45, 46] or return with recovery of T-tubule structures in treated heart failure [45]. In these same studies, BIN1 decreased with decrease in T-tubule density in heart failure [45, 46], and BIN1 recovered along with T-tubule density when heart failure was treated [45]. When isolated mature ventricular cells are placed in culture, they lose their T-tubules within 1–3 days. Interesting, pharmacologic stabilization of actin filaments can extend the life-span of T-tubules in cultured myocytes [59, 60]. These data support the concept that actin is important to T-tubule maintenance, but do not explain the mechanism. It is worth noting that loss of BIN1 to levels that occur in end-stage heart failure result in impaired actin association with T-tubules, and in BIN1-deificient mice T-tubule remodeling similar to that of failing hearts [9].
Internalization
Healthy Physiology
Given the short half live of channel proteins, just as with forward trafficking, internalization from the plasma membrane represents an important regulatory step in determining the level of gap junction coupling. Posttranslational modification of plasma membrane proteins leads to internalization, and the c-terminus of Cx43 has many residues known to be subjected to ubiquitin, acetylation, and phosphorylation based modifications, of which phosphorylation has been most intensively studied.
The importance of phosphorylation of connexin is highlighted by recent findings that casein kinase-dependent phosphorylation alters gap junction remodeling and decreases arrhythmic susceptibility [61]. The specifics of the kinases acting on other residues, and the trafficking consequence of specific residues being phosphorylated, are being actively investigated. It is useful to consider that, with 22 serines, 5 tyrosines, and 4 threonines, many residues on the c-terminus of Cx43 are potentially subject to phosphorylation. Phosphorylation based modification of the c-terminus is complex, with upstream sites that, once modified, cause structural changes to the c-terminus, eliciting gain or loss of binding partners. To make matters even more complex, Cx43 exists as a hexamer on the plasma membrane, and it is currently not known how phosphorylation differs between individual connexin protomers of the same connexon. Ubiquitination of Cx43 occur following activation of protein kinase C, leading to channel internalization [62].
Endocytosis of Cx43 can occur either through internalization of uncoupled hemichannels or entire gap junctions, which entails engulfment of the opposing cells plasma membrane, and generation of double-membrane intracellular structures termed annular gap junctions. Both the lysosome and the proteasome have been implicated in degradation of Cx43 and interestingly, autophagy is now known to be involved in degradation of annular gap junctions in failing hearts [63]. Studies have shown recycling of gap junctions to occur during cell cycle progression in cell lines [64], but whether gap junctions are recycled in cardiomyocytes remains a controversial issue. It is exciting to consider the possibility that posttranslational modifications of Cx43 may be acting as checkpoints within the same connexin molecule, or connexon hemichannel, requiring a specific series of events to permit ubiquitination and internalization of a hemichannel, or annular gap junction.
Pathophysiology
Our experience to date, mostly with Cx43 protein, is that cytoskeletal proteins regulate forward trafficking whereas post-translational modification such as phosphorylation changes affect retrograde trafficking or internalization. Historically, altered phosphorylation of Cx43 has been the hallmark of the pathological remodeling of gap junctions during disease [65–67]. Rather than individual phosphorylation events of different residues on the same channel being independent of each other, it is likely that internalization results from a sophisticated cascade of posttranslational modifications. The Cx43 C-terminus contains a phosphorylation-dependent 14-3-3 binding motif at Serine 373 (within 10 amino acids of the end of the protein). 14-3-3 proteins are known to regulate protein transport and have been implicated in facilitating de novo Cx43 transport from ER to Golgi apparatus [68, 69]. Phosphorylation of Ser373 and subsequent 14-3-3 binding provide a gateway to a signaling cascade of downstream post-translational modification(s), leading to gap junction ubiquitination, internalization and degradation during acute cardiac ischemia [17]. (Figure 2).
Cx43 has many binding partners within the cell, and the majority of these protein–protein interactions occur via the Cx43 C-terminus [70]. In close proximity to the Cx43 14-3-3 binding motif is a PDZ domain encompassing the distal end of the C-terminus. It is through this PDZ domain that Cx43 interacts with ZO-1 [71], and this interaction has been demonstrated to regulate Cx43 gap junction plaque size and assembly [51, 72]. Disruption of Cx43/ZO-1 complexing has been reported to increase gap junction plaque size in cultured cells [73, 74]. Phosphorylation of Cx43 Serine373 can disrupt interaction with ZO-1 [75], and indeed it would be sterically unlikely for both 14-3-3 and ZO-1 to bind the same Cx43 protomer simultaneously. However, increased Cx43/ZO-1 interaction has also been associated with gap junction remodeling, highlighting the complex nature of these dynamic posttranslational and protein complexing events [54, 76].
Pharmacologic Rescue of Trafficking Pathways
In this review, we have focused on recent investigations involving the Cx43 cell-cell communication channels important for electrical transmission and the L-type calcium channel important for regulating the calcium transient and contraction. In this section, we explore efforts to rescue diminished cell-cell coupling and diminished L-type calcium channel (and hence contractile) activity in failing hearts. Table 1 summarizes potential therapeutic interventions related to protein trafficking for heart failure. We emphasize that our discussion of many of the potential treatments is preliminary. We include this discussion to both underscore the knowledge gained so far and help guide development of new therapies.
Table 1.
Potential trafficking related therapeutic interventions for heart failure.
| Targeted pathway | Mechanism of intervention | Potential treatments | Ref | |
|---|---|---|---|---|
| Rescue Cx43 plaque density | Increase forward trafficking | Increase translation of GJA1-20k | mTOR inhibitors, Mnk1/2 inhibitors | [37, 39] |
| Stabilize actin | [33, 34] | |||
| Stabilize microtubule | [18, 19] | |||
| Decrease Cx43 internalization | Increase casein kinase phosphorylation of Cx43 | Mineralocorticoid receptor antagonists | [61, 79] | |
| Block interaction with ZO-1 | αCT1 peptide mimetic, rotigaptide | [73, 80, 81, 83, 86] | ||
| Block 14-3-3 binding | [17, 80] | |||
| Rescue failing Ca2+ transient | Reduce basal intracellular Ca2+ | Rescue leaky RyR2 | JTV-519 (1,4- benzothiazepine), Rycals (Ca2+ release channel stabilizers) | [84] |
| Rescue SERCA2 expression and activity | SERCA2a gene therapy, PP1 inhibitory peptide (I-1), S100A1, Istaroxime | [85] | ||
| Rescue LTCC localization | Rescue forward trafficking | Bin1 gene therapy | [27, 28] | |
| Restore T-tubule membrane folds | Rescue Bin1 expression | Bin1 gene therapy | [9] |
For Cx43 based gap junction communication, plaque density can be improved by either increasing forward trafficking to cell-cell border or limiting internalization. The microtubule disrupting agent colchicine was found, in a substudy of the COPPS trial, to reduce post-operative atrial fibrillation in postpericardiotomy patients [77]. However in the recent COPPS-2 trial with postoperative atrial fibrillation as a primary endpoint, colchicine reduced the incidence of postpericardiotomy syndrome, but did not reduce postoperative atrial fibrillation [78].
Given the recent findings of alternative translation of smaller Cx43 isoforms, which facilitate the forward trafficking of full length Cx43, and the increases in such alternative translation by inhibiting mTOR and Mnk1/2 [37, 39], use of existing mTOR and Mnk1/2 inhibitors could increase plaque density. As mTOR inhibitors are already widely used in the fields of organ transplant and immunosuppression, therapeutic rescue could potentially be available as a novel use of an existing agent. Actin and microtubule stabilization could also be employed to rescue forward trafficking [18, 19, 33, 47], although it is less clear whether current cytoskeleton drugs used in research studies can be safely applied to humans.
Three potential avenues of therapy exist to decrease Cx43 plaque internalization. Enhanced casein kinase phosphorylation by antagonizing aldosterone/mineralocorticoid receptors could limit stress induced Cx43 dephosphorylation and internalization [61, 79], maintaining plaque density. Using Cx43 c-terminal peptides to block Cx43 interaction with ZO-1 is already undergoing clinical evaluation of therapeutic impact [73, 80–83]. With the discovery of endogenous production of truncated C-terminal isoforms and their assistance with forward trafficking [37], improving forward trafficking could be an additional mechanism by which the peptides exert their effect. Finally, 14-3-3 inhibition appears to be a potent potential rescue of Cx43 internalization [17, 80], albeit a safe pharmacologic approach needs to be developed.
Current clinical efforts to rescue altered calcium transient in failing myocytes focus on calcium release (ryanodine) and reuptake (SERCA) channels in the sarcoplasmic reticulum. Leaky ryanodine receptors can be stabilized by drugs [84] and excess cytoplasmic calcium can be re-sequestered by enhancing SERCA2a reuptake by virus based gene therapy that targets new SERCA2a expression and activity to the heart [85].
Newer studies indicate inhibition of the L-type calcium channel trafficking alone can result in an increased diastolic calcium levels and limited systolic calcium transient [27, 28]. Rescuing diminished cardiac BIN1 expression could both rescue L-type calcium channel trafficking [27, 28] and restore normal T-tubule membrane morphology [9]. It is theoretically possible to restore cardiac BIN1 by virus mediated gene therapy in an approach similar to that used for SERCA2 restoration [85].
Conclusions
The individual cardiomyocyte is a highly complex and dynamic cell with internal organization designed to maintain efficient cell-cell communication and excitation-contraction coupling. Cardiomyocyte structures and organization are negatively affected by environmental conditions of stress. Existing therapies such as beta-blockers, ace-inhibitors, and aldosterone inhibitors, focus on blocking these external signals. New therapies for failing heart will be focused on the specific organelles and pathways that regulate cardiomyocyte protein trafficking. Cytoskeleton based protein trafficking pathways are being elucidated and with this knowledge, targets developed for therapeutic intervention.
Supplementary Material
Supplemental Video 1: Rapid population of Cx43 in Plasma Membrane.
Ninety minute movie of a single cell containing fluorescently tagged Cx43. Imaging was performed using total internal reflection fluorescence microscopy (TIRFm) which limits resolution depth to about 50 nm, capturing only what is occurring at the membrane and just underneath. Transcription of tagged Cx43 in this cell was under pharmaceutical control, and the movie initiated when Cx43 had been transcribed/translated and was in the Golgi loading dock but not yet in the membrane. Over the course of 90 minutes, Cx43 is trafficked to and populates the membrane. Applied color is a heat map, in which brighter colors correspond to greater intensity of Cx43 signal.
Acknowledgments
This work was supported by NIH/NHLBI R01 HL094414 and AHA EIA 13EIA4480016 (RMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. Int J Biochem Cell Biol. 2012;44:266–74. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Gu F, Crump CM, Thomas G. Trans-Golgi network sorting. Cell Mol Life Sci. 2001;58:1067–84. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luini A, Mironov AA, Polishchuk EV, Polishchuk RS. Morphogenesis of post-Golgi transport carriers. Histochem Cell Biol. 2008;129:153–61. doi: 10.1007/s00418-007-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang SJ, Kim W. Mitochondrial dynamics in the heart as a novel therapeutic target for cardioprotection. Chonnam Med J. 2013;49:101–7. doi: 10.4068/cmj.2013.49.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beikoghli Kalkhoran S, Hall AR, Whittington H, Davidson SM, Yellon DM, Hausenloy DJ. 4 Characterisation of Mitochondrial Morphology in the Adult Rodent Heart. Heart. 2014;100:A2–A3. [Google Scholar]
- 6.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–43. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 7.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca(2)(+) and reactive oxygen species signaling. J Cell Sci. 2013;126:2965–78. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc Res. 2008;77:667–75. doi: 10.1093/cvr/cvm048. [DOI] [PubMed] [Google Scholar]
- 9.Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, et al. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20:624–32. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noorman M, van der Heyden MA, van Veen TA, Cox MG, Hauer RN, de Bakker JM, et al. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47:23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, et al. Most LQT2 mutations reduce Kv11. 1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–73. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 13.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1. 5 on the surface of cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel DM, Dubash AD, Kreitzer G, Green KJ. Disease mutations in desmoplakin inhibit Cx43 membrane targeting mediated by desmoplakin-EB1 interactions. J Cell Biol. 2014;206:779–97. doi: 10.1083/jcb.201312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw RM. Desmosomal hotspots, microtubule delivery, and cardiac arrhythmogenesis. Dev Cell. 2014;31:139–40. doi: 10.1016/j.devcel.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–35. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 17.Smyth JW, Zhang SS, Sanchez JM, Lamouille S, Vogan JM, Hesketh GG, et al. A 14-3-3 mode-1 binding motif initiates gap junction internalization during acute cardiac ischemia. Traffic. 2014;15:684–99. doi: 10.1111/tra.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, et al. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–79. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–60. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith S, Curran J, Hund TJ, Mohler PJ. Defects in cytoskeletal signaling pathways, arrhythmia, and sudden cardiac death. Front Physiol. 2012;3:122. doi: 10.3389/fphys.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1. 3) ion channel half-life and surface expression. Neuroscience. 2007;144:531–46. doi: 10.1016/j.neuroscience.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, et al. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–44. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 23.Di Biase V, Tuluc P, Campiglio M, Obermair GJ, Heine M, Flucher BE. Surface traffic of dendritic CaV1. 2 calcium channels in hippocampal neurons. J Neurosci. 2011;31:13682–94. doi: 10.1523/JNEUROSCI.2300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Porzig H, Niggli E, Schwaller B. Rapid turnover of the “functional” Na(+)-Ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell Calcium. 2005;37:233–43. doi: 10.1016/j.ceca.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1. 5 in cardiomyocytes. Circ Res. 2011;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 26.Maltsev VA, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1. 5 antisense inhibition. Am J Physiol Heart Circ Physiol. 2008;295:H667–76. doi: 10.1152/ajpheart.00111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong TT, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, et al. BIN1 is reduced and Cav1. 2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9:812–20. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8:808–19. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 30.Levy JR, Holzbaur EL. Special delivery: dynamic targeting via cortical capture of microtubules. Dev Cell. 2007;12:320–2. doi: 10.1016/j.devcel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Hendricks AG, Lazarus JE, Perlson E, Gardner MK, Odde DJ, Goldman YE, et al. Dynein tethers and stabilizes dynamic microtubule plus ends. Curr Biol. 2012;22:632–7. doi: 10.1016/j.cub.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, et al. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm. 2012;9:1133–1140. e6. doi: 10.1016/j.hrthm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, et al. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012;110:978–89. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang SS, Hong S, Kleber AG, Lee LP, Shaw RM. A micropatterning approach for imaging dynamic Cx43 trafficking to cell-cell borders. FEBS Lett. 2014;588:1439–45. doi: 10.1016/j.febslet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell. 2012;23:4032–40. doi: 10.1091/mbc.E12-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J Cell Biol. 2003;161:845–51. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013;5:611–8. doi: 10.1016/j.celrep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ul-Hussain M, Olk S, Schoenebeck B, Wasielewski B, Meier C, Prochnow N, et al. Internal ribosomal entry site (IRES) activity generates endogenous carboxyl-terminal domains of Cx43 and is responsive to hypoxic conditions. J Biol Chem. 2014;289:20979–90. doi: 10.1074/jbc.M113.540187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salat-Canela C, Sese M, Peula C, Ramon y Cajal S, Aasen T. Internal translation of the connexin 43 transcript. Cell Commun Signal. 2014;12:31. doi: 10.1186/1478-811X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180:173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dun W, Lowe JS, Wright P, Hund TJ, Mohler PJ, Boyden PA. Ankyrin-G participates in INa remodeling in myocytes from the border zones of infarcted canine heart. PLoS One. 2013;8:e78087. doi: 10.1371/journal.pone.0078087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunha SR, Mohler PJ. Ankyrin-based cellular pathways for cardiac ion channel and transporter targeting and regulation. Semin Cell Dev Biol. 2011;22:166–70. doi: 10.1016/j.semcdb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hennessey JA, Wei EQ, Pitt GS. Fibroblast growth factor homologous factors modulate cardiac calcium channels. Circ Res. 2013;113:381–8. doi: 10.1161/CIRCRESAHA.113.301215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Hennessey JA, Kirkton RD, Wang C, Graham V, Puranam RS, et al. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circ Res. 2011;109:775–82. doi: 10.1161/CIRCRESAHA.111.247957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, et al. Plasticity of Surface Structures and beta2-Adrenergic Receptor Localization in Failing Ventricular Cardiomyocytes During Recovery from Heart Failure. Circ Heart Fail. 2012 doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, et al. Dependence of Cardiac Transverse Tubules on the BAR Domain Protein Amphiphysin II (BIN-1) Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang SS, Hong S, Kleber AG, Lee LP, Shaw RM. A micropatterning approach for imaging dynamic Cx43 trafficking to cell-cell borders. FEBS Lett. 2014 doi: 10.1016/j.febslet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schumacher-Bass SM, Vesely ED, Zhang L, Ryland KE, McEwen DP, Chan PJ, et al. A Role for Myosin V Motor Proteins in the Selective Delivery of Kv Channel Isoforms to the Membrane Surface of Cardiac Myocytes. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–51. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 51.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oxford EM, Danko CG, Kornreich BG, Maass K, Hemsley SA, Raskolnikov D, et al. Ultrastructural changes in cardiac myocytes from Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. J Vet Cardiol. 2011;13:101–13. doi: 10.1016/j.jvc.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delmar M, Liang FX. Connexin43 and the regulation of intercalated disc function. Heart Rhythm. 2012;9:835–8. doi: 10.1016/j.hrthm.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, et al. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–12. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–46. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 56.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–31. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, et al. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–14. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, et al. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation. 2014;129:1742–50. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38:515–26. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, et al. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J Mol Cell Cardiol. 2012;52:113–24. doi: 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, et al. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–66. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem. 2004;279:50089–96. doi: 10.1074/jbc.M402006200. [DOI] [PubMed] [Google Scholar]
- 63.Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–63. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 2010;11:1471–86. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–62. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 66.Hesketh GG, Van Eyk JE, Tomaselli GF. Mechanisms of gap junction traffic in health and disease. J Cardiovasc Pharmacol. 2009;54:263–72. doi: 10.1097/FJC.0b013e3181ba0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818:1985–92. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majoul IV, Onichtchouk D, Butkevich E, Wenzel D, Chailakhyan LM, Duden R. Limiting transport steps and novel interactions of Connexin-43 along the secretory pathway. Histochem Cell Biol. 2009;132:263–80. doi: 10.1007/s00418-009-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Batra N, Riquelme MA, Burra S, Jiang JX. 14-3-3theta facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J Cell Sci. 2014;127:137–46. doi: 10.1242/jcs.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–45. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–4. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 72.Laing JG, Chou BC, Steinberg TH. ZO-1 alters the plasma membrane localization and function of Cx43 in osteoblastic cells. J Cell Sci. 2005;118:2167–76. doi: 10.1242/jcs.02329. [DOI] [PubMed] [Google Scholar]
- 73.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunter AW, Jourdan J, Gourdie RG. Fusion of GFP to the carboxyl terminus of connexin43 increases gap junction size in HeLa cells. Cell Commun Adhes. 2003;10:211–4. doi: 10.1080/cac.10.4-6.211.214. [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Pan L, Wei Z, Zhao Y, Zhang M. Domain-swapped dimerization of ZO-1 PDZ2 generates specific and regulatory connexin43-binding sites. EMBO J. 2008;27:2113–23. doi: 10.1038/emboj.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res. 2008;77:757–65. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imazio M, Brucato A, Ferrazzi P, Rovere ME, Gandino A, Cemin R, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124:2290–5. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 78.Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–23. doi: 10.1001/jama.2014.11026. [DOI] [PubMed] [Google Scholar]
- 79.Qu J, Volpicelli FM, Garcia LI, Sandeep N, Zhang J, Marquez-Rosado L, et al. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circ Res. 2009;104:365–71. doi: 10.1161/CIRCRESAHA.108.184044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127:455–64. doi: 10.1242/jcs.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108:704–15. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhein S, Hagen A, Jozwiak J, Dietze A, Garbade J, Barten M, et al. Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:221–34. doi: 10.1007/s00210-009-0473-1. [DOI] [PubMed] [Google Scholar]
- 84.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588:1423–9. doi: 10.1016/j.febslet.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: Rapid population of Cx43 in Plasma Membrane.
Ninety minute movie of a single cell containing fluorescently tagged Cx43. Imaging was performed using total internal reflection fluorescence microscopy (TIRFm) which limits resolution depth to about 50 nm, capturing only what is occurring at the membrane and just underneath. Transcription of tagged Cx43 in this cell was under pharmaceutical control, and the movie initiated when Cx43 had been transcribed/translated and was in the Golgi loading dock but not yet in the membrane. Over the course of 90 minutes, Cx43 is trafficked to and populates the membrane. Applied color is a heat map, in which brighter colors correspond to greater intensity of Cx43 signal.