Abstract
Behavioral inhibition (BI) is a temperament characterized by social reticence and withdrawal from unfamiliar or novel contexts and conveys risk for social anxiety disorder. Developmental outcomes associated with this temperament can be influenced by children’s caregiving context. The convergence of a child’s temperamental disposition and rearing environment is ultimately expressed at both the behavioral and neural levels in emotional and cognitive response patterns to social challenges. The present study used functional neuroimaging to assess the moderating effects of different parenting styles on neural response to peer rejection in two groups of adolescents characterized by their early childhood temperament (Mage = 17.89 years, N= 39, 17 males, 22 females; 18 with BI; 21 without BI). The moderating effects of authoritarian and authoritative parenting styles were examined in three brain regions linked with social anxiety: ventrolateral prefrontal cortex (vlPFC), striatum, and amygdala. In youth characterized with BI in childhood, but not in those without BI, diminished responses to peer rejection in vlPFC were associated with higher levels of authoritarian parenting. In contrast, all youth showed decreased caudate response to peer rejection at higher levels of authoritative parenting. These findings indicate that BI in early life relates to greater neurobiological sensitivity to variance in parenting styles, particularly harsh parenting, in late adolescence. These results are discussed in relation to biopsychosocial models of development.
Keywords: Behavioral inhibition, Social anxiety, Parenting, Peer rejection, Brain function
The temperament of behavioral inhibition (BI) is a predisposition to react with fearfulness and hyper-vigilance to novelty that can be identified in infancy and early childhood. Children with this temperament express social reticence in middle childhood and adolescence, and can have poor peer relationships (Fox, Henderson, Marshall, Nichols, & Ghera, 2005). Given these social difficulties, it is not surprising that BI conveys increased risk for internalizing problems, as 40–50% of children with a history of BI develop social anxiety disorder in adolescence and adulthood (Chronis-Tuscano et al., 2009; Clauss & Blackford, 2012). As such, BI does not predetermine negative outcomes, but is viewed as an inherent vulnerability factor for later difficulties. At the biological level, vulnerability for the social problems associated with BI may be expressed in terms of a heightened neural responding by anxiety-related circuitry to salient stimuli, particularly socially challenging stimuli.
One factor that may moderate outcomes associated with childhood BI, including neural responding, is the social context in which development takes place. Parenting style is a foundational social context insofar as distinct approaches to parenting interact with variance in children’s behavioral tendencies to jointly contribute to developmental outcomes (Darling & Steinberg, 1993). Indeed, different types of parenting styles moderate a range of negative outcomes associated with BI, including internalizing and externalizing difficulties (Degnan & Fox, 2007; Kiff, Lengua, & Zalewski, 2011; Phillips et al., 2012; Williams et al., 2009). Parenting practices also influence specific characteristics of BI such as social wariness (Rubin, Burgess, & Hastings, 2002). The present study examined the combined influence of early childhood BI and parenting styles on brain function during a socially challenging experience in adolescents, with a focus on the ventrolateral prefrontal cortex (vlPFC), amygdala, and striatum, three brain regions associated with both BI and social anxiety (Guyer et al., 2014; Guyer, Choate, Detloff, et al., 2012; Guyer et al., 2008; Guyer et al., 2006; Perez-Edgar et al., 2007).
The effects of different parenting styles on children’s well-being and social functioning have long been a focus of developmental psychology. One way that parenting style is thought to influence children’s social and emotional development is by shaping the emotional climate of the parent-child relationship, which can then generalize to other social situations (Darling & Steinberg, 1993). Two such widely-studied styles are authoritative and authoritarian parenting. These styles are characterized by markedly different emotional tones that parents engage in when interacting with their children. Importantly, these styles are not simply opposite ends of the same dimension, but represent orthogonal profiles of parenting characteristics.
Authoritative parenting is characterized by high levels of emotional warmth, support, and responsiveness, and communicating expectations in a manner that is firm, democratic, and transparent; authoritarian parenting, in contrast, is characterized by a lack of emotional warmth and support for the child, non-transparent declaration of rules, and high levels of control (Baumrind, 1991; Robinson, Mandelco, Olsen, & Hart, 2001). Since parenting style sets the affective tone in which a child matures, it can impact a child’s response to social challenges. This is a particularly important process in adolescence given the heightened salience of peer rejection and acceptance at that developmental stage (Steinberg & Morris, 2001). Indeed, the warm and supportive climate provided by authoritative parenting is largely associated with social benefits in adolescence, such as peer acceptance, social competence, and self-regulatory skills, whereas the failure to scaffold a positive emotional climate associated with authoritarian parenting is detrimental to building rewarding social relationships (Steinberg, 2001).
Research has demonstrated direct negative effects of authoritarian over authoritative parenting styles on social development (Steinberg, 2001). These differences are highly salient to the development of children with BI, as they already face high risk for social anxiety. Indeed, associations between parenting style, whether harsh or supportive, and outcomes, including later psychopathology, are stronger in children with BI relative to children without BI (Degnan, Almas, & Fox, 2010; Hastings et al., 2008; Kertes et al., 2009; Rubin, Burgess, Dwyer, & Hastings, 2003; van der Voort et al., 2014; Williams et al., 2009), consistent with a diathesis-stress model of development (Hankin & Abela, 2006).
At a mechanistic level, parenting effects may be associated with the activity of certain brain areas, specifically with neural responses to social stimuli in brain networks that play a key role in affective responding. Because of the multi-level, complex nature of social interactions, parenting effects may be detected in the response patterns of neural structures that underlie social cognition and social affect relative to overt expressions of behavior, particularly as behavior becomes more nuanced in adolescence and adulthood. Both BI and social anxiety disorder have been characterized by differential response tendencies in affective neural circuits involving prefrontal cortex (PFC), amygdala, and striatum, consistent with the idea that both conditions involve difficulty regulating emotional responses to social stimuli. For example, functional magnetic resonance imaging (fMRI) research has shown that childhood BI is associated with dysregulated engagement of regions within PFC (Jarcho et al., 2013), amygdala (Perez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003), and striatum (Bar-Haim et al., 2009; Guyer et al., 2014; Guyer et al., 2006; Perez-Edgar et al., 2014). Likewise, harsh parenting, low maternal warmth, and maternal negative affect measured in other at-risk groups have all been associated with atypical structure (e.g., larger regional volumes) and functional engagement (e.g., increased activation) in these same brain regions (Casement et al., 2014; Tan et al., 2014; Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, 2006; Whittle et al., 2009; Yap et al., 2008). Thus, the interactive effects of early childhood BI and authoritarian parenting, which comprise a compounded risk, may generate unique patterns of neural responding to social challenges. Authoritative parenting may facilitate adaptive neural responding to such challenges in youth characterized in childhood with BI.
The present study examined the combined effects of temperament and parenting styles in childhood on neural response to negative and positive evaluation from peers in adolescence. Based on prior research, we focused on the vlPFC, amygdala, and striatum as these regions each play an integral role in generating emotional responding to social cues and integrating affect with behavior. For example, the vlPFC has been associated with functions involving cognitive control or flexibility, even in social contexts (Nelson & Guyer, 2011). The amygdala aids in the detection of salience in the environment, particularly in the case of emotionally-laden stimuli (LeDoux, 2000). The striatum is involved in motivated action, reinforcement learning, and reward processing (Delgado, 2007). Although differential responses to peer evaluation in these regions are found in adolescents with childhood BI (Guyer et al., 2014) and with social anxiety (Guyer et al., 2008), and in relation to parenting styles (Casement et al., 2014; Tan et al., 2014; Whittle et al., 2012), no prior work has examined temperament and parenting together. We hypothesized that parenting would differentially moderate the link between BI and engagement of the vlPFC, striatum, and amygdala in an evaluative context involving peers, such that:
Adolescents with a history of BI and authoritarian parenting will show dampened function during social stress in cortical brain regions implicated in cognitive control and flexibility, based on data showing vlPFC hypoactivation in relation to distress felt during social exclusion (Eisenberger, Lieberman, & Williams, 2003) and in highly anxious youth when viewing social threats (Monk et al., 2006). Specifically, we hypothesized that higher levels of authoritarian parenting would be associated with lower vlPFC response to peer rejection than acceptance, particularly among youth characterized with BI in childhood.
Adolescents with a history of BI and authoritarian parenting will have heightened sub-cortical responses to social stress. Given past work showing that aberrant amygdala function relates to experiences of harsh parenting (Taylor et al., 2006) and to BI during social threat cue processing (Perez-Edgar et al., 2007), we expected that higher levels of authoritarian parenting would be associated with greater amygdala response to peer rejection than acceptance, particularly among youth characterized by BI in childhood. Maternal negative affect has also been associated with striatal response to peer evaluation (Tan et al., 2014). As such, altered striatal response to peer rejection vs. acceptance was expected among youth characterized by a history of BI who also experienced higher levels of authoritarian parenting.
Adolescents with a history of BI and authoritative parenting will evidence a buffering effect from social stress. Positive parenting promotes emotional well-being in shy, reticent youth (Rubin et al., 2002; Williams et al., 2009), and may promote adaptive neural responding to negative and positive cues from peers. Given the striatum’s role in processing reward stimuli and promoting adaptive behavior, including heightened striatal response to peer acceptance (Guyer, Choate, Pine, & Nelson, 2012), we hypothesized that higher levels of authoritative parenting would result in lower striatal activation to peer rejection (a social threat), relative to peer acceptance (a social reward), signaling an overall protective effect in adolescents with a history of BI in childhood. We also expected reduced amygdala and heightened vlPFC response to peer rejection in adolescents with a history of BI and higher levels of authoritative parenting.
Method
Participants
Participants were drawn from 433 infants who were screened on reactivity to novel sensory stimuli at 4 months old. Infants with extreme reactivity scores (N=153) were enrolled in a longitudinal study of temperament (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Based on their early BI grouping (see Measures), 58 of the 153 participants were eligible for a neuroimaging assessment in late adolescence. Participants were excluded if taking psychotropic medications or if determined to have serious, untreated psychopathology based on an expert clinician’s respective interviews of the participant and parent using the Schedule for Affective Disorders and Schizophrenia for School Aged Children–Present and Lifetime Version (Kaufman et al., 1997). These two exclusion criteria limited the number of acutely symptomatic participants in the present sample. A subset of participants (n=4) had clinical diagnoses that did not meet exclusionary criteria, although the distribution did not differ by BI group.
Of the 58 eligible participants for the neuroimaging study, nine were excluded due to dental braces, leaving 49 who were scanned. Of the 49 who were scanned, 10 participants were excluded due to excessive motion (>3mm) and/or lack of task deception (for 3 of the 10). The present analyses included 39 adolescents (Mage=17.89 years, 17 males, 22 females): a BI group (n=18, 9 males, 9 females) characterized in childhood by high BI scores and a behavioral non-inhibition (BN) group (n=21, 8 males, 13 females) characterized in childhood by low BI scores. Participants’ BI scores were representative of the scores in the cohort from which they were recruited (0.09 for participants vs. −0.03 for the remaining cohort, p=0.44). All participants were Caucasian and from middle-class families, which reflected the full cohort’s demographics. Data from these 39 participants were reported in past work (Guyer et al., 2014), which examined the association between BI and neural responding without any consideration of parenting. The current study tests independent hypotheses about the moderating role of parenting on temperament-related differences in neural responses to the receipt of peer rejection.
The present study was approved by the ethical review boards at the National Institute of Mental Health and the University of Maryland, College Park. Participants’ informed verbal assent and their parents’ informed written consent were obtained prior to study participation.
Measures
Behavioral Inhibition
At ages 14 and 24 months, children’s reactions to novel objects and people were assessed during laboratory assessments (Fox et al., 2001). Inter-coder reliability of these behaviors yielded Pearson correlations ranging from .85 to 1.00. At ages 4 and 7 years, children’s reticent behavior with unfamiliar peers was measured using Rubin’s Play Observation Scale (Rubin, 1989) for which inter-coder reliability kappa estimates ranged from .81 to .94. Mothers rated their child’s social fear at 14 (α=.92) and 24 (α=.87) months on the Toddler Behavior Assessment Questionnaire (Goldsmith, 1996) and shyness at ages 4 (α=.82) and 7 (α=.87) years on the Colorado Child Temperament Inventory (Rowe & Plomin, 1977). Observed behavior and maternal-report scores collected at these four time points were standardized in the full cohort. The average of these Z scores was then used to create a composite BI score, M=−0.008, SD=0.64, range: −1.33 to 2.82. A median split of the composite score was used to create BI and BN groups within the full cohort.
Maternal Parenting Styles
Parenting styles were assessed through mothers’ self-report on the Parenting Practices Questionnaire (Robinson et al., 2001) when their child was 7 years old. We focused on the subscales of authoritative and authoritarian parenting styles according to Baumrind’s (1991) dimensions. The Authoritative subscale consists of 27 items measuring warmth and involvement, inductive reasoning, democratic participation, and general pleasantness (α=88). The Authoritarian subscale consists of 20 items measuring verbal hostility, corporal punishment, punitive strategies, and directiveness (α=77). These subscales are widely-used and reliable assessments of parenting school-age children (Robinson et al., 2001). Parents rated from 1=never to 5=always how often they exhibited each parenting behavior. In this sample, the mean was 4.03 (SD=0.29) for authoritative parenting and 1.83 (SD=0.21) for authoritarian parenting. Authoritative and authoritarian parenting were negatively but not significantly correlated, r=−.24, p=0.14, as expected given their orthogonal nature. No differences in either parenting style were found between BI groups (see Results).
Peer Evaluation Neuroimaging Task
Participants completed a variant of the neuroimaging task called The Chat Room, which simulates several aspects of peer evaluation (Guyer et al., 2014; Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Guyer et al., 2008). The task was administered during two laboratory visits. At visit one, participants were told that the study was designed to learn about teenagers’ online interactions, and that during the subsequent visit they would chat online with another participant deemed a good match for them. Participants were photographed and asked for basic biographical information to be shared with other study participants. Participants then sorted photographs displayed on a laptop of 60 peers into a group of 30 with whom they would and 30 with whom they would not like to chat online. Stimuli were 60 digital headshots of 11–20 year-old actors (30 males) posing happy expressions with direct gaze (Egger et al., 2011). E-prime software was used for task programming and administration (Psychological Software Tools, Pittsburgh, PA).
At visit two, 2 weeks later, participants returned for a functional neuroimaging scan. Although participants believed they would receive positive or negative feedback from each peer, valence of feedback was manipulated via computer algorithm. All 60 photographs were viewed in random order during each of two tasks. For task one, participants rated how much they thought each person in the photograph was interested in them. For task two, participants received accepting or rejecting evaluation from each peer. Given our focus on neural response during peer evaluation, only results from task two are considered here. Each trial started with an anticipation event (3 s), during which a peer’s photograph was shown and participants were reminded by text stating whether they selected or rejected the peer for a chat. Next was an evaluation event (2 s) during which the same peer’s photograph was shown along with text stating the peer’s purported selection or rejection of the participant. Participants rated from 0=not at all to 100=totally how much they expected that outcome. Variable duration jitter (a fixation cross presented for 0–8000 ms) occurred between these two events to dissociate neural responses to each event.
After the scan, participants rated from 1=not at all to 10=very how happy they felt when someone was interested in chatting with them and how upset they felt when someone was disinterested in chatting with them. They then underwent a standardized debriefing procedure used in prior studies involving deceptive feedback (Guyer et al., 2014). No adverse effects associated with deception occurred after participants were debriefed post-scan.
fMRI Acquisition
Scanning occurred in a Signa 3 Tesla magnet (General Electric, Waukesha, WI). Stimuli were projected onto a screen at the end of the scanner bed and viewed with mirrors mounted on the head coil. Foam padding was used to constrain head motion. A hand-held two-button response device was used to record participants’ ratings (Research Services Branch, NIMH, Bethesda, MD). Each brain volume (367 total) consisted of 36 interleaved slices, acquired axially using a T2*-weighted gradient echo pulse sequence with 2300 ms repetition time (TR), 25 ms echo time (TE), 90° flip angle, 2.5 × 2.5 × 2.6 mm voxels, 96 × 96 matrix, and 24 cm field-of-view (FOV). Four dummy acquisitions were obtained before task onset for signal stabilization, and excluded from analyses. A high-resolution anatomical scan was acquired using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence: 124 axial slices 1.2 mm thick, 7.816 TR, 3.024 TE, 6° flip angle, 256 × 192 matrix, and 22 cm FOV.
Data Analysis
Analysis of Functional and Neural Images (AFNI) software (Cox, 1996) was used to conduct pre-processing and first-level analyses on fMRI data. Pre-processing included corrections for slice timing and motion, re-slicing to 2 mm isotropic voxels, warping into standard space, spatial smoothing to a 6 mm full-width half-maximum Gaussian kernel, and normalizing blood-oxygen-level-dependent signal intensity to percentage signal change.
A general linear model was used to determine the beta value and t-statistic of each task event at the single subject level. This model applied a gamma variate basis function convolved with the hemodynamic response function provided in AFNI. Movement artifact was mitigated by including six motion correction parameters in the model as nuisance covariates along with a covariate for mean intensity and linear drift. Events of interest were modeled using the onset time of the picture shown for each of the acceptance/rejection evaluation events, with our main focus on neural response to receipt of evaluation. Anticipation of evaluation included two event types (participant accepted peer; participant rejected peer). Receipt of evaluation included four event types (participant accepted peer, peer accepted participant; participant rejected peer, peer accepted participant; participant accepted peer, peer rejected participant; participant rejected peer, peer rejected participant). Contrasts of these events were generated at the individual subject level. Our main contrast of interest was peer rejection of participant (averaged across participant selection/rejection of peer) minus peer acceptance of participant (averaged across participant selection/rejection of peer). Peer acceptance and rejection were also each contrasted to baseline.
Group-level analyses were conducted using an a priori region of interest (ROI) approach. Using the Talairach Daemon software provided in AFNI, we created anatomically-defined ROIs (see Figure 1): bilateral vlPFC (encompassing inferior frontal gyrus and middle frontal gyrus inferior to z=0 and Brodmann areas 10, 11, and 47), striatum (caudate, nucleus accumbens, and putamen), and amygdala. Multiple comparisons were mitigated by confining our analyses to these a priori ROIs. In contrast to a voxel-based approach, we obtained a single mean response across voxels for each ROI to further reduce risk of making Type I errors. Mean percent signal change values were extracted from each bilateral ROI per subject for the contrasts of peer rejection vs. acceptance, peer rejection vs. baseline, and peer acceptance vs. baseline, and entered into SPSS. Hierarchical regression analyses were then conducted to test whether parenting styles moderated the association between BI group and neural responses to peer rejection minus acceptance. Separate models were conducted for group-by-authoritarian and group-by-authoritative parenting for each ROI. Each model included group (BI vs. BN) and continuous scores on parenting style on step one and the interaction term of group x parenting style on step two. All models included parenting scores centered on the sample mean for both main and interaction effects. For significant interaction effects, we examined scatterplots of neural response to rejection vs. baseline and acceptance vs. baseline to aid interpretation.
Figure 1.
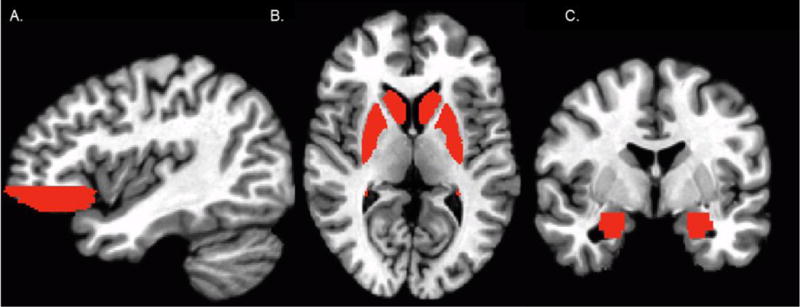
Masks of the anatomically-defined regions of interest. Bilateral (A) ventrolateral prefrontal cortex, (B) striatum (including caudate, putamen, and nucleus accumbens), and (C) amygdala.
Correlations were calculated between post-task self-reported affective response to evaluation and activation in each ROI. Associations between distress when rejected and neural activation to peer rejection vs. acceptance and between happiness when accepted and neural activation to peer acceptance vs. rejection were examined within each group.
Results
Sample characteristics and task-related data
By design, the groups differed significantly in composite temperament score, with a large effect size, d=3.42, but did not differ significantly on demographic characteristics, parenting styles, self-reported expectations of evaluation received during scanning, or post-scan affective responses to peer evaluation (Table 1). Based on the diagnostic psychiatric interview, one BI participant had co-morbid social anxiety and depression, one had attention-deficit hyperactivity disorder, and two BN participants had generalized anxiety disorder. To maximize representativeness, these participants were retained in all analyses; however, separate analyses in which these subjects were excluded did not change the overall patterns of significance.
Table 1.
Sample characteristics and task ratings
| Characteristic | Behavioral inhibition group (n=18) |
Behavioral non-inhibition group (n=21) |
Statistic | ||
|---|---|---|---|---|---|
| Males | n=9 | 50% | n=8 | 38% | χ2 (1, 39)=0.56, p=0.46 |
| Mean | SD | Mean | SD | ||
| Age (years) | 17.85 | 1.38 | 17.93 | 1.80 | t(37)=0.17, p=0.87 |
| IQ | 114.28 | 10.74 | 113.52 | 12.50 | t(37)=0.20, p=0.84 |
| Behavioral inhibition composite score | 0.89 | 0.64 | −0.63 | 0.18 | t(37)=10.39, p<0.001 |
| Age 7 authoritative parenting style (1–5) | 3.95 | 0.29 | 4.10 | 0.28 | t(37)=1.71, p=0.10 |
| Age 7 authoritarian parenting style (1–5) | 1.89 | 0.19 | 1.78 | 0.21 | t(37)=1.81, p=0.08 |
| In scanner rating: expectation of peer acceptance (1–100) | 56.23 | 10.84 | 53.89 | 9.71 | t(37)=0.71, p=0.48 |
| In scanner rating: expectation of peer rejection (1–100) | 51.94 | 11.60 | 49.90 | 9.93 | t(37)=0.59, p=0.56 |
| Post-scan rating: happy when peer interested in chatting (1–10) | 8.06 | 1.51 | 8.25 | 1.39 | t(32)=0.39, p=0.70 |
| Post-scan rating: upset when peer disinterested in chatting (1–10) | 3.33 | 2.38 | 3.81 | 1.64 | t(32)=0.68, p=0.50 |
Neuroimaging data
Authoritarian parenting
The unique contributions of group and authoritarian parenting each were at a trend level of significance for right vlPFC response to peer rejection vs. acceptance, Beta=−0.30, t (35)=−1.92, p=0.06 and Beta =−0.37, t (35)=1.79, p=0.08, respectively. However, a significant interaction of authoritarian parenting and group was found in right vlPFC, Beta =−0.47, t (35)=−2.33, p=0.03. Harsh parenting style had a differential impact on vlPFC response to peer evaluation in the two groups as shown in Figure 2a. To help interpret this interaction effect, both the rejection and acceptance conditions were plotted relative to baseline (Figure 2b). Consistent with hypothesis one, the interaction effect was driven by decreased vlPFC response to rejection at higher levels of authoritarian parenting for youth characterized in childhood by BI. For BN youth, vlPFC response to rejection did not vary as a function of authoritarian parenting.
Figure 2.
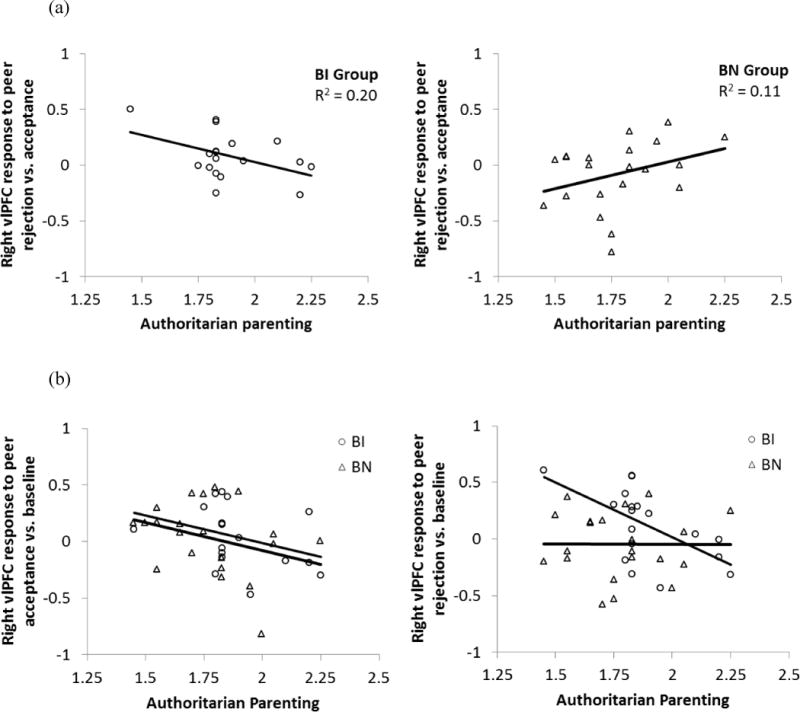
(a) Interaction of behavioral inhibition group and authoritarian parenting on signal change in the right vlPFC to peer rejection vs. acceptance. (b) Illustrative depiction of signal change in the right vlPFC to peer acceptance vs. baseline and to peer rejection vs. baseline. BI=behavioral inhibition. BN=behavioral non-inhibition.
We did not find significant main or interactive effects of group, authoritarian parenting, or their combined influence on amygdala response, thus finding no support for hypothesis two, nor did we find effects in striatal regions.
Authoritative parenting
The interaction of group x authoritative parenting and of group alone did not significantly predict caudate response to peer rejection vs. acceptance. The interaction term was then dropped from the model and a second model was run with group on step one and authoritative parenting score on step two. This model showed a significant main effect of authoritative parenting on activity in the right caudate that accounted for 12% more of the variance in caudate response above and beyond group, Beta=−0.36, t (35)=−2.19; p=0.04. This effect was driven by a decreased caudate response to rejection as authoritative parenting increased (Figure 3), providing partial support for our third hypothesis.
Figure 3.
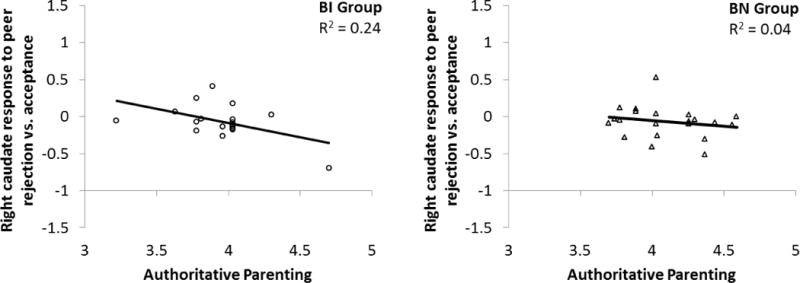
Main effect of authoritative parenting on signal change in the right caudate to peer rejection vs. acceptance. BI=behavioral inhibition. BN=behavioral non-inhibition.
At a trend level of significance, there was an interaction effect of group x authoritative parenting on left amygdala activation that accounted for an additional 9% of the variance in amygdala response, Beta=−0.43, t (35)=−1.89, p=0.07. Neither group nor authoritative parenting alone accounted for a significant amount of the variance. Left amygdala activation to peer rejection vs. acceptance was plotted as a function of authoritative parenting for each group (Figure 4). Increased amygdala response was found at lower levels of authoritative parenting in the BI group. At higher levels of authoritative parenting, amygdala response was greater to acceptance than rejection. In other words, adolescents characterized by childhood BI showed an amygdala response that tended to increase to acceptance and decrease to rejection, as authoritative parenting increased. The BN group did not show variation in amygdala response to peer acceptance vs. rejection at different levels of authoritative parenting, even at a trend level.
Figure 4.
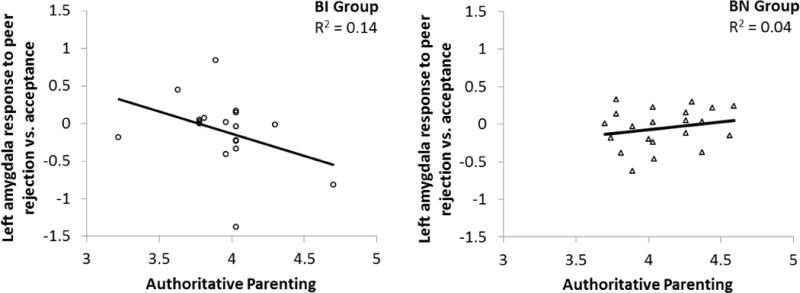
Interaction of behavioral inhibition group and authoritative parenting on signal change in the left amygdala to peer rejection vs. acceptance. BI=behavioral inhibition. BN=behavioral non-inhibition.
We did not find significant main and interactive effects of group, authoritative parenting, or their combined influence on activation in putamen, nucleus accumbens, or vlPFC.
Post-scan self-reported affective response to peer evaluation
Greater distress was associated with increased bilateral amygdala activity to rejection vs. acceptance in the BI group (n=18), left: r=.55, p=0.02, right: r=.56, p=0.02. Associations with amygdala response were not significant in the BN group (n=16), left: r=−.11, p=0.68, right: r=.21, p=0.44. Fisher’s Z transformations indicated that the group differences were significant in left amygdala, z=1.93, p=0.05, but not right amygdala, z=1.12, p=0.26. Significant associations were not found for any other ROIs. No significant associations were found between happiness and neural response to acceptance vs. rejection for any ROIs in either group.
Discussion
The present study used a prospective, longitudinal, multi-level approach to assess the interwoven relations among temperament, family processes, neurobiology, and social context. We examined whether associations between temperament and brain responses to a social challenge vary as a function of parenting styles. Specifically, we tested hypotheses examining the moderating influence of two different parenting styles as measured in middle childhood on the relation between BI in early childhood and neural response to experiences of social evaluation in adolescence. Using an a priori ROI approach based on past work, we focused on the modulation of neural activity within the vlPFC, striatum, and amygdala to peer rejection. By focusing on neural mechanisms and sensitivity to caregiving, we can better pinpoint which individuals possess temperament-based vulnerability to specific kinds of caregiving environments, manifesting in outcomes such as brain function during a social challenge.
We found that in youth with a history of early childhood BI, higher levels of authoritarian parenting were associated with diminished responses to peer rejection in right vlPFC whereas authoritarian parenting was not related to vlPFC response differences in youth without such a history. The vlPFC is involved in stimulus-response learning whereby prescribed rules and contextual information are integrated with input from subcortical temporal lobe regions to guide behavioral responding (Bunge, 2004). These processes include modulating affective response to threat or reward stimuli and updating goals and guides for action, and are supported by strong anatomical connections found between the vlPFC and amygdala (Price, 2007; Sakagami & Pan, 2007). Using a task similar to the one in this study, differential vlPFC activity was found in response to receiving feedback from peers that adolescents were likely not expecting to receive feedback from (Guyer, Choate, Pine, et al., 2012), which suggests that vlPFC plays a key role in response flexibility and updating expectations about social information (Nelson & Guyer, 2011).
In youth with affective disorders, differential response patterns have been found in the vlPFC during affective responding to social threats (Guyer et al., 2008; Maslowsky et al., 2010; Monk et al., 2006). For example, relative to responses measured pre-treatment, right vlPFC response to angry faces increased in clinically anxious adolescents after receiving either cognitive behavioral therapy or pharmacological treatment (Maslowsky et al., 2010). Research on healthy adolescent (Masten et al., 2009) and adult (Eisenberger et al., 2003) samples documents a negative association of higher social distress and lower right vlPFC response to social exclusion. Consistent with these findings, results from the present study indicated a negative association between harsh parenting style and right vlPFC response to peer rejection among youth with a history of BI, but not those without such a history. For youth who were inhibited, higher levels of harsh parenting in childhood were related to less engagement of right vlPFC during a challenging social experience. Although socially anxious adolescents have shown positive amygdala-vlPFC co-activation when anticipating peers’ evaluations (Guyer et al., 2008), increased vlPFC activity may have been instantiated in tandem with increased amygdala activity because the evaluative outcome was unknown. That is, an anticipatory process was engaged in prior work (Guyer et al., 2008) rather than one involving updating of social rules upon learning an outcome (e.g., a peer does not want to interact) as examined in this study.
Although speculative, harsh parenting may interrupt the ability of children with BI to update their expectations and goals about distressing social experiences, such as peers’ disinterest. Right vlPFC is thought to serve a regulatory function through modulating affective response or promoting social learning, and these processes may become disrupted for youth with a history of BI who experienced harsher emotional climates with their parents in childhood. When children with BI encounter low levels of warmth and support from their parents, an inherent sensitivity to negative caregiving may impede their ability to update the value they place on an unsupportive person even from another social domain (i.e., rejecting peers) (Behrens, Hunt, & Rushworth, 2009). It may also be the case that the reduced vlPFC response to peer rejection occurred because this evaluative outcome is consistent with the harsh parenting that these youth experienced and to which they are especially sensitive. In other words, more vlPFC activation would be instantiated in situations where the peer outcomes are inconsistent with prior experiences with parents because the vlPFC serves to invoke top-down processing to engage other regions in generating context-appropriate behavior (Levy & Wagner, 2011). Indeed, in healthy adolescents, increased right vlPFC to social exclusion has been associated with less distress over being excluding (Masten et al., 2009). Finally, because the vlPFC responds to social distress, it may be that increased vlPFC activity is associated with harsh parenting. However, because we assessed neural response to real-time peer rejection events, our expectation for the direction of the vlFPC response was based on rejection as the source of social distress rather than past harsh parenting, which was not measured in tandem with brain function.
As expected, no effect of authoritarian parenting was found on right vlFPC activity to peer rejection for adolescents without a temperament of BI. Negative parenting appears to have been less influential on neural responding in this prefrontal region to a challenging social experience in youth without a history of BI, possibly due to an inherently blunted sensitivity to their surroundings (i.e., low reactivity to novelty in infancy and low social reticence in childhood), including even harsh caregiving.
A different pattern of results was found for the more favorable parenting style. At higher levels of authoritative parenting, all adolescents demonstrated decreased activity in the caudate to peer rejection. The caudate has been implicated in reward processing, learning, and habitual patterns of behavior (Delgado, 2007). There is also evidence for differential patterns of striatal engagement to social events of a negative valence. For example, research on adolescents with depression has documented increased striatal response to peer rejection (Silk et al., 2014) and work on healthy adolescents has shown that increased striatal response to social exclusion relative to inclusion relates to less distress (Masten et al., 2009). Results from the present study indicate that, in both groups, experiencing higher levels of warm, supportive parenting was associated with reduced caudate activation to negative feedback from peers. This finding may indicate a reduced salience of negative peer experiences because of exposure to positive parenting across development. In past work, adolescents without a history of BI showed greater caudate activation to acceptance versus rejection from peers they were interested in, but this pattern was not seen in those with a history of BI (Guyer et al., 2014). In related work, nurturing parenting protects anxious children from the negative effects of peer victimization (Affrunti, Geronimi, & Woodruff-Borden, 2014). As such, authoritative parenting may provide a similar buffering effect manifest in brain function and regardless of BI history.
Finally, there was a trend effect of authoritative parenting moderating the link between BI and amygdala response to social rejection. Specifically, lower amygdala response was found at higher levels of positive parenting within the BI but not the BN group. Amygdala response to peer rejection was influenced differentially by group and reduced response was associated with positive parenting, as expected. However, contrary to our prediction, increased amygdala response did not relate to negative parenting. The present results are also in contrast with work showing that adults raised in families with few stressors had heightened amygdala response to social threats (Taylor et al., 2006), whereas those from risky families had blunted amygdala response and positively correlated activity between the amygdala and vlPFC in response to threats, suggesting dysregulated fear-circuitry as a result of harsh rearing environment. We did find, however, that greater self-reported distress over being rejected was significantly associated with increased bilateral amygdala engagement to peer rejection in the BI but not the BN group, indicating that peer rejection held greater salience both behaviorally and neurally for youth characterized by a history of BI. The amygdala, typically implicated in attention and threat processing, is engaged by socially evaluative events. For example, amygdala hyperactivity has been found in both socially anxious and healthy adolescents when anticipating peer feedback, but after receiving negative feedback, this activity declined in healthy but not anxious adolescents (Lau et al., 2011). Supportive parenting may help youth with BI better regulate emotional responding to a social stressor, such as peer rejection, via the amygdala.
In the present study, we found some evidence to support a diathesis-stress model of development whereby adolescents with BI showed decreased vlPFC response to social stress when they had experienced higher levels of harsh parenting assessed in middle childhood. However, rates of psychopathology, including social anxiety, in the BI group were low and similar to the BN group. This limited our ability to examine in this sample whether harsh parenting style moderates the link between BI and social anxiety. Nonetheless, we found altered neural response patterns in the BI group that are consistent with patterns documented in samples of adolescents with clinical social anxiety.
Other biopsychosocial models of development posit that some individuals are highly sensitive to both positive and negative socialization experiences, which lead to beneficial and detrimental outcomes, respectively (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011). It may be that youth characterized by a history of BI are more susceptible to not only the negative effects of adverse environments on brain function, but also may be more sensitive to the beneficial effects of positive social environments and thrive. This latter idea reflects the differential susceptibility to environment model (Ellis et al 2011), which is supported by evidence indicating a differential impact of parenting styles on children with or without genetic sensitivity to social contexts and vulnerability to psychopathology (Belsky & Pluess, 2009; Boyce & Ellis, 2005; Taylor et al., 2006). Our prediction that youth with a history of BI would generally exhibit greater modulation of neural response to peer rejection as a function of their caregiving experiences, both positive and negative, was upheld. We found that neural response in the BI but not BN group was moderated by both negative and positive parenting in some (e.g., vlPFC) but not all (e.g., striatum) regions. By examining parenting as a moderator of temperament (a susceptibility factor) on brain function, we have better identified for whom a specific temperament (i.e., BI) relates to brain function and under what conditions. Because interactions between genes and environments are consistently viewed as established mechanisms of development, BI may operate similarly to genes. That is, BI may act as a biologically-based indicator of susceptibility to both adverse and facilitative caregiving experiences (Ellis et al., 2011). Furthermore, BI as a biological susceptibility factor may express itself more strongly indexed by brain function than genes because it is closely tied to the coding of environmental cues and associated behavior and emotional responses to those cues. Again, however, rates of psychopathology were low and did not vary between the groups in this study. Nonetheless, because youth with childhood BI may experience poor peer relationships, social reticence, low self-esteem, and increased risk for social anxiety into adulthood, identifying adolescents vulnerable to poor social outcomes vis-a-vis childhood temperament and socialization influences may generate new insights into the etiology and mechanisms of internalizing problems.
Our study had several strengths. We employed a longitudinal design that used temperament classifications based on multi-informant and multi-method data collected at four time points in early childhood, parenting assessed in middle childhood, and brain function measured in late adolescence. We also accounted for multiple levels of contributing factors (e.g., temperament, brain function, and parental socialization). Through the use of a longitudinal study design, we were able to identify associations between children’s early-appearing temperament and childhood socialization experiences and later assessments of brain function, highlighting the effects of parenting on the brain a decade later, even in a relatively low risk sample (i.e., non-clinical). These results may inform intervention efforts targeted to those at risk for internalizing problems, particularly given the overlap in brain regions involved and known social problems experienced by those with a history of BI and those with social anxiety.
Our study was not without limitations. First, the sample size was relatively small due to our use of neuroimaging to study a unique sample followed from infancy into adolescence. Given its small size, we did not control for multiple comparisons on participants’ affect in response to rejection and acceptance. Although these data may motivate future work, it will be important to replicate our findings in larger samples, particularly for results at a trend level of significance. Future work using a larger sample will also be needed to test the influence of additional factors such as gender, past peer experiences, and clinical levels of anxiety. Second, the sample was limited in the number of subjects with psychopathology, particularly social anxiety. Third, the middle-class Caucasian nature of the sample may limit the generalizability of findings to youth of other racial/ethnic groups or socioeconomic strata. For example, highly controlling parenting may be viewed as harsh in some middle-class families, but serve to protect youth from negative outcomes in minority and/or impoverished families living in dangerous neighborhoods. Nonetheless, harsh discipline strategies such as spanking are largely found to be harmful (Gershoff, 2013). Fourth, caution must be taken when interpreting these findings as brain function does not reside in these structures, but is a product of dynamic interactions among structures. Finally, further work is needed to determine the specificity of effects that emerge in the striatum and amygdala, as both regions may respond to rejection and acceptance. Events where peers had no chance to rate participants could serve as a neutral condition against which acceptance and rejection feedback could be compared in future research.
Our study highlights the importance of relationships with parents in shaping how adolescents respond to experiences with peers. The present results underscore the utility in having clinicians help parents understand that parent-child relationships are dynamic interaction processes with lasting effects, including on brain development. By assessing a child’s temperament, clinicians may be able to optimize the fit between a parent’s approach to parenting and a child’s behavioral tendencies. The present results also speak to the importance of peer evaluation for adolescents in a clinical context. Children with BI may benefit from interventions that teach them to adaptively perceive, interpret, respond, and manage their reactions to peer feedback. This is especially important given that for adolescents, peer interactions are highly salient, negative peer evaluation is a common concern, and the potential for negative peer evaluation leads youth with BI to avoid social situations. Finally, identifying for whom neural responses to a peer stressor vary based on temperament and parenting can help pinpoint mechanisms that may lead to later psychopathology and inform the design of interventions for high-risk youth. For example, cognitively-based interventions could train youth with BI whose parents fail to model warm, supportive social interactions, to interpret and respond more adaptively to social bids from others.
The present study presented novel data on how two different types of parenting styles shape the neural coding of children’s subsequent social development. For children who are temperamentally inhibited and reticent with peers, harsh parenting experiences appear to shape neural mechanisms of social development at an age when peer evaluation is highly salient. The multi-level, longitudinal approach taken in this study may provide new avenues for future work on the pathways to internalizing problems. For example, the diminished cortically-based cognitive flexibility we found in response to socially distressing experiences in conjunction with BI and harsh parenting may impair the integration of affective reactions with behavioral outputs, and in turn, be an important mechanism in the development of internalizing problems.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), an NIMH Seymour S. Kety Memorial Award (AEG), and NIMH grants MH080076 (AEG) and MH074454 (NAF). The authors thank all of the families who participated in the study.
Footnotes
Ethical standards: All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- Affrunti NW, Geronimi EM, Woodruff-Borden J. Temperament, peer victimization, and nurturing parenting in child anxiety: a moderated mediation model. Child Psychiatry and Human Development. 2014;45:483–92. doi: 10.1007/s10578-013-0418-2. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–18. doi: 10.1111/j.14679280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumrind D. The influence of parenting styles on adolescent competence and substance use. The Journal of Early Adolescence. 1991;11:56–95. [Google Scholar]
- Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/A0017376. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cognitive, Affective and Behavioral Neuroscience. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1066–1075 e1061. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Darling N, Steinberg L. Parenting style as context: An integrative model. Psychological Bulletin. 1993;113:487–496. doi: 10.1037/0033-2909.113.3.487. [DOI] [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry. 2010;51:497–517. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Egger HL, Pine DS, Nelson EE, Leibenluft E, Ernst M, Towbin K, Angold A. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research. 2011;20:145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gershoff ET. Spanking and child development: We know enough now to stop hitting our children. Child Development Perspectives. 2013;7:133–137. doi: 10.1111/cdep.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67:218–235. doi: 10.1111/j.1467-8624.1996.tb01730. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology. 2014;26:229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archvies of General Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abela JRZ. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA: Sage; 2006. [Google Scholar]
- Hastings PD, Sullivan C, McShane KE, Coplan RJ, Utendale WT, Vyncke JD. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: direct mothers and moderated fathers. Child Development. 2008;79:45–64. doi: 10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, Ernst M. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology. 2013;92:306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Donzella B, Talge NM, Garvin MC, Van Ryzin MJ, Gunnar MR. Inhibited temperament and parent emotional availability differentially predict young children’s cortisol responses to novel social and nonsocial events. Development Psychobiology. 2009;51:521–532. doi: 10.1002/dev.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, Zalewski M. Nature and nurturing: parenting in the context of child temperament. Clinical Child and Family Psychology Review. 2011;14:251–301. doi: 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Guyer AE, Tone EB, Jenness J, Parrish J, Pine DS, Nelson EE. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. International Journal of Behavioral Development. 2011;36:36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, Monk CS. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:105–111. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience. 2011;1:233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, et al. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience. 2014;9:445–453. doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Crowell NA, Gunnar M, Fox NA, Sussman AL, Hane AA, Bisgaier J. Reactive temperament and sensitivity to context in child care. Social Development. 2012;21:628–643. [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Robinson CC, Mandelco B, Olsen SF, Hart CH. Parenting styles and dimensions questionnaire. In: Perlmutter BF, Touliatos J, Holdem GW, editors. Handbook of Family Measurement Techniques: Instruments and Index. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Rowe DC, Plomin R. Temperament in early childhood. Journal of Personality Assessment. 1977;41:150–156. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) Canada: University of Waterloo; 1989. [Google Scholar]
- Rubin KH, Burgess KB, Dwyer KM, Hastings PD. Predicting preschoolers’ externalizing behaviors from toddler temperament, conflict, and maternal negativity. Developmental Psychology. 2003;39:164–176. [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, Hastings PD. Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development. 2002;73:483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Current Opinions in Neurobiology. 2007;17:228–233. doi: 10.1016/j.conb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive Affective Neuroscience. 2014;9:1798–807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. We know some things: adolescent-parent relationships in retrospect and prospect. Journal of Research on Adolescence. 2001;11:1–19. [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Tan PZ, Lee KH, Dahl RE, Nelson EE, Stroud LJ, Siegle GJ, et al. Associations between maternal negative affect and adolescent’s neural response to peer evaluation. Developmental Cognitive Neuroscience. 2014;8:28–39. doi: 10.1016/j.dcn.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- van der Voort A, Linting M, Juffer F, Bakermans-Kranenburg MJ, Schoenmaker C, van Ijzendoorn MH. The development of adolescents’ internalizing behavior: longitudinal effects of maternal sensitivity and child inhibition. Journal of Youth and Adolescence. 2014;43:528–540. doi: 10.1007/s10964-013-9976-7. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Yucel M, Sheeber L, Simmons JG, Pantelis C, Allen NB. Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Social Cognitive and Affective Neuroscience. 2009;4:247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yucel M, Forbes EE, Davey CG, Harding IH, Sheeber L, et al. Adolescents’ depressive symptoms moderate neural responses to their mothers’ positive behavior. Social Cognitive and Affective Neuroscience. 2012;7:23–34. doi: 10.1093/scan/nsr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Degnan KA, Perez-Edgar KE, Henderson HA, Rubin KH, Pine DS, et al. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology. 2009;37:1063–1075. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MB, Whittle S, Yucel M, Sheeber L, Pantelis C, Simmons JG, Allen NB. Interaction of parenting experiences and brain structure in the prediction of depressive symptoms in adolescents. Archives of General Psychiatry. 2008;65:1377–1385. doi: 10.1001/archpsyc.65.12.1377. [DOI] [PubMed] [Google Scholar]


