Abstract
Objective
To test the hypothesis that negative social interaction in old age is associated with increased risk of mild cognitive impairment and rate of cognitive decline.
Methods
Participants are 529 older people without cognitive impairment at study onset. They completed annual evaluations that included assessment of negative social interaction frequency in the previous month (e.g., unsympathetic behavior, rejection), cognitive testing, and clinical classification of mild cognitive impairment.
Results
During a mean of 4.8 years of follow-up (SD=2.5), 198 individuals (37.4%) developed mild cognitive impairment. In a proportional hazards model adjusted for age, sex, and education, higher baseline frequency of negative social interactions (mean=1.51, SD=0.43, skewness=1.60) was associated with higher risk of developing mild cognitive impairment (hazard ratio =1.53, 95% confidence interval: 1.13, 2.07). Results were similar after adjustment for depressive symptoms, social network size, social activity level, and loneliness. This association was mainly due to neglect and rejection. There was no change in negative social interaction rate over time (estimate =− 0.003, SE=0.004, p=0.508). Higher baseline level of negative social interaction was associated with lower initial level of global cognition (estimate=− 0.096, SE=0.034, p=0.005) but not with cognitive decline (estimate= − 0.018, SE=0.011, p=0.098). Higher mean level of negative interactions across the study period was robustly related to lower level of global cognition (estimate =− 0.154, SE=0.037, p<0.001) and faster rate of cognitive decline (estimate =− 0.036, SE=0.012, p=0.002).
Conclusion
Frequent negative social interactions may be a risk factor for mild cognitive impairment and cognitive decline in old age.
Keywords: negative social interaction, longitudinal study, mild cognitive impairment, cognitive decline
INTRODUCTION
Psychosocial stressors include major negative life events that are very upsetting but relatively infrequent (e.g., divorce) and daily irritants that may be less upsetting but are more common (e.g., family argument). Exposure to major negative life events has been associated with higher risk of cognitive impairment and dementia in old age (Persson & Skoog, 1996; Johansson et al., 2013), but knowledge about the association of daily stressors with late-life cognitive health is limited. The most common (Almeida, 2005) and upsetting (Bolger, DeLongis, Kessler, & Schlling, 1989) daily stressors are interpersonal arguments and tensions. Negative social interactions such as being rejected or receiving unwanted advice predict subsequent levels of depressive symptoms (Finch & Zautra, 1992; Mavandadi, Sorkin, Rook, & Newson, 2007; Stafford, McNunn, Zaninotto, & Nazroo, 2011), well being (Rook, 1984; Newsom, Nishishiba, Morgan, & Rook, 2003), and disability (Mavandadi, Rook, & Newsom, 2007; Newsom, Mahan, Rook, & Krause, 2008). Research on the acute effects of daily stress suggests that attention demanding processes such as working memory are most vulnerable (Sliwinski, Smyth, Hofer, & Stawski, 2006). Cross-sectional data from epidemiologic studies has supported the attention depletion hypothesis in some cases (Seeman et al., 2011;Tun et al., 2013), but the overall evidence has been mixed (Seeman, Lusignolo, Albert, & Berkman, 2001; Okabayashi, Liang, Krause, Akiyama, & Sugisawa, 2004; Hughes, Andel, Small, Borenstein, & Mortimer, 2008; Seeman et al., 2011; Tun, Miller-Martinez, Lachman, & Seeman, 2013; Windsor, Gerstorf, Pearson, Ryan, & Anstey, 2014). A more critical question is whether higher level of exposure to daily stressors contributes to late-life cognitive lossbe cause it would suggest that programs of stress reduction (Khoury et al., 2013) and reappraisal (Hofmann, Asnaani, Vonk, Sawyer, & Fang, 2012) might help maintain cognitive health in old age. Longitudinal studies of daily stress and cognition have been inconclusive (Seeman, Lusignolo, Albert, & Berkman, 2001; Hughes, Andel, Small, Bornstein, & Mortimer, 2008; Windsor, Gerstorf, Pearson, Ryan, & Anstey, 2014), but methodological factors may have limited the opportunity to observe an association in these studies.
In the present study, we test the hypothesis that higher level of negative social interactions is associated with increased incidence of mild cognitive impairment (MCI) and more rapid cognitive decline. More than 500 older individuals without cognitive impairment at baseline underwent yearly assessments that included a standard measure of the frequency of negative social interactions, cognitive testing, and clinical classification of MCI. We tested for the hypothesized associations of negative social interactions with development of MCI in proportional hazards models and with rate of cognitive decline in mixed-effects models.
METHODS
Participants
All data are based on individuals participating in the Rush Memory and Aging Project, an ongoing longitudinal cohort study initiated in 1997 (Bennett et al., 2005; Bennett et al., 2012). Presentations about the project were made at retirement homes, churches, subsidized housing complexes, and social service organizations in the Chicago metropolitan area. Interested individuals met with project staff for a more detailed discussion about participation and provided written informed consent. There were 3 eligibility criteria: age at baseline > 50, no diagnosis of dementia prior to enrollment, and agreement to yearly clinical examination sand brain autopsy at death. The institutional review board of Rush University Medical Center approved the study.
The present analyses are based on data collected between 2004, when the negative social interaction scale was added to the protocol of the Rush Memory and Aging Project, and 2014. In this period, 858 individuals completed the negative social exchange scale at least once. We excluded individuals who already had dementia (n= 63) or MCI (n=174), 12 persons who died before the first yearly follow–up visit, and 44 persons who had been enrolled for less than 1 year. Follow–up data were available for5 29 of the remaining 565 people (93.6 %). Their mean age at study baseline was 81.4 years (SD=7.1), their mean educational attainment was 15.0 years (SD=2.9), 78.9% were women. They were followed for a mean of 4.8 years (SD=2.5) and completed a mean of 5.53 cognitive assessments (SD=2.43, range: 2–9) with a mean of 0.06 assessments (SD=0.27, range: 0–3) missing for reasons other than death.
Clinical Evaluation
At annual intervals, participants had a uniform evaluation that contained a medical history, neurological examination, and assessments of cognition and other behaviors (Bennett et al., 2005; Bennett et al., 2012). Following each evaluation, a clinician who was unaware of previously collected data classified individuals with respect to MCI following previously reported procedures (Bennett et al., 2002; Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006). To minimize random variation in the diagnosis of MCI across time and clinicians, we developed an algorithm to rate impairment in 5 cognitive domains: orientation, attention, memory, language, and perception (Bennett, Schneider, Aggarwal, et al., 2006). For 11 cognitive measures, we specified cutoff scores for impairment at 4 education levels (Table 1 in Wilson, Boyle, Yang, James, & Bennett, in press) and rules for deriving cognitive domain ratings from the individual test ratings. The algorithmic results were compared to expert clinical judgment in a pilot test, the algorithm was revised and retested, and this process was repeated until agreement between the algorithm and clinician was maximized.
After each clinical evaluation, a neuropsychologist reviewed each algorithmic cognitive domain rating and either agreed with it or revised it. The neuropsychologist had access to all cognitive data, education, occupation, ratings of sensory and motor problems, and comments about deviations from test procedures, but not to age. Cognitive impairment was rated without reference to age because most late-life cognitive change reflects disease related (e.g., neurodegenerative and cerebrovascular conditions [Boyle et al., 2013]) and mortality related (i.e., terminal cognitive decline [Wilson, Beckett, Bienias, Evans, & Bennett, 2003]) factors rather than a maturational process.
Clinical classification of MCI was done by an experienced physician or nurse practitioner after review of the medical history, neurological examination, neuropsychological data, and cognitive domain impairment ratings and a brief evaluation of the participant. A diagnosis of MCI was made either if 1 cognitive domain was impaired or if 2 cognitive domains were impair ed, but there was insufficient evidence of a meaningful decline in cognitive function from a previously higher level to warrant a diagnosis of dementia. These MCI criteria have been shown to be associated with subsequent clinical (e.g., rate of cognitive decline [Bennett et al., 2002; Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006]) and neuropathological (e.g., neurofibrillary tangles [Bennett, Schneider, Arvanitakis, et al., 2006]) features of late-life dementia.
Assessment of Cognitive Function
Each year, 19 performance tests were administered by a research assistant in a session lasting about 60 minutes. Episodic memory measures included immediate and delayed recall of the East Boston Story (Albert et al., 1991; Wilson et al., 2002) and Story A from Logical Memory (Wechsler, 1987) and Word List Memory, Word List Recall, and Word List Recognition (Welsh et al., 1994). Semantic memory tests included a 15-item version of the Boston Naming Test (Welsh et al., 1994), a 15-item word reading test using words with atypical spelling sound correspondence, and a verbal fluency task requiring naming examples of animals and vegetables in 60strials (Welsh et al., 1994). Working memory was assessed with Digit Span Forward, Digit Span Backward, and Digit Ordering (Weschler, 1987; Wilson et al., 2002). Perceptual speed was assessed with the oral form of the Symbol Digit Modalities Test (Smith, 1982), a modified version (Wilson et al., 2002) of Number Comparison (Ekstrom, French, Harman, & Kermen, 1976), and 2 performance measures from a modified form (Wilson et al., 2005) of the Stroop Neuropsychological Screening Test (Trenerry, Crosson, Deboe, & Leber, 1989): number of words read in 30s minus errors and number of colors named in 30s minus errors. A 15-item form of Judgment of Line Orientation (Benton, Sivan, Hamsher, Varney, & Spreen, 1994) and a 16-item form of Standard Progressive Matrices (Raven, Court, & Raven, 1992) were used to assess visuospatial ability.
To maximize measurement precision, analyses are based on composite measures of 2 o r more individual tests. The primary outcome was a composite measure of global cognition based on all 19 tests. It required valid scores on a minimum of 6 of the 19 component tests. The 3, 042 global cognitive scores used in the present analyses were based on a mean of 18.5 valid component scores (SD =2.1, range: 6–19). Supported by factor analyses in this (Wilson, Barnes, & Bennett, 2003; Wilson et al., 2005) and other (Wilson et al., 2002; Wilson, Aggarwal, et al., 2009; Krueger, Wilson, Bennett, & Aggarwal, 2009) cohorts, we also constructed composite measures of episodic memory (based on 7 tests), semantic memory (3 tests), working memory (3 tests), perceptual speed (4 tests), and visuospatial ability (2 tests). Individual test scores were transformed to z scores, using raw score means and standard deviations from the initial evaluation, and the z scores of component tests were averaged to calculate the composite score. Previous publications provide more information about the individual tests and composite measures (Wilson et al., 2003; Wilson et al., 2005).
Assessment of Negative Social Interactions
We used a standard psychometrically established scale to assess frequency of negative social interactions (Newsom et al., 2003; Krause & Rook, 2003). On each of 12 items, the participant rated the frequency of a specific kind of social interaction in the last month on a 5-point scale from 1 (never) to 5 (very often). The scale addresses 4 domains of negative interaction with 3 items per domain: neglect or rejection by others (“In the past month, how often did the people you know leave you out of activities that you would have enjoyed”), others’ unwanted intrusion or advice (“… give you unwanted advice?”), failure by others to provide help (“…let you down when you needed help?”), and unsympathetic or insensitive behavior by others (“…act angry or upset with you?”). Scores for the total scale and each domain were obtained by averaging the item scores. In previous research, the scale has been shown to be internally consistent (Newsom et al., 2003; Krause & Rook, 2003; Sorkin & Rook 2008), temporally stable (Krause & Rook, 2003; Shaw, Krause, Liang, & Bennett, 2007), and related to subsequent health (Mavandadi et al., 2007; Newsom et al., 2008; Chiang, Eisenberger, Seeman, & Taylor, 2012) and well being (Rook, 1984; Newsom et al., 2003).
Assessment of Other Covariates
Social network size was the number of children, family, and friends interacted with at least monthly (Bennett, Schneider, Tang, et al., 200 6). Social activity was the mean frequency of participation during the past year in common social activities rated on a 5-point scale (Wilson et al., 2007). There were 6 activities: going to restaurants, sporting events, or bingo; going on day trips or overnight trips; doing unpaid community volunteer work; visiting at relatives’ or friends’ houses; participating in groups; attending church or religious services. Loneliness was assessed with a modified 5-item version (Wilson et al., 2007) of the De Jong-Gierveld Loneliness Scale (De Jong-Gierveld & Kamphuis, 1985). Depressive symptoms were measured with a 10-item form (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993) of the Center for Epidemiological Studies Depression scale (Radloff, 1977). The ability to cope with stress was assessed with the Vulnerability scale from the NEO Personality Inventory-Revised (Costa & McCrae, 1992). The 8 items of this facet of neuroticism were rated from 0 to 4 and item scores were summed to yield a total score (Wilson, Begeny, Boyle, Schneider, & Bennett, 2011). Negative life events were assessed with an 18-item scale modified from the Established Populations for Epidemiologic Studies of the Elderly version to include events particularly relevant to old age such as loss of a driver’s license or assuming care giving responsibilities (Murrel & Norris, 1984). Because these events occur relatively in frequently, this measure had modest internal consistency (Cronbach’s coefficient alpha = 0.51) and temporal stability (12 month test-retest correlations: baseline-year 1 r = 0.35; year 1-year 2 r =0.38; year 2-year3 r = 0.48; year 3-year 4 r= 0.44; year 4-year 5 r = 0.40, all p < 0.001). At baseline, it was positively correlated with depressive symptoms on the Center for Epidemiological Studies Depression scale (r= 0.21, p<0.001), supporting the validity of the measure.
Statistical Analysis
All analyses controlled for age, sex, and education. The association of negative social interaction score with risk of MCI was assessed in proportional hazards models. The initial model included a term for baseline negative social interaction score. We repeated the model excluding those with baseline age <65;adding a term for age x negative social interaction score; adding terms for depressive symptoms and aspects of social engagement; and adding terms for vulnerability and vulnerability x negative social interaction score. Subsequent analyses included terms for negative social interaction sub scores separately and then together. We used a cause- specific relative hazards model (Lau, Cole, & Gange, 2009) to simultaneously estimate the relation of negative social interaction score to 2 MCI subtypes. Change in negative social interaction score was assessed in a mixed-effects model, and a final fully adjusted proportional hazards model assessed the relation of mean negative social interaction score to MCI risk. The association of negative social interaction score with level of and change in cognitive function was estimated in mixed-effects models, with separate analyses for baseline interaction score and mean interaction score. The primary outcome was a composite measure of global cognition and subsequent analyses used measures of specific cognitive functions. The relation of negative life events to MCI risk was assessed in a proportional hazards model. A second model added a term for baseline negative social interaction score.
RESULTS
At the initial evaluation, negative social interactions cores ranged from 1.00 (indicating that none of the 12 negative interactions occurred in the past month) to 4.33 (indicating that the interactions were occurring often to very often), with a mean of 1.51 (SD= 0.43, skewness= 1.60). Higher negative social interaction score was associated with younger age (r= − 0.14, p<0.001) but was not related to education (r=0.04, p=0.376) or sex (t[209.4]=0.4, p =0.728). Cronbach’s coefficient alpha was 0.87, indicating that the scale is internally consistent, in line with prior research (Newsom et al., 2003; Krause & Rook, 2003; Sorkin & Rook, 2008).
Negative Social Interactions and Mild Cognitive Impairment
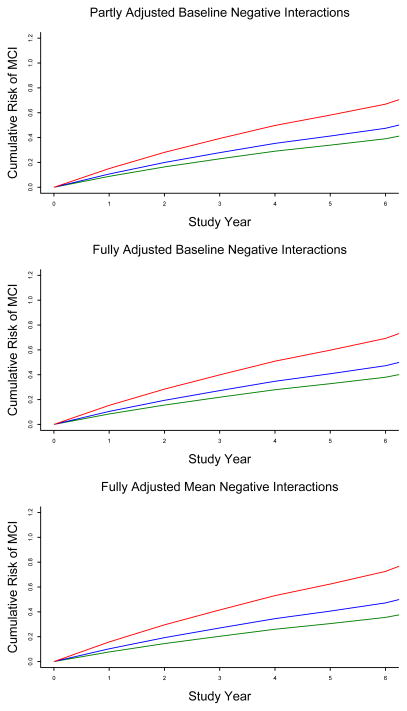
Participants were followed for a mean of 4.8 years (SD= 2.5, range: 0.7 – 8.1) during which 198 of them (37.4%) developed MCI. Those with incident MCI were older at baseline than those who remained cognitively healthy (83.4 vs 80.1, t[467.0]= 5.7, p<0.001) but did not differ in education (15.1 vs 14.9, t[527]= 0.7, p =0.499) or percent of men ( 21.7 vs 21.1, χ2[1] = 0.0, p = 0.877). In a proportional hazards model adjusted for age, sex, and education, higher frequency of negative social interactions at baseline was associated with higher risk of developing MCI (hazard ratio [HR]=1.53, 95% confidence interval [CI]: 1.13, 2.07). As shown in the upper portion of figure1, compared to a person reporting no negative interactions at baseline (score = 1.00, 10th percentile, green line), the MCI hazard ratio was approximately 20% higher with a negative social interactions core at the 50th percentile ( score = 1.42, blue line) and 58% higher with a score at the 90th percentile ( score = 2.08, red line).
Figure 1.
Cumulative risk of developing mild cognitive impairment in persons with low (green line), medium (blue line), or high (red line) levels of negative social interaction at baseline (upper and middle figures) or averaged across evaluations (lower figure), adjusted for age, sex, and education (each figure) and baseline level (middle figure) or mean level averaged across evaluations (lower figure) of depressive symptoms, social network size, social interaction frequency, and loneliness.
Five participants were under the age of 65 at baseline. To see if this subgroup affected the findings, we repeated the analysis with them excluded and results were not substantially changed (HR =1.63, 95% CI: 1.18, 2.26). In a subsequent analysis, there was an interaction between age and negative social interaction score (estimate = 0.060, SE = 0.029, p = 0.034) such that the association of negative social interaction with risk of developing MCI was stronger among older participants than younger ones.
Depressive symptoms (Barnes, Alexopoulos, Lopez, Williamson, & Yaffe, 2006) and social engagement (Wilson et al., 2007) have been associated with risk of mild cognitive impairment and were related to negative social interaction score at baseline. Therefore, we added terms to the initial model for baseline levels of depressive symptoms (mean = 0.93, SD = 1.43) and 3 indicators of social engagement: size of social network (mean = 6.67, SD=5.36), frequency of social interaction (mean = 2.64, SD=0.59), and loneliness (mean = 2.10, SD=0.63). The results of this analysis were virtually identical to the original model, with higher negative social interaction score continuing to predict subsequent development of MCI (HR = 1.58, 95% CI: 1.15, 2.18; middle portion of figure 1).
At baseline, participants completed a standard 8-item measure of vulnerability to stress (mean = 9.56, SD =4.20 ). To test whether stress vulnerability modified the relation of negative social interaction to risk of MCI, we repeated the initial proportional hazards model with terms added for vulnerability and its interaction with negative social interaction score. There was no interaction (estimate = − 0.010, SE = 0.028, p=0.724).
The negative social interaction scale assesses 4 types of interactions: neglect or rejection by others (mean = 1.49, SD = 0.53, skewness 1.70, range: 1.00–4.33), others’ unwanted intrusion (mean = 1.70, SD = 0.58, skewness 1.10, range: 1.00–5.00), others’ failure to provide help (mean = 1.42, SD = 0.52, skewness 1.70, range: 1.00–4.33), and unsympathetic behavior by others (mean = 1.49, SD = 0.55, skewness 1.58, range: 1.00–4.67). To assess whether the types of interactions were differentially related to risk of MCI, we examined the relation of each subscore to MCI, first in separate analyses and then in a single analysis (table 1). When separately analyzed, 3 of the subscores were at least marginally related to risk of MCI but when simultaneously analyzed, only neglect or rejection by others was related to MCI risk.
Table 1.
Relation of baseline levels of different types of negative social interaction to risk of mild cognitive impairment
| Negative interaction type | Separate Models | Single Model | ||
|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
| Neglect or rejection | 1.513 | 1.196, 1.914 | 1.523 | 1.121, 2.069 |
| Unwanted intrusion | 1.269 | 0.999, 1.610 | 1.076 | 0.792, 1.463 |
| Failure to help | 1.100 | 0.830, 1.460 | 0.728 | 0.505, 1.051 |
| Unsympathetic | 1.374 | 1.085, 1.740 | 1.218 | 0.868, 1.708 |
Note. Estimated from 5 proportional hazards models adjusted for age at baseline, sex, and education. CI, confidence interval.
We assessed whether negative social interactions were differentially related to amnestic versus nonamnestic MCI substypes in a cause-specific relative hazard model. In this analysis, negative social interactions were related to nonamnestic MCI (n=101; HR = 1.73, 95% CI: 1.13, 2.65) but not amnestic MCI (n=97; HR = 1.43, 95% CI: 0.90, 2.27).
Repeated Measurement of Negative Social Interactions
We constructed a mixed-effects model to see if the frequency of negative social interactions changed over time. There was no evidence of change (estimated change per year = − 0.003, SE=0.004, p=0.508). Because of the stability in the frequency of negative social interactions over time in this and previous (Krause & Rook, 2003; Shaw et al., 2007) longitudinal studies, we computed each person’s mean score across evaluations to capture the enduring disposition to be involved in negative social interactions (mean = 1.51, SD = 0.40, skewness = 1.42, range: 1.00–3.84). Higher score on this mean negative social interaction measure was robustly related to risk of MCI in a model adjusted for mean levels of covariates across the study period (HR=1.89, 95% CI:1.28, 2.79; lower portion of figure 1).
Negative Social Interactions and Cognitive Decline
Table 2 shows the cognitive scores at baseline in the 2 subgroups. To determine whether negative social interactions predictedMCI because of an association with level of cognit ive function, rate of cognitive decline, or both, we constructed a mixed-effects model. To make use of all cognitive data, the primary outcome was acomposite measure of global cognition based on all 19 individual tests (baseline mean = 0.340, SD = 0.411, skewness = 0.09, range: − 0.856 – 1.350). In this analysis, the global cognitive score declined a mean of 0.070-unit per year (SE = 0.006, p < 0.001). Higher negative social interaction score at baseline had an association with lower baseline cognitive score (estimate = − 0.096, SE = 0.034, p = 0.005) but not with rate of cognitive decline (estimate = − 0.018, SE = 0.011, p = 0.098). When the analysis was repeated using mean level of negative social interaction rather than baseline level, higher interaction score was robustly related to lower level of global cognition (estimate = − 0.154, SE=0.037, p<0.001) and faster rate of decline (estimate =− 0.036, SE=0.012, p=0.002).
Table 2.
Baseline cognitive scores in no cognitive impairment and incident mild cognitive impairment subgroups
| Cognitive Measure | No Cognitive Impairment (n=331) | Incident Mild Cognitive Impairment (n=198) |
|---|---|---|
| Immediate story recall | 10.0 (1.7) | 9.4 (1.8) |
| Delayed story recall | 9.9 (1.7) | 9.1 (1.8) |
| Logical Memory Ia | 13.7 (3.8) | 12.1 (4.0) |
| Logical Memory IIa | 12.6 (3.9) | 10.8 (4.3) |
| Word List Memory | 20.0 (4.1) | 18.4 (3.9) |
| Word List Recall | 6.8 (1.9) | 5.8 (1.9) |
| Word List Recognition | 9.9 (0.3) | 9.8 (0.4) |
| Boston Naming Test | 14.4 (0.9) | 14.0 (1.1) |
| Verbal Fluency | 37.1 (8.3) | 33.2 (9.0) |
| Word reading test | 13.2 (2.4) | 12.9 (2.6) |
| Digit Span Forward | 8.7 (1.9) | 8.3 (1.9) |
| Digit Span Backward | 6.7 (2.0) | 6.1 (2.0) |
| Digit Ordering | 7.5 (1.5) | 7.2 (1.6) |
| Symbol Digit Modalities Test | 42.4 (8.9) | 36.7 (9.5) |
| Number Comparison | 26.5 (7.0) | 24.2 (7.4) |
| Stroop word reading | 51.4 (11.5) | 48.2 (13.6) |
| Stroop color naming | 21.6 (6.4) | 17.5 (7.7) |
| Judgment of Line Orientation | 10.9 (2.8) | 10.3 (2.7) |
| Standard Progressive Matrices | 10.9 (1.7) | 10.1 (1.9) |
| Mini–Mental State Examination | 28.8 (1.4) | 28.3 (1.6) |
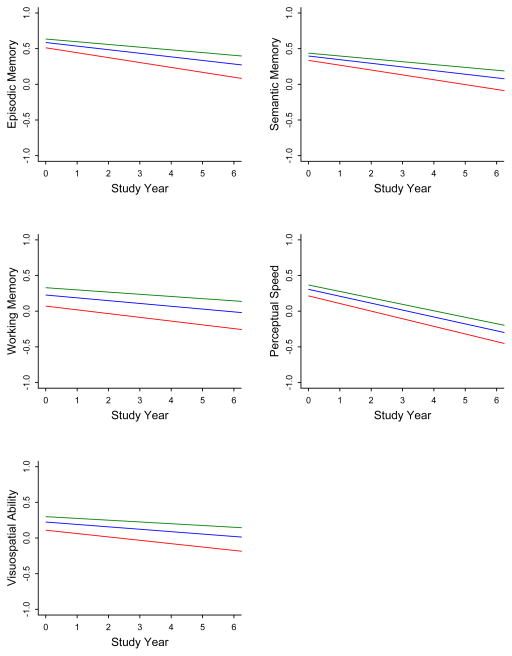
Because prior research has suggested that negative social interactions are related to some cognitive functions more than others (Seeman et al., 2011; Tun et al., 2013), we examined the association of baseline and mean levels of negative social interaction score to paths of change in different cognitive domains (Table 3). Higherbase line negative social interaction score was associated with lower levels of working memory and visuospatial ability at baseline but not with decline in any domain. Bycontrast, higher mean negative social interaction score was associated with lower level of function in all domains and more rapid decline in episodic, semantic, and working memory.
Table 3.
Relation of baseline and mean levels of negative social interaction to level of and change in cognitive abilities
| Cognitive outcome | Model term | Baseline NSI | Mean NSI | ||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | ||
| Episodic memory | Time | − 0.061 | 0.007 | <0.001 | − 0.061 | 0.007 | <0.001 |
| NSI | − 0.081 | 0.043 | 0.062 | − 0.128 | 0.047 | 0.007 | |
| NSI x time | − 0.014 | 0.014 | 0.333 | − 0.032 | 0.015 | 0.035 | |
| Semantic memory | Time | − 0.059 | 0.006 | <0.001 | − 0.059 | 0.006 | <0.001 |
| NSI | − 0.050 | 0.044 | 0.257 | − 0.102 | 0.048 | 0.032 | |
| NSI x time | − 0.010 | 0.011 | 0.386 | − 0.029 | 0.012 | 0.015 | |
| Working memory | Time | − 0.045 | 0.006 | <0.001 | − 0.045 | 0.006 | <0.001 |
| NSI | − 0.175 | 0.062 | 0.005 | − 0.271 | 0.067 | <0.001 | |
| NSI x time | − 0.013 | 0.011 | 0.207 | − 0.023 | 0.012 | 0.044 | |
| Perceptual speed | Time | − 0.110 | 0.006 | <0.001 | − 0.109 | 0.006 | <0.001 |
| NSI | − 0.085 | 0.060 | 0.156 | − 0.155 | 0.065 | 0.018 | |
| NSI x time | − 0.011 | 0.012 | 0.352 | − 0.017 | 0.013 | 0.164 | |
| Visuospatial ability | Time | − 0.038 | 0.006 | <0.001 | − 0.038 | 0.006 | <0.001 |
| NSI | − 0.127 | 0.058 | 0.029 | − 0.193 | 0.063 | 0.002 | |
| NSI x time | − 0.012 | 0.011 | 0.300 | − 0.023 | 0.012 | 0.063 | |
Note. Estimated from 10 mixed–effects models adjusted for age at baseline, sex, and education. NSI, negative social interaction; SE, standard error.
Negative Life Events
At baseline, participants reported experiencing a mean of 2.60 negative life events in the previous year (SD=1.89) and negative life events were correlated with negative social interactions (r = 0.20, p <0.001). However, negative life events were not related to subsequent risk of MCI (HR = 1.05, 95% CI: 0.97, 1.13) and controlling for them did not affect the association of negative social interactions with MCI (HR = 1.49, 95% CI: 1.09, 2.04).
DISCUSSION
More than 500 older persons who were cognitively healthy at enrollment underwent annual evaluations that included assessment of negative social interactions, cognitive function, and MCI. During a mean of 4.8 years of observation, higher level of negative social interactions was associated with higher incidence of MCI and more rapid cognitive decline. The results indicate that chronic negative social interactions may be a risk factor f orMCI and cognitive decline in old age.
There has been little evidence of an association between negative social interactions and cognitive decline in previous longitudinal studies (Seeman et al., 2001; Hughes et al., 2008; Windsor et al., 2014). However, cognition was only assessed twice in 2 of these studies (Seeman et al., 2001; Hughes et al., 2008), making it difficult to separate level of cognitive function from rate of change. A third study assessed cognition at 3 time points but excluded those with impairment at any point (Windsor et al., 2014), truncating variability in cognitive slopes and making it difficult to identify factors related to individual difference in slopes. Another issue is that participants in the previous studies were younger than participants in the present study by a mean of 7 to 19 years at baseline (Seeman et al., 2001; Hughes et al., 2008; Windsor et al., 2014). Because there was probably less cognitive decline in these younger participants and because the present data show thatthe association is stronger at older compared to younger ages, the previous studies were not well positioned to observe an association between negative social interactions and cognitive decline. The present results not only suggest that negative social interactions are associated with late-life cognitive decline but also underscore the clinical importance of this association by showing that they also predict development of MCI, widely recognized as the precursor of dementia.
Acute exposure to daily stressors has been shown to have a selective association with cognitive function, with higher exposure related to impairment in working memory but not in tasks with minimal working memory demand (Sliwinski, Smyth, Hofer, & Stawski, 2006), suggesting that acute exposure to daily stressors drains attentional resources. In the present study, frequency of negative social interactions did not change over time, and previous studies have found no change (Krause & Rook, 2003; Shaw et al., 2007) or a slight decrease (Windsor et al., 2014). The stability of negative social interaction rates across time and types of relationships (Krause & Rook, 2003) suggests that the disposition to be involved in interpersonal conflict or to interpret daily social interactions in a negative manneris a relatively stable trait. In old age, therefore, individual differences in this disposition likely represent substantial cumulative differences over time in daily stress exposure. We found that higher exposure to negative social interactions during the course of the study was associated with lower initial level of working memory and more rapid working memory decline. However, this indicator of chronic daily relationship stress was also related to level of function and rate of decline in multiple other cognitive domains (Table 3). This mixed support for the attention–depletion hypothesis (Sliwinski et al., 2006) is consistent with previous epidemiologic research, with some evidence linking negative social interaction with lower performance on attention demanding tasks (Seeman et al., 2011; Tun et al., 2013) but other cross-sectional (Hughes et al., 2008; Seeman et al., 2001; Seeman et al., 2011; Okabayashi et al., 2004; Windsor et al., 2014) and longitudinal (Seeman et al., 2001; Hughes et al., 2008; Windsor et al., 2014) data suggesting no association or an association with other cognitive domains. Thus, chronic exposure to daily stressors may have a less selective association with cognition than acute exposure.
The factors underlying the association of negative social interactions with cognitive impairment and decline are not clear. The temporal stability of these interactions makes it unlikely that they are an early manifestation of cognitive aging. The association persisted after adjustment for related factors such as depressive symptoms, social engagement, and negative life events. There was no evidence that the association was due to stress sensitivity in a subset of participants. Daily relationship stress has been associated with physiologic changes with the potential to impair cognition, including higher levels of cortisol (Van Eck, Berkhof, Nicholson, & Sulon, 1996; Stawski, Cichy, Piazza, & Almeida, 2013) and proinflammatory cytokine activity (Kiecolt-Glaser et al., 2005; Chiang et al., 2012; Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012) and more rapid progression of atherosclerosis (Everson et al., 1997; Kamarck, Shiffman, Sutton-Tyrrell, Muldoon, & Tepper, 2012). Regardless of the mechanisms linking chronic psychosocial stress to cognition, the present results suggest that interventions targeting how individuals manage and appraise daily stress (Khoury et al., 2013; Hofmann et al., 2012) may benefit cognitive health in old age.
This study has notable strengths and limitations. The diagnosis of MCI was based on a uniform clinical evaluation and widely accepted criteria applied by experienced clinicians, minimizing diagnostic misclassification. The high rate of participation in follow-up makes it unlikely that selective attrition biased results. The temporal instability of MCI and its subtypes is a study limitation, but because the diagnosing clinician was blinded to previously collected data, incident MCI and change in cognitive functions were independent outcomes, and the consistency of results across these outcomes suggests that study findings are reliable. Another limitation is that the cohort is selected and so it will be important to replicate these results in other groups. In addition, there was not a lot of cognitive decline during the relatively brief follow-up period, and this likely limited our power to detect associations of baseline negative social interaction with cognitive decline and of negative life events with cognitive outcomes.
Figure 2.
Predicted 5-year paths of change indifferent cognitive abilities in persons with low (green line), medium (blue line), or high (red line) levels of negative social interaction averaged across evaluations, adjusted for age, sex, and education.
Acknowledgments
The authors thank the Rush Memory and Aging Project participants; Traci Colvin, MPH, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; and Donna Esbjornson, MS, and Liping Gu, MS, for statistical programming.
References
- Albert M, Scherr P, Taylor J, Evans D, Funkensten H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International Journal of Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Almelda DM. Resilience and vulnerability to daily stressors assessed via diary methods. Current Directionsin Psychologcial Science. 2005;14:62–68. [Google Scholar]
- Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Archives of General Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias LL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurology. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, de S, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2. New York: Oxford University Press; 1994. [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of daily stress on negative mood. Journal of Personality and Social Psychology. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer's disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Annals of Neurology. 2013;74:478–489. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proceedings of the National Academy of SciencesUSA. 2012;109:1878–1882. doi: 10.1073/pnas.1120972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. NEO Personality Inventory-Revised. Lutz, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- De Jong-Gierveld J, Kamphuis F. The development of a Rasch-type loneliness scale. Applied Psychological Measurement. 1985;5(9):289–299. [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Kermen D. Manual for kit of factor- referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Everson SA, Lynch JW, Chesney MA, Kaplan GA, Goldberg DE, Shade SB, Cohen RD, Salonen R, Salonen JT. Interaction of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis: population based study. British Medical Journal. 1997;314:553–558. doi: 10.1136/bmj.314.7080.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J, Zautra AJ. Testing latent longitudinal model of social ties and depression among the elderly. Psychology and Aging. 1992;7:107–118. doi: 10.1037/0882-7974.7.1.107. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology. 2012;31:264, 268. doi: 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive Therapy Research. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TF, Andel R, Small BJ, Borenstein AR, Mortimer JA. The Association between social resources and cognitive change in older adults: evidence from the Charlotte County Healthy Aging Study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2008;63:241–244. doi: 10.1093/geronb/63.4.p241. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Hällström T, Norton MC, Waern M, Östling S, Bengtsson C, Skoog I. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: a 38-year longitudinal population study. British Medical Journal Open. 2013;3:e003142. doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarck TW, Shiffman S, Sutton-Tyrrell K, Muldoon MF, Tepper P. Daily psychological demands are associated with 6-year progression of carotid artery atherosclerosis: the Pittsburgh Healthy Heart Project. Psychosomatic Medicine. 2012;74:432–439. doi: 10.1097/PSY.0b013e3182572599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, Chapleau MA, Paquin K, Hofmann SG. Mindfulness-based therapy: a comprehensive meta–analysis. Clinical Psychology Review. 2013;33:763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Kiecolt–Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health. 1993;5:79–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Krause N, Rooks KS. Negative interaction in late life: issues in the stability and generalizability of conflict across relationships. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58:88–99. doi: 10.1093/geronb/58.2.p88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Bennett DA, Aggarwal NT. A battery of tests for assessing cognitive function in older Latino persons. Alzheimer Disease Association Disorder. 2009;23:384–388. doi: 10.1097/WAD.0b013e31819e0bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Coles SR, Gange SJ. Competing risk regression models for epidemiologic data. American Journal of Epidemiology. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavandadi S, Rook KS, Newsom JT. Positive and negative social exchanges and disability in later life: an investigation of trajectories of change. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:361–370. doi: 10.1093/geronb/62.6.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavandadi S, Sorkin DH, Rook KS, Newsom JT. Pain, positive and negative social exchanges, and depressive symptomatology in later life. Journal of Aging and Health. 2007;7:813–830. doi: 10.1177/0898264307305179. [DOI] [PubMed] [Google Scholar]
- Murrell SA, Norris FH. Resources, life events, and changes in positive affect and depression in older adults. American Journal of Community Psychology. 1984;12:445–464. doi: 10.1007/BF00896505. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Mahan TL, Rook KS, Krause N. Stable negative social exchanges and health. Health Psychology. 2008;27:78–86. doi: 10.1037/0278-6133.27.1.78. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Nishishiba M, Morgan DL, Rook KS. The relative importance of three domains of positive and negative social exchanges: a longitudinal model with comparable measures. Psychology and Aging. 2003;18:746–754. doi: 10.1037/0882-7974.18.4.746. [DOI] [PubMed] [Google Scholar]
- Okabayashi H, Liang J, Krause N, Akiyama H, Sugisawa H. Mental health among older adults in Japan: do sources of social support and negative interaction make a difference? Social Science & Medicine. 2004;59:2259–2270. doi: 10.1016/j.socscimed.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Persson G, Skoog I. A prospective population study of psychosocial risk factors for late onset dementia. International Journal of Geriatric Psychiatry. 1996;11:15–22. [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary: Standard Progressive Matrices. Oxford, England: Oxford Psychologists Press; 1992. [Google Scholar]
- Rook KS. The negative side of social interaction: impact on psychological well-being. Journal of Personality and Social Psychology. 1984;46:1097–1108. doi: 10.1037//0022-3514.46.5.1097. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology. 2001;20:243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Miller-Martinez DM, Merkin SS, Lachman ME, Tun PA, Karlamangla AS. Histories of social engagement and adult cognition: midlife in the U.S. study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66:141–152. doi: 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BA, Krause N, Liang J, Bennett J. Tracking changes in social relations throughout late life. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:90–99. doi: 10.1093/geronb/62.2.s90. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Smyth JM, Hofer SM, Stawski RS. Intraindividual coupling of daily stress and cognition. Psychology and Aging. 2006;21:545–557. doi: 10.1037/0882-7974.21.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test manual-revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Sorkin DH, Rook KS. Interpersonal control strivings and vulnerability to negative social exchanges in later life. Psychology and Aging. 2004;19:555–564. doi: 10.1037/0882-7974.19.4.555. [DOI] [PubMed] [Google Scholar]
- Stafford M, McNunn A, Zaninotto P, Nazroo J. Positive and negative exchanges in social relationships a s predictors of depression: evidence from the English Longitudinal Study of Aging. Journal Aging and Health. 2011;23:607–628. doi: 10.1177/0898264310392992. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Cichy KE, Piazza JR, Almeida DM. Associations among daily stressors and salivary cortisol: findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. The Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Tun PA, Miller–Martinez D, Lachman ME, Seeman T. Social strain and executive function across the lifespan: the dark (and light) sides of social engagement. Aging, Neuropsychology, and Cognition. 2013;20:320–338. doi: 10.1080/13825585.2012.707173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part V: a normative study of the neuropsychologic al battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer’s disease. Archives of Neurology. 2009;66:767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. American Journal of Geriatric Psychiatry. 2011;19:327–334. doi: 10.1097/JGP.0b013e31820119da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yang J, James BD, Bennett DA. Early life instruction in foreign language and music and incidence of mild cognitive impairment. Neuropsychology. doi: 10.1037/neu0000129. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer’s disease. Archives of General Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Windsor TD, Gerstorf D, Pearson E, Ryan LH, Anstey KJ. Positive and negative social exchanges and cognitive aging in young-old adults: differential associations across family, friend, and spouse domains. Psychology and Aging. 2014;29:28–43. doi: 10.1037/a0035256. [DOI] [PubMed] [Google Scholar]