Abstract
Executive functions (EF) are a complex set of neurodevelopmental, higher-ordered processes that are especially salient during adolescence. Disruptions to these processes are predictive of psychiatric problems in later adolescence and adulthood. The objectives of the current study were to characterize the latent structure of EF using bifactor analysis and to investigate the independent and interactive effects of genes and environments on EF during adolescence. Using a representative young adolescent sample, we tested the interaction of a polymorphism in the serotonin transporter gene (5-HTTLPR) and parental supervision for EF through hierarchical linear regression. To account for the possibility of a hierarchical factor structure for EF, a bifactor analysis was conducted on the eight subtests of the Delis-Kaplan Executive Functions System (D-KEFS). The bifactor analysis revealed the presence of a general EF construct and three EF subdomains (i.e., conceptual flexibility, inhibition, and fluency). A significant 5-HTTLPR by parental supervision interaction was found for conceptual flexibility, but not for general EF, fluency or inhibition. Specifically, youth with the L/L genotype had significantly lower conceptual flexibility scores compared to youth with S/S or S/L genotypes given low levels of parental supervision. Our findings indicate that adolescents with the L/L genotype were especially vulnerable to poor parental supervision on EF. This vulnerability may be amenable to preventive interventions.
Keywords: Executive functions, Bifactor model, Adolescence, Gene-environment interaction, 5-HTTLPR, Parental supervision
INTRODUCTION
Adolescence is a critical period for cognitive and emotional development, particularly for executive functioning (EF; Crone, 2009), which are neurocognitive processes that regulate and maintain higher-order actions and goal oriented behaviors (Barkley, 1997). During adolescence, typically developing youth improve in their abilities to regulate and plan their actions and thoughts (Huizinga, Dolan, & Van der Molen, 2006). The degree of maturation in adolescent regulatory abilities is thought to reflect neurobiological development and influences risk behaviors, including disruptive behavior (Hobson, Scott & Rubia, 2011; Matthys, Vanderschuren, & Schutter, 2013) and substance use disorders (Clark et al., 2013; Giancola & Tarter, 1999). Adolescent development is also strongly influenced by environmental factors, such as parenting behaviors (Clark, Thatchere, & Maisto, 2004; Clark, Kirisci, Mezzich, & Chung, 2008) and deviant peers (Huizinga et al., 2006). However, relatively little is known about how environmental and heritable factors interact to influence EF during this developmental epoch.
Regarding the taxonomy of EF, a tripartite framework has been proposed (Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001), consisting of three distinct but moderately correlated factors. These dimensions include set-shifting [i.e., the ability to shift back and forth between multiple tasks, operations or mental sets (Monsell, 1996)], updating and monitoring [i.e., the ability to monitor and code information relevant to the task and manipulate the information appropriately when new information is provided; also similar to working memory (Goldman-Rakic, 1996)], and inhibition [i.e., the ability to deliberately suppress a dominant response in the presence of a nonessential stimuli (Logan, Schachar, & Tannock, 1997)]. However, emerging research suggests that the factor structure of EF may vary by age, particularly across childhood and adolescence (Huizinga et al., 2006; Lee, Bull, & Ho, 2013; Prencipe et al., 2011; Zelazo, Craik, & Booth, 2004). A two factor structure, representing inhibition and switching, was the best fit to the data during early to late childhood, but a three factor model, representing inhibition, updating, and switching, became the best fit to the data during adolescence (Lee et al., 2013). Prencipe and colleagues (2011) distinguished between “hot” (i.e., motivationally salient) and “cool” (i.e., abstract) EF tasks in a typically developing sample between 8 and 15 years of age and found that improvements in cool EF tasks (i.e., Color-Word Stroop, Backward Digit Span) began during the earlier aged cohorts, whereas improvements in “hot” tasks (i.e., gambling task, delay-discounting) developed more gradually and were most robust in the adolescent cohort. However, in their exploratory factor analysis for all tasks, a single factor model emerged as the best fit to the data. This suggests that the factor structure of EF may be organized hierarchically, such that the covariation among EF components may be modeled as a single latent factor (Alarcón, Plomin, Fulker, Corley, & DeFries, 1998; Friedman et al., 2008), whereas each sub-dimension of EF may be defined by unique genetic and environmental pathways.
Twin studies have established the important role of genetic influences for variation in EF, with heritability estimates for inhibition, set-shifting, and monitoring/working memory ranging from 43% to 77% (Ando, Ono, & Wright, 2001; Coolidge, Thede, & Young, 2000; Kuntsi et al., 2006). While the search for specific genes associated with EF have been elusive, one particular candidate system with implications for EF is serotonin (5-HT; see Logue & Gould, 2014). The role of 5-HT in the development of EF is partly related to the expression of 5-HT in the prefrontal cortex (PFC; Puig & Gulledge, 2011), a region of the brain that is known to regulate higher order functions such as learning, working memory, and behavioral flexibility (Fuster, 2001; also see Blakemore & Choudhury, 2006). Serotonergic receptors are largely expressed in the PFC, which regulate 5-HT activity (Enge, Fleischhaauer, Lesch, Reif, & Strobel, 2011). Variations in extracellular 5-HT in the PFC have been associated with performance in response inhibition, reversal learning tasks and other EF tasks across human (Cools, Roberts, & Robbins, 2008; Crean, Richards, & de Wit, 2002) and nonhuman primate models (Homberg et al., 2007; Walker, Mikheenko, Argyle, Robbins, & Roberts, 2006), although associations with set-shifting abilities have been equivocal (Logue & Gould, 2014). Given the primacy of 5-HT regulation and EF performance in general, the functional polymorphism in the promoter region of the 5-HT transporter gene (5-HTTLPR) is a plausible candidate for EF, as the short (S) allele is known to convey reduced 5-HT transporter transcription (i.e., lower transporter levels) and subsequently reduced 5-HT reuptake than the long (La) allele (Hu et al., 2006). The A > G single nucleotide polymorphism (SNP) has also been identified within the L allele and is functionally similar to the S allele (Hu et al., 2006).
Genetic association studies have shown a link between the S allele and increased sensitivity to stress and higher risk for depression (see meta-analysis by Karg, Burmeister, Shedden, & Sen, 2011), but better performance on EF (Weikum et al., 2013). However, it is unclear whether 5-HTTLPR functionality is specific to any single domain of EF, or whether it is generally associated with EF performance. For example, a meta-analysis of youth with attention-deficit/hyperactivity disorder found an association between the L/L genotype and worse performance on measures of impulsivity, inattention, and working memory (Gizer, Ficks, & Waldman, 2009). Youth with the L/L genotype performed worse than non-L/L youth on EF tasks when their mothers endorsed high levels of depression symptoms, although they were also better than non-L/L youth on these tasks when their mothers endorsed few depression symptoms (Weikum et al., 2013). Adults carrying the L/L genotype performed worse on a tasks of risky decision making and visual planning (Roiser, Rogers, Cook, & Sahakian, 2006), set-shifting (Borg et al., 2009) and inhibition (Roiser et al., 2006) compared to individuals without this genotype. Taken together, these findings suggest that allelic variation in 5-HTTLPR may also be associated with EF performance (Weikum et al., 2013). However, more research is needed to disentangle the possibility of specific 5-HTTLPR effects as they relate to the various dimensions of EF.
Genetic influences for complex phenotypes are also widely believed to act in conjunction with environmental factors (i.e., gene-environment interaction; GxE), whereby genetic influences on a phenotype may be enhanced or attenuated as a function of the environment (or vice versa). An abundance of studies have examined GxE effects involving 5-HTTLPR and harsh or severe parenting, including for depression (Gibb, Uhrlass, Grassia, Benas, & McGeary, 2009; Kaufman et al., 2004), aggression (Li & Lee, 2010; Reif et al., 2007), and attention-deficit/hyperactivity disorder (Retz et al., 2008). However, GxE studies for EF have yet to emerge. One particular environmental factor that may moderate the association between 5-HTTLPR and EF is parental supervision (i.e., knowledge of child’s whereabouts, availability). Parental supervision is a critical component of adolescent development, given its association with socioemotional (Li, Berk, & Lee, 2013; Wang, Pomerantz, & Chen, 2007), behavioral (Clark, Thatcher, & Maisto, 2005; Dishion & McMahon, 1998), and academic achievement (e.g., Li, Walker, & Armstrong, 2014; Soenens, Vansteenkiste, Luyckx, & Goossens, 2006) outcomes. The extant literature on parental supervision and adolescent cognitive and academic achievement has been mixed, as although some studies have found an association between higher parental supervision and better performance (e.g., Rankin & Quane, 2002), others have found null or inverse associations with performance (e.g., Li et al., 2014; Weiss & Schwarz, 1996). Despite evidence suggesting a role of parenting on the development of EF and related phenotypes, studies regarding the potential interplay of 5-HTTLPR genotype and EF are lacking.
The aims of this study were to elucidate the latent architecture of EF and to investigate the interplay of 5-HTTLPR and parental supervision on EF in adolescents. A hierarchical three-factor structure for EF was predicted, characterized by dimensions corresponding to those reported by previous studies (i.e., inhibition, working memory, and set-shifting; Friedman et al., 2008; Miyake et al., 2001), as a well as a higher-order general factor that would account for the covariation among the dimensions. Youth exposed to low parental supervision were predicted to have worse EF performance compared to youth reporting comparably higher parental supervision. In line with recent GxE findings (e.g., Weikum et al., 2013), it was also predicted that individuals carrying the L/L genotype would be more sensitive to environmental influences, such that youth carrying the L/L genotype would perform worse on EF in the presence of poor parental supervision compared to youth with the S/S or S/L genotypes.
METHOD
Participants
Adolescent participants (N = 142; ages 12–15 years) were recruited from the Pittsburgh area. All participants were a representative sample stratified by year of birth, sex, and race-ethnicity. Among these participants, genotype data were available for 116. All descriptive data can also be found in Table 1. Adolescents were identified through a neighborhood-based targeted random dialing telephone procedure. Successfully contacted families were screened for eligibility by staff at the University Center for Social and Urban Research (UCSUR) at the University of Pittsburgh. Eligible participants and their parents completed informed consent, a psychological assessment, and DNA collection. Written informed consent was obtained in person from a parent and assent from the adolescent before conducting any of the study procedures. The study protocol was approved by the university’s Institutional Review Board.
Table 1.
Demographic information and D-KEFS mean scores by 5-HTTLPR genotype
| L/L | S/S + S/L | Test statistic | p | Cohen’s d | |
|---|---|---|---|---|---|
| N* | 28 | 88 | — | — | — |
| Male (n) | 14 | 46 | χ2(1) = .27 | .61 | — |
| Mean age (SD) | 14.11 (1.21) | 14.19 (1.28) | t = .51 | .66 | .09 |
| Racial-ethnic groups (n) | — | — | χ2(3) = 1.02 | .80 | — |
| European-American | 21 | 62 | — | — | — |
| African-American | 8 | 27 | — | — | — |
| Other | 2 | 4 | — | — | — |
| Parental education (n) | — | — | χ2(4) = 5.32 | .26 | — |
| GED | 4 | 10 | — | — | — |
| Partial college | 5 | 20 | — | — | — |
| College graduate | 5 | 19 | — | — | — |
| Partial graduate school | 13 | 21 | — | — | — |
| Masters level degree | 4 | 23 | — | — | — |
| Doctoral level degree | 0 | 0 | — | — | — |
| Mean LQAS composite score (SD) | 10.91 (1.25) | 10.62 (1.84) | t = .94 | .35 | .18 |
| Mean D-KEFS scaled scores (SD) | — | — | — | — | — |
| Trails | 9.52 (2.75) | 9.97 (3.15) | t = .75 | .45 | .14 |
| VF letter | 9.37 (1.86) | 9.42 (2.47) | t = .09 | .93 | .02 |
| VF category | 9.87 (2.99) | 10.23 (3.13) | t = .57 | .57 | .10 |
| VF switch | 9.71 (2.83) | 9.74 (3.70) | t = .05 | .96 | .01 |
| VF accuracy | 10.26 (2.56) | 10.48 (3.17) | t = .40 | .69 | .07 |
| Design Fluency | 10.67 (3.97) | 11.13 (3.31) | t = .73 | .47 | .13 |
| CW inhibition | 9.86 (2.88) | 10.65 (3.26) | t = 1.27 | .21 | .23 |
| CW switch | 9.04 (3.28) | 10.65 (2.47) | t = 2.49 | .01 | .45 |
| Sorting correct | 9.31 (2.69) | 10.00 (3.00) | t = 1.20 | .23 | .22 |
| Sorting description | 9.37 (2.74) | 9.90 (3.00) | t = .92 | .36 | .17 |
| Sorting recognition | 8.92 (3.47) | 10.06 (3.56) | t = 1.58 | .12 | .29 |
| Twenty Questions | 11.33 (2.09) | 11.48 (2.91) | t = .33 | .74 | .06 |
| Word Context | 10.57 (3.08) | 10.81 (3.17) | t = .37 | .71 | .07 |
| Tower | 10.55 (1.95) | 10.58 (2.20) | t = .08 | .94 | .01 |
| Mean D-KEFS factor scores (SD) | — | — | — | — | — |
| Fluency | −0.09 (2.52) | −0.02 (2.82) | t = .13 | .90 | .02 |
| Inhibition | −0.05 (.70) | 0.21 (.57) | t = 1.90 | .06 | .34 |
| Conceptual flexibility | 0.03 (2.28) | 0.07 (1.95) | t = .09 | .93 | .02 |
| General factor | −0.07 (1.41) | 0.28 (1.73) | t = 1.11 | .27 | .20 |
Note. Categorical variables were compared using Pearson’s chi-squared tests, continuous variables were compared using an independent samples t-test; VF = Verbal Fluency; CW = Color-Word.
Genotypic information was not available on the full sample (n = 116 out of 142).
Genotyping
We extracted DNA from saliva using a mouthwash protocol (King et al., 2002). Samples were subjected to whole genome amplification using the genomiphi protocol (Dean et al., 2002), quantified by the PicoGreen protocol, and diluted to 40 ng/μL for storage. A polymerase chain reaction protocol followed by double restriction endonuclease digestion was used to identify the 5-HTTLPR and rs25531 variants: S, La, and Lg (Wendland, Martin, Kruse, Lesch, & Murphy, 2006). The primer sequences were: (forward) 5′-TCCTCCGCTTTGGCGCCTCTTCC-3′, and (reverse) 5′-TGGGGGTTGCAGGGGAGATCCTG-3′. The L allele was subtyped for rs25531. The A > G SNP of rs25531 was concurrently detected by digesting the amplified fragments with MspI (New England Biolabs, Beverly, MA), where the A > G substitution creates an additional MspI site. Amplification products were simultaneously resolved by electrophoresis on 3.5% agarose gels. The La variant (528 bp) has approximately three times the basal activity of the S promoter (484 bp) with the deletion (Lesch et al., 1996).
The genotype distribution for the available sample was: S/S (16.4%; n = 19), S/Lg (10.3%; n = 12), Lg/Lg (1.7%; n = 2), (12.1%; n = 14) S/La (35.3%; n = 41), and La/La (24.1%; n = 28). Because the rare Lg and S allele are functionally equivalent, we combined the rs24431 SNP and 5-HTTLPR polymorphism so that the variable had three levels: (1) “S/S,” which includes S/S and S/Lg genotypes, (2) “S/L,” which includes S/La and Lg/La genotypes, and (3) “L/L,” which includes the La/La genotype. Following empirical precedent (Greenberg et al., 1999; Little et al., 1998), we dummy coded 5-HTTLPR genotype where individuals carrying at least one copy of the low transcription alleles (i.e., S/S and S/L) were coded 0 and individuals carrying zero low transcription alleles (i.e., L/L) were coded 1. Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium (χ2 = .17; df = 1).
Measures
Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001)
The D-KEFS is a standardized neuropsychological assessment protocol with excellent psychometric properties. We used eight subtests of the D-KEFS: (1) Trail Making (attention, conceptual flexibility), (2) Verbal Fluency (processing speed, lexical organization), (3) Design Fluency (nonverbal processing speed), (4) Color-Word Interference (response inhibition, conceptual flexibility), (5), Sorting (conceptual flexibility), (6) Twenty Questions (deductive reasoning, working memory), (7) Word Context (deductive reasoning, conceptual flexibility, working memory), and (8) Tower (planning). Each test is described in greater detail in the D-KEFS manual (Delis et al., 2001). In line with previous studies and empirical precedent (i.e., Delis et al., 2001; Latzman & Markon, 2010), we used the D-KEFS Total Achievement Scaled scores (i.e., mean = 10; SD = 2) for our analyses. Means, standard deviations and effect sizes are presented in Table 1.
Loeber Youth Questionnaire, Supervision Subscale (LYQ; Jacob, Moser, Windle, Loeber, & Stouthamer-Loeber, 2000)
The 58-item LYQ was completed by the adolescent. We used the supervision subscale, which consists of four items including (1) whether parents know where and (2) with whom he/she is with when away from home, (3) when he/she will return, and (4) whether he/she would be able to contact the parents when the parents are away from home. Adolescents responded to these questions by selecting the frequency of these items: “almost never,” “sometimes,” “almost always,” and “does not apply.” Psychometric properties of the LYQ have been described elsewhere (Loeber, Farrington, Stouthamer-Loeber, & Van Kammen, 1998). The internal consistency (Cronbach’s alpha) of the scale in the current sample was adequate (.71).
Statistical Analyses
Analyses were conducted in Mplus 6.11 (Muthén & Muthén, 2010) using the full sample (N = 142). In the first step, we established the optimal factor structure for the D-KEFS by conducting an exploratory factor analysis (EFA) with correlated factors (i.e., oblimin rotation) on the full correlation matrix using maximum likelihood estimation (see Satorra, 2003). A scree plot was examined for visual inspection of the best fitting factor structure. The Bayesian Information Criterion (BIC) was used to assess goodness-of-fit from models comprising of one to eight factors. In step 2, we fit a bifactor model using the best fitting factor model from the EFA. The bifactor model allows each item to have a positive loading on the general trait (which is assumed to underlie all items) as well as loadings on one or more “group” factors, which is assumed to be more conceptually narrow (Reise, Morizot, & Hays, 2007). Factor scores were derived based on the results of the bifactor model. In the final step, we conducted a hierarchical linear regression using the available genotypic sample (n = 116) to model: (1) main effects of parental supervision and 5-HTTLPR genotype and (2) main effects plus the interaction of 5-HTTLPR genotype and parental supervision for bifactor-derived EF variables. In all models, child age, sex (1 = male, 2 = female), self-reported race-ethnicity (1 = European-American, 2 = African-American, 3 = other), and parental education (1 = GED, 2 = partial college, 3 = college graduate, 4 = partial graduate school, 5 = masters level degree, 6 = doctoral level degree) were controlled.
RESULTS
Factor Analysis and Factor Score Derivation
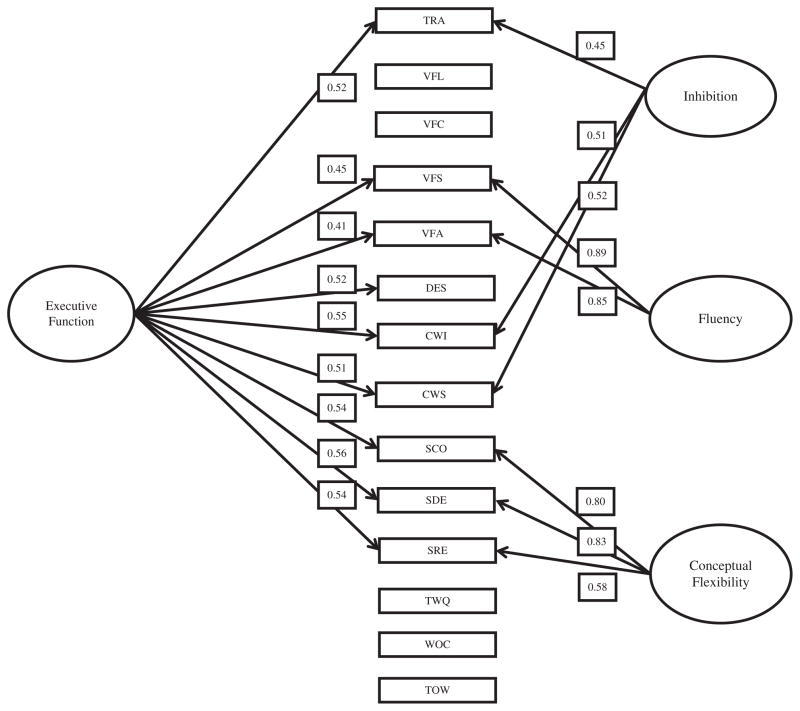
Factor loadings for the best fitting EFA model are shown in Table 2. Comparison of the BIC values for one through eight factor models indicated that the three-factor model was optimal (i.e., smallest BIC value). Results of the scree plot also suggested that the three factor solution provided the best fit to the data (i.e., based on number of factors with eigenvalues >1) (table and figure are available upon request). Our findings are almost entirely consistent with those reported by Latzman and Markon (2010) among their 8- to 19-year-old subgroup. The Sorting tests, including Free Sort Correct (.96), Free Sort Description (.99), and Sort Recognition (.70), uniformly loaded onto a single dimension, which was labeled as “conceptual flexibility,” because these tests require flexibility in thinking and behavior, manipulation of both verbal and nonverbal processes, and the ability to initiate problem solving, among other abilities (Greve, Farrell, Besson, & Crouch, 1995; Latzman & Markon, 2010). The second factor consisted of high factor loadings contributed by Trail Making (.63), Verbal Fluency Category Fluency (.42), Design Fluency (.56), Color-Word Inhibition (.74), and Color-Word Inhibition/Switching (.79). This domain was labeled “inhibition,” given that these tasks measure the ability to inhibit overlearned responses across a variety of visual-motor tasks (Latzman & Markon, 2010). Finally, the third factor was represented by two tasks: Verbal Fluency Category Switching (.99) and Verbal Fluency Accuracy (.78). In contrast to Latzman and Markon (2010), factor loadings for Verbal Fluency Letter (−.04) and Category Fluency (.26) did not significantly load onto this dimension. We labeled this factor “fluency.”
Table 2.
Factor analysis of D-KEFS
| Variable | Three factor EFA model
|
Three factor bifactor model
|
|||||
|---|---|---|---|---|---|---|---|
| Conceptual flexibility | Inhibition | Fluency | g | Conceptual flexibility | Inhibition | Fluency | |
| Trails | .11 | .63 | −.01 | .52 | .09 | .45 | −.04 |
| VF letter | .02 | .36 | −.04 | .28 | .01 | .21 | .11 |
| VF category | −.21 | .42 | .26 | .31 | −.20 | .27 | .31 |
| VF switch | −.01 | −.02 | .99 | .45 | .01 | .01 | .89 |
| VF accuracy | .05 | .05 | .78 | .41 | .01 | −.02 | .85 |
| Design Fluency | .10 | .56 | .09 | .52 | .10 | .38 | .11 |
| CW inhibition | .02 | .74 | .03 | .55 | .01 | .51 | −.01 |
| CW switch | −.05 | .79 | −.06 | .51 | −.03 | .52 | −.04 |
| Sorting correct | .96 | −.02 | .01 | .54 | .80 | −.01 | .00 |
| Sorting description | .99 | −.03 | .01 | .56 | .83 | −.01 | .01 |
| Sorting recognition | .70 | .18 | .02 | .54 | .58 | .12 | .02 |
| Twenty Questions | .29 | .22 | −.07 | .29 | .20 | .17 | −.05 |
| Word Context | .25 | .32 | .04 | .40 | .20 | .19 | .14 |
| Tower | .12 | .17 | .01 | .19 | .11 | .12 | −.03 |
Note. EFA = exploratory factor analysis; g = general factor; VF = Verbal Fluency; CW = Color-Word.
Next, we fit a three-factor bifactor model, with the purpose of determining whether the D-KEFS tests could be represented by a single general factor, whereas each subdomain (i.e., conceptual flexibility, inhibition, and fluency) could be represented by unique group factors. Factor loadings from the bifactor analysis are shown in Table 2 and graphically represented in Figure 1. Factor loadings on the general factor were consistently high (i.e., >.40) for most subtests of the D-KEFS, with the exceptions of Verbal Fluency Letter (.28), Category (.31), Twenty Questions (.29), and Tower (.19). As expected, factor loadings on the three group factors were relatively consistent with the three-factor EFA solution, although two subtests no longer loaded highly onto the inhibition domain: Verbal Fluency Category (.27) and Design Fluency (.38). The general factor accounted for 38% of the explained variance, whereas the conceptual flexibility, inhibition, and fluency group factors accounted for 24, 15, and 23% of the remaining variance, respectively. These findings indicate that a general factor is a significant contributor to subtest scores on the D-KEFS. Factor scores for the EF general factor, conceptual flexibility, inhibition and fluency were derived based on these results.
Fig. 1.
Bifactor model and factor loadings. Factor loadings above >.40 are shown. TRA = Trail Making; VFL = Verbal Fluency Letters; VFC = Verbal Fluency Category; VFS = Verbal Fluency Switching; VFA = Verbal Fluency Accuracy; DES = Design Fluency; CWI = Color-Word Inhibition; CWS = Color-Word Switching; SCO = Sorting Correct; SDE = Sorting Description; SRE = Sorting Recognition; TWQ = Twenty Questions; WOC = Word Context; TOW = Tower.
Gene-Environment Interaction
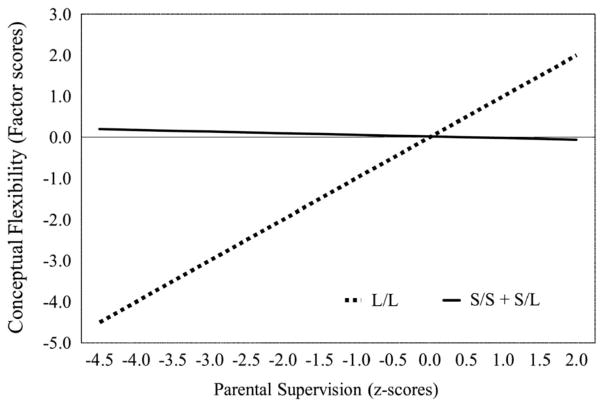
The bifactor solution was used to regress the general factor and the group factors on 5-HTTLPR genotype, parental supervision, and their interaction within hierarchical linear regression models. In all models, race-ethnicity, sex, parental education, and child age were statistically controlled. Parameter estimates from these models are presented in Table 3. In the main effects models, we found a significant main effect for 5-HTTLPR L/L genotype (B = −.55; SE = .27; p = .05), but not for parental supervision (B = .15; SE = .14; p = .31) on the EF general factor. Specifically, individuals with the L/L genotype had lower scores on the EF general factor. No main effects for 5-HTTLPR or parental supervision were detected for conceptual flexibility, inhibition, or fluency. In the final (interaction) models, we detected a significant interactive effect of 5-HTTLPR and parental supervision for conceptual flexibility (B = −1.18; SE = .45; p <.01) (Figure 2). Post hoc analyses indicated that parental supervision was significantly associated with conceptual flexibility among carriers of the L/L genotype (B = .94; SE = .34; p <.01), but not among S/S or S/L individuals (B = −.18; SE = .30; p = .59). We then examined regions of significance using the Johnson-Neyman method (Preacher, Curran, & Bauer, 2006), revealing that conceptual flexibility scores did not differ between L/L versus S/L and S/S genotype groups at parental supervision Z-scores greater than −1.51. In other words, despite the association with conceptual flexibility as a function of increasing parental supervision, the L/L genotype group had significantly lower scores on conceptual flexibility at very low self-reported levels of parental supervision compared to the S/L and S/S groups. No significant 5-HTTLPR by parental supervision interactions emerged for the general EF factor (B = −.38; SE = .25; p = .14) or inhibition (B = −.04; SE = .14; p = .78), although a marginally significant interaction effect was found for fluency (B = 1.00; SE = .57; p = .08).
Table 3.
Hierarchical regression parameter estimates
| Estimate | SE | p | R2 | ||
|---|---|---|---|---|---|
| Executive function (General Factor) | Step 1 | — | — | — | .24 |
| Parental Supervision | .15 | .14 | .31 | — | |
| 5-HTTLPR genotype | −.55 | .27 | .05 | — | |
| Step 2 | — | — | — | .26 | |
| 5-HTTLPR × Parental Supervision | −.38 | .25 | .14 | — | |
| Conceptual Flexibility (Factor 1) | Step 1 | — | — | — | .03 |
| Parental Supervision | .29 | .26 | .27 | — | |
| 5-HTTLPR genotype | −.09 | .50 | .86 | — | |
| Step 2 | — | — | — | .08 | |
| 5-HTTLPR × Parental Supervision | −1.18 | .45 | <.01 | — | |
| Inhibition (Factor 2) | Step 1 | — | — | — | .08 |
| Parental Supervision | .12 | .08 | .10 | — | |
| 5-HTTLPR genotype | −.17 | .15 | .24 | — | |
| Step 2 | — | — | — | .08 | |
| 5-HTTLPR × Parental Supervision | −.04 | .14 | .78 | — | |
| Fluency (Factor 3) | Step 1 | — | — | — | .01 |
| Parental Supervision | −.29 | .31 | .36 | — | |
| 5-HTTLPR genotype | .07 | .61 | .90 | — | |
| Step 2 | — | — | — | .04 | |
| 5-HTTLPR × Parental Supervision | 1.00 | .57 | .08 | — |
Note. Covariates are not presented in the table but were modeled in both steps.
Fig. 2.
5-HTTLPR genotype × parental supervision interaction.
DISCUSSION
As hypothesized, three factors of EF emerged that reflected domains related to conceptual flexibility, inhibition, and fluency. The covariation between EF factors was associated with a single general factor, evidence that EF may be comprised of both unitary and disparate components. There was a significant main effect of 5-HTTLPR genotype on the general EF factor, such that individuals with the L/L genotype had lower scores on this factor than individuals without this genotype. Additionally, a significant 5-HTTLPR genotype by parental supervision interaction emerged that was specific to conceptual flexibility, even after controlling for race-ethnicity, sex, parental education, and child age. Specifically, youth with the L/L genotype performed worse on conceptual flexibility given very low levels of parental supervision compared to youth with S/S or S/L genotypes.
The factor structure of EF is relatively consistent with previous factor analytic studies (e.g., Latzman & Markon, 2010; Lee et al., 2013). During adolescence, distinct factors representing conceptual flexibility, inhibition, and fluency emerged in prior studies, including a general factor that largely accounted for the covariation between these dimensions. However, there is likely to be factorial variance outside of this developmental epoch, particularly in younger children where a two-factor structure has been reported (e.g., Huizinga et al., 2006; van der Sluis, de Jong, & van der Leij, 2007). Over the course of development, particularly from childhood to young adulthood, different neural circuits and brain regions mature along distinct trajectories (Ernst, 2014); the PFC, in particular, is crucial in regulating EF process and its development typically follows a linear trajectory of maturation as a function of age, such that certain abilities and functions to do not fully come online until adolescence (Ernst, 2014). This may explain why adolescent and adult samples typically converge on three factors of EF, whereas younger samples typically converge on two factors. The PFC may be associated with the general factor of EF, regions within the PFC and other subcortical structures (e.g., striatum, amygdala) may regulate specific dimensions of EF (Ernst, 2014; Monchi, Petrides, Strafella, Worsley, & Doyon, 2006; Stuss & Alexander, 2000; Taylor et al., 2004). Inhibition, conceptual flexibility, and updating were preferentially activated in the posterior regions of the left superior parietal gyrus and right intraparietal sulcus in a neuroimaging study (Collette et al., 2005). Conceptual flexibility was associated with activation in the inferior frontal gyrus (Hirschorn & Thompson-Schill, 2006; Periáñez et al., 2004), whereas inhibition was associated with activation of the right orbitofrontal gyrus (Collette et al., 2005).
We found a main effect of 5-HTTLPR genotype on the EF general factor, whereby individuals with the L/L genotype had lower scores on the EF general factor than individuals without this genotype, again suggesting that 5-HT regulation plays a generally important role in EF (Logue & Gould, 2014). Studies have shown an inverse association between the L allele, which is more transcriptionally active in coding 5-HT transporter proteins compared to the S allele, and performance on conceptual flexibility tasks in humans (Borg et al., 2009; Jedema et al., 2010), rodents (Birrell & Brown, 2000) and nonhuman primates (Clarke, Dalley, Crofts, Robbins, & Roberts, 2004; Lapiz-Bluhm et al., 2008). In addition, dimensions of EF may be influenced by different (and overlapping) neurochemical and genetic pathways in the PFC (Anderson, Northam, Hendy, & Wrenall, 2001; Jurado & Roselli, 2007). Genes associated with dopamine receptors and transporters have been linked to performance in response inhibition (Ghahremani et al., 2012; Krämer et al., 2009) and working memory (Bertolino et al., 2006; Blanchard, Chamberlain, Roiser, Robbins, & Müller, 2011). Furthermore, a functional polymorphism in the catechol-O-methyltransferase (COMT) gene was associated with sustained attention and conceptual flexibility (Logue & Gould, 2014). These other genetic pathways, not examined here, also warrant consideration.
Previous genetic association studies on EF have largely ignored the potential contribution of environmental influences. We found that the association of 5-HTTLPR genotype was moderated by parental supervision for conceptual flexibility, but not for fluency or inhibition, and no longer for the EF general factor. Specifically, individuals with the L/L genotype may be more sensitive to parental influences in the context of their cognitive maturation trajectories (Ernst, 2014). Human neuroimaging studies suggest that these associations may be mediated by the distinct neural pathways, whereby environmental stressors may be increase the activation of the amygdala, which in turn relays signals to regulatory circuits in the PFC (see Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Hackman, Farah, & Meaney, 2010). However, the fact that the L allele conveyed increased sensitivity to parental supervision for conceptual flexibility is in contrast to the prevailing GxE literature for 5-HTTLPR and psychopathology, which consistently show that S allele carriers are more sensitive to environmental influences for internalizing and externalizing phenotypes than individuals carrying the L allele (Gibb et al., 2009; Kaufman et al., 2004; Li & Lee, 2010; Reif et al., 2007; Retz et al., 2008). One explanation is that certain genes are known to be pleiotropic (i.e., genes that effect multiple traits; Chesler et al., 2005) and their associations may differ depending on the phenotype. For example, the Val/Met polymorphism in the COMT gene was differentially associated with emotion-regulation versus cognitive phenotypes (Mier, Kirsch, & Meyer-Lindenberg, 2010). Similarly, there may also be functional variation in 5-HTTLPR with respect to emotion versus cognition, such that S allele homozygotes performed better on cognitive tasks but were more vulnerable to depression and anxiety than L allele carriers (Gizer et al., 2009; Wiekum et al., 2013). Allelic functionality may also diverge depending on the environment (Borg et al., 2009), where certain genotypes that were previously believed to confer risk in an adverse environment may also be simultaneously beneficial in the context of an enriched or supportive environment (Belsky & Pluess, 2009). The phenotypic and genetic complexity of EF warrants additional study, as different genetic and environmental influences may be at play for specific dimensions of EF.
Although parental supervision has been well-studied across a variety of developmental phenotypes, including delinquency (Murray & Farrington, 2010) and substance use (Bogenschneider, Wu, Raffaelli, & Tsay, 1998; Clark et al., 2004, 2005, 2008), few studies have focused on parental supervision in the context of EF development. Previous empirical and meta-analytic studies have produced mixed results for parental supervision and academic achievement (Li et al., 2014; Stattin & Kerr, 2000; Weiss & Schwarz, 1996;), which is robustly related to EF abilities (Best, Miller, & Naglieri, 2011; Clark, Prior, & Kinsella, 2002). It is possible that the inconsistency in the literature is due to the relevance of parental influences on EF, which has been understudied. In addition, our findings suggest that parental influences continue to play a crucial role in the development of EF beyond childhood, which is in line with developmental theories (Galambos, Barker, & Almeida, 2003; Steinberg & Silk, 2002). Future studies should explore the association between EF development during adolescence and other aspects of parenting, including support, warmth, and involvement, given previous work showing that these factors are associated with socioemotional and brain development in young children (Conger, Ge, Elder, Lorenz, & Simons, 1994; Tucker-Drob & Harden, 2012).
Several study limitations should be noted. First, our investigation focused on a single aspect of parenting (i.e., parental supervision). Although the importance of parental supervision in relation to adolescent outcomes is well-established, other dimensions of parenting, such as parental warmth, support, and involvement, have also been shown to be associated with EF development in younger populations (Hughes & Ensor, 2009) and may be relevant to cognitive development in adolescents as well. Second, cultural factors may have played a role in the GxE. Although race-ethnicity was statistically controlled in our analyses, 5-HTTLPR alleles may be nonrandomly distributed by race and ethnicity in much larger populations and may have affected the genetic associations in our study (Gelernter, Cubells, Kidd, Pakstis, & Kidd, 1999). Racial-ethnic differences may also have influenced the magnitude of the association between parental supervision and EF, as one study found that parental supervision was inversely related to academic achievement among Asian Americans students but not with Caucasian students (Mau, 1997). Thus, racial-ethnic issues may be important to address in larger samples. Third, like most candidate gene studies, our sample was underpowered to detect genetic main effects. Genome-wide association studies (GWAS) of psychiatric and behavioral phenotypes have established that individual SNPs convey very small effects individually and account for only a fraction of the overall variance in the phenotype (Plomin, Haworth, & Davis, 2009). Indeed, variation in 5-HTTLPR genotype may exert only a small effect on conceptual flexibility and EF in general; other genes may potentially be identified using GWAS, which have yet to be conducted for EF. We await future studies of EF that will use more powerful approaches for gene identification. Fourth, our study of EF was limited to measures assessed by the D-KEFS. This precluded us from investigating other salient aspects of EF, such as those that involve decision-making and risk-taking (i.e., “hot” EF; Kerr & Zelazo, 2004). These functions have been implicated in the orbitofrontal cortex (Kerr & Zelazo, 2004), a region in the brain that is also sensitive to variations in 5-HT and environmental stimuli (Kalin et al., 2008). Additionally, a wider array of measures for EF may potentially uncover a more heterogeneous factor structure for EF than we derived. Finally, the data presented in the current investigation were not assessed longitudinally. Previous longitudinal investigations of EF have established variability in the factor structure of EF as a function of age (Huizinga et al., 2006; Lee et al., 2013; Prencipe et al., 2011; Zelazo et al., 2004). Using longitudinal strategies, such as latent growth curve modeling or structural equation modeling, may allow researchers to examine how genetic influences predict phenotypic patterns over time, while taking into account individual differences in initial status and trajectories. Longitudinal approaches should be prioritized in future investigations of EF.
The current study characterized the latent structure of EF in a typically developing adolescent population with evidence that 5-HTTLPR genotype interacted with parental supervision in the prediction of conceptual flexibility. Our findings illustrate the utility of using a latent variable framework in the study of complex phenotypes and suggest that the dimensions of EF may be characterized by different genetic and environment pathways. Furthermore, we anticipate that integrated models of EF that incorporate genetic and environmental influences may potentially facilitate the development and implementation of targeted interventions. There is emerging evidence that 5-HTTLPR genotype may confer differential sensitivity to parenting behaviors, such that genetically susceptible individuals may develop simultaneously better and worse outcomes in the context of positive and negative parenting conditions, respectively (Hankin et al., 2011; Li et al., 2013). Future studies that incorporate gene-environment models may potentially identify populations that are not only at greater risk for developing negative outcomes, but may also benefit the most from interventions (Jaffee & Price, 2007; Brody, Beach, Philibert, Chen, & Murry, 2009).
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA014357, K02AA018195, R21AA017128, R01AA13397, U01AA021690), National Institute on Drug Abuse (P50 DA005605) and the Commonwealth of Pennsylvania (PA-HEAL SPH00010). The first author (J.J.L.) was supported by the National Institute of Mental Health (T32MH20030-14).
Footnotes
The authors disclose no conflicts of interest.
References
- Alarcón M, Plomin R, Fulker DW, Corley R, DeFries JC. Molarity not modularity: Multivariate genetic analysis of specific cognitive abilities in 16-year-old children in the Colorado Adoption Project. Cognitive Development. 1998;14:175–193. [Google Scholar]
- Anderson V, Northam E, Hendy J, Wrenall J. Developmental neuropsychology: A clinical approach. New York: Psychology Press; 2001. [Google Scholar]
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behavior Genetics. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Dallapiccola B. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. The Journal of Neuroscience. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Miller PH, Naglieri JA. Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learning and Individual Differences. 2011;21:327–336. doi: 10.1016/j.lindif.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Chamberlain SR, Roiser J, Robbins TW, Müller U. Effects of two dopamine-modulating genes (DAT1 9/10 and COMT Val/Met) on n-back working memory performance in healthy volunteers. Psychological Medicine. 2011;41:611–618. doi: 10.1017/S003329171000098X. [DOI] [PubMed] [Google Scholar]
- Bogenschneider K, Wu MY, Raffaelli M, Tsay JC. Parent influences on adolescent peer orientation and substance use: The interface of parenting practices and values. Child Development. 1998;69:1672–1688. [PubMed] [Google Scholar]
- Borg J, Henningsson S, Saijo T, Inoue M, Bah J, Westberg L, Farde L. Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. The International Journal of Neuropsychopharmacology. 2009;12:783–792. doi: 10.1017/S1461145708009759. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Philibert RA, Chen YF, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene x environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, Williams RW. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nature Genetics. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Clark C, Prior M, Kinsella G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. Journal of Child Psychology and Psychiatry. 2002;43:785–796. doi: 10.1111/1469-7610.00084. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Maisto SA. Adolescent neglect and alcohol use disorders in two-parent families. Child Maltreatment. 2004;9:357–370. doi: 10.1177/1077559504269533. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Maisto SA. Supervisory neglect and adolescent alcohol use disorders: Effects on AUD onset and treatment outcome. Addictive Behaviors. 2005;30:1737–1750. doi: 10.1016/j.addbeh.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Mezzich A, Chung T. Parental supervision and alcohol use in adolescence: Developmentally specific interactions. Journal of Developmental and Behavioral Pediatrics. 2008;29:285–292. doi: 10.1097/DBP.0b013e31816e22bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Chung T, Pajtek S, Zhai Z, Long E, Hasler B. Neuroimaging methods for adolescent substance use disorder prevention science. Prevention Science. 2013;14:300–309. doi: 10.1007/s11121-012-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25:409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH, Lorenz FO, Simons RL. Economic stress, coercive family process, and developmental problems of adolescents. Child Development. 1994;65:541–561. [PubMed] [Google Scholar]
- Coolidge FL, Thede LL, Young SE. Heritability and the comorbidity of attention deflcit hyperactivity disorder with behavioral disorders and executive function deflcits: A preliminary investigation. Developmental Neuropsychology. 2000;17:273–287. doi: 10.1207/S15326942DN1703_1. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends in Cognitive Sciences. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behavioural Brain Research. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Crone EA. Executive functions in adolescence: Inferences from brain and behavior. Developmental Science. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Dean FB, Hosono S, Fang L, Wu X, Faruqi AF, Bray-Ward P, Lasken RS. Comprehensive human genome amplification using multiple displacement amplification. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Dishion TJ, McMahon RJ. Parental monitoring and the prevention of child and adolescent problem behavior: A conceptual and empirical formulation. Clinical Child and Family Psychology Review. 1998;1:61–75. doi: 10.1023/a:1021800432380. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain and Cognition. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Reif A, Strobel A. Serotonergic modulation in executive functioning: Linking genetic variations to working memory performance. Neuropsychologia. 2011;49:3776–3785. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex–An update: Time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Galambos NL, Barker ET, Almeida DM. Parents do matter: Trajectories of change in externalizing and internalizing problems in early adolescence. Child Development. 2003;74:578–594. doi: 10.1111/1467-8624.7402017. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Cubells JF, Kidd JR, Pakstis AJ, Kidd KK. Population studies of polymorphisms of the serotonin transporter protein gene. American Journal of Medical Genetics. 1999;88:61–66. [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, London ED. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. The Journal of Neuroscience. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999;10:203–205. [Google Scholar]
- Gibb BE, Uhrlass DJ, Grassia M, Benas JS, McGeary J. Children’s inferential styles, 5-HTTLPR genotype, and maternal expressed emotion-criticism: An integrated model for the intergenerational transmission of depression. Journal of Abnormal Psychology. 2009;118:734–745. doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. American Journal of Medical Genetics. 1999;88:83–87. [PubMed] [Google Scholar]
- Greve KW, Farrell JF, Besson PS, Crouch JA. A psychometric analysis of the California Card Sorting Test. Archives of Clinical Neuropsychology. 1995;10:265–278. [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Translational Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Hobson CW, Scott S, Rubia K. Investigation of cool and hot executive function in ODD/CD independently of ADHD. Journal of Child Psychology and Psychiatry. 2011;52:1035–1043. doi: 10.1111/j.1469-7610.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schiepers OJ, Schoffelmeer AN, Cuppen E, Vanderschuren LJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacology. 2007;195:175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CH, Ensor RA. How do families help or hinder the emergence of early executive function? New Directions for Child and Adolescent Development. 2009:35–50. doi: 10.1002/cd.234. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Jacob T, Moser RP, Windle M, Loeber R, Stouthamer-Loeber M. A new measure of parenting practices involving preadolescent-and adolescent-aged children. Behavior Modification. 2000;24:611–634. doi: 10.1177/0145445500245001. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Price T. Gene–environment correlations: A review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Molecular Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A, Zelazo PD. Development of “hot” executive function: The children’s gambling task. Brain and Cognition. 2004;55:148–157. doi: 10.1016/S0278-2626(03)00275-6. [DOI] [PubMed] [Google Scholar]
- King IB, Satia-Abouta J, Thornquist MD, Bigler J, Patterson RE, Kristal AR, White E. Buccal cell DNA yield, quality, and collection costs: Comparison of methods for large-scale studies. Cancer Epidemiology, Biomarkers, and Prevention. 2002;11:1130–1133. [PubMed] [Google Scholar]
- Krämer UM, Rojo N, Schüle R, Cunillera T, Schöls L, Marco-Pallarés J, Münte TF. ADHD candidate gene (DRD4 exon III) affects inhibitory control in a healthy sample. BMC Neuroscience. 2009;10:150. doi: 10.1186/1471-2202-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Börger N, Meere JVD, Rijsdijk F, Asherson P. Reaction time, inhibition, working memory and delay aversion performance: Genetic influences and their interpretation. Psychological Medicine. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Bondi CO, Doyen J, Rodriguez GA, Bédard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. Journal of Neuroendocrinology. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzman RD, Markon KE. The factor structure and age-related factorial invariance of the Delis-Kaplan Executive Function System (D-KEFS) Assessment. 2010;17:172–184. doi: 10.1177/1073191109356254. [DOI] [PubMed] [Google Scholar]
- Lee K, Bull R, Ho RM. Developmental changes in executive functioning. Child Development. 2013;84:1933–1953. doi: 10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li H, Walker R, Armstrong D. International note: Parenting, academic achievement and problem behaviour among Chinese adolescents. Journal of Adolescence. 2014;37:387–389. doi: 10.1016/j.adolescence.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Latent class analysis of antisocial behavior: Interaction of serotonin transporter genotype and maltreatment. Journal of Abnormal Child Psychology. 2010;38:789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Berk MS, Lee SS. Differential susceptibility in longitudinal models of gene–environment interaction for adolescent depression. Development and Psychopathology. 2013;25:991–1003. doi: 10.1017/S0954579413000321. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. American Journal of Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Antisocial behavior and mental health problems: Explanatory factors in childhood and adolescence. Mahwah, NJ: L. Erlbaum; 1998. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Logue SF, Gould TJ. The neural and genetic basis of executive function: Attention, cognitive flexibility, and response inhibition. Pharmacology, Biochemistry, and Behavior. 2014;123:45–54. doi: 10.1016/j.pbb.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys W, Vanderschuren LJ, Schutter DJ. The neurobiology of oppositional defiant disorder and conduct disorder: Altered functioning in three mental domains. Development and Psychopathology. 2013;25:193–207. doi: 10.1017/S0954579412000272. [DOI] [PubMed] [Google Scholar]
- Mau WC. Parental influences on the high school students’ academic achievement: A comparison of Asian immigrants, Asian Americans, and White Americans. Psychology in the Schools. 1997;34:267–277. [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Molecular Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. Journal of Experimental Psychology: General. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Annals of Neurology. 2006;59:257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Monsell S. Control of mental processes. In: Bruce V, editor. Unsolved mysteries of the mind: Tutorial essays in cognition. New York, NY: Erlbaum; 1996. pp. 93–148. [Google Scholar]
- Murray J, Farrington DP. Risk factors for conduct disorder and delinquency: Key findings from longitudinal studies. Canadian Journal of Psychiatry. 2010;55:633–642. doi: 10.1177/070674371005501003. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus (Version 6) Los Angeles. CA: Muthen & Muthen; 2010. [Google Scholar]
- Periáñez JA, Maestú F, Barceló F, Fernández A, Amo C, Ortiz Alonso T. Spatiotemporal brain dynamics during preparatory set shifting: MEG evidence. Neuroimage. 2004;21:687–695. doi: 10.1016/j.neuroimage.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD. Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology. 2011;108:621–637. doi: 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Molecular Neurobiology. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin BH, Quane JM. Social contexts and urban adolescent outcomes: The interrelated effects of neighborhoods, families, and peers on African-American youth. Social Problems. 2002;49:79–100. [Google Scholar]
- Reif A, Rösler M, Freitag CM, Schneider M, Eujen A, Kissling C, Retz W. Nature and nurture predispose to violent behavior: Serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Reise SP, Morizot J, Hays RD. The role of the bifactor model in resolving dimensionality issues in health outcomes measures. Quality of Life Research. 2007;16:19–31. doi: 10.1007/s11136-007-9183-7. [DOI] [PubMed] [Google Scholar]
- Retz W, Freitag CM, Retz-Junginger P, Wenzler D, Schneider M, Kissling C, Rösler M. A functional serotonin transporter promoter gene polymorphism increases ADHD symptoms in delinquents: Interaction with adverse childhood environment. Psychiatry Research. 2008;158:123–131. doi: 10.1016/j.psychres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Rogers RD, Cook LJ, Sahakian BJ. The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology. 2006;188:213–227. doi: 10.1007/s00213-006-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A. New developments in psychometrics. Tokyo: Springer; 2003. Power of χ2 goodness-of-fit tests in structural equation models: The case of non-normal data; pp. 57–68. [Google Scholar]
- Soenens B, Vansteenkiste M, Luyckx K, Goossens L. Parenting and adolescent problem behavior: An integrated model with adolescent self-disclosure and perceived parental knowledge as intervening variables. Developmental Psychology. 2006;42:305–318. doi: 10.1037/0012-1649.42.2.305. [DOI] [PubMed] [Google Scholar]
- Stattin H, Kerr M. Parental monitoring: A reinterpretation. Child Development. 2000;71:1072–1085. doi: 10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Silk JS. Parenting adolescents. Handbook of Parenting. 2002;1:103–133. [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Luan Phan K, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM, Harden KP. Intellectual interest mediates Gene × Socioeconomic Status interaction on adolescent academic achievement. Child Development. 2012;83:743–757. doi: 10.1111/j.1467-8624.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S, de Jong PF, van der Leij A. Executive functioning in children, and its relations with reasoning, reading, and arithmetic. Intelligence. 2007;35:427–449. [Google Scholar]
- Walker SC, Mikheenko YP, Argyle LD, Robbins TW, Roberts AC. Selective prefrontal serotonin depletion impairs acquisition of a detour-reaching task. European Journal of Neuroscience. 2006;23:3119–3123. doi: 10.1111/j.1460-9568.2006.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Pomerantz EM, Chen H. The role of parents’ control in early adolescents’ psychological functioning: A longitudinal investigation in the United States and China. Child Development. 2007;78:1592–1610. doi: 10.1111/j.1467-8624.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Weikum WM, Brain U, Chau CM, Grunau RE, Boyce WT, Diamond A, Oberlander TF. Prenatal serotonin reuptake inhibitor (SRI) antidepressant exposure and serotonin transporter promoter genotype (SLC6A4) influence executive functions at 6 years of age. Frontiers in Cellular Neuroscience. 2013;7:1–12. doi: 10.3389/fncel.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LH, Schwarz JC. The relationship between parenting types and older adolescents’ personality, academic achievement, adjustment, and substance use. Child Development. 1996;67:2101–2114. [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Craik FI, Booth L. Executive function across the life span. Acta Psychologica. 2004;115:167–183. doi: 10.1016/j.actpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]