Abstract
Previous studies indicate that topiramate reduces alcohol use among problem drinkers, with one study showing that the effect was moderated by a polymorphism (rs2832407) in GRIK1, the gene encoding the GluK1 kainate subunit. We examined whether the interactive effect of medication and genotype (a) altered the association between daily self-efficacy and later day drinking and (b) had an indirect effect on drinking via self-efficacy.
Methods
In a 12-week, placebo-controlled trial of topiramate, we used daily interactive voice response technology to measure self-efficacy (i.e., confidence in avoiding heavy drinking later in the day) and drinking behavior in 122 European-American heavy drinkers.
Results
Topiramate’s effects on both self-efficacy and drinking level were moderated by rs2832407. C-allele homozygotes treated with topiramate showed higher levels of self-efficacy and lower levels of nighttime drinking across the 12-week trial. Further, the interactive effect of topiramate and genotype on mean nighttime drinking levels was mediated by mean levels of self-efficacy.
Conclusion
By modeling topiramate’s effects on nighttime drinking across multiple levels of analysis, we found that self-efficacy, a key psychological construct, mediated the effect of topiramate, which was moderated by rs2832407 genotype. Thus, it may be possible to use an individualized assessment (i.e., genotype) to select treatment (i.e., topiramate or psychotherapy aimed at enhancing self-efficacy) to optimize the reduction in heavy drinking to provide a personalized treatment approach.
Keywords: Topiramate, Self-Efficacy, GRIK1, Pharmacogenetics, Mediated Moderation, Personalized Treatment
INTRODUCTION
Self-efficacy, the confidence in one’s ability to achieve a desired goal, plays a key role in determining behavioral change (Bandura 1977), with greater self-efficacy to change being associated with a higher probability that the desired behavioral change will occur. In recent decades, studies have shown that self-efficacy is one of the most consistent predictors and/or mediators of treatment outcomes for a variety of pathologies (Annesi 2011; Brown et al. 2014; Marceaux & Melville 2011). Self-efficacy is seen as particularly relevant to the treatment of alcohol and drug use disorders (Kadden & Litt 2011).
Despite a growing number of studies demonstrating the importance of self-efficacy in determining the outcomes of psychosocial treatments for substance use disorders (Kadden & Litt, 2011), few studies have examined the influence of self-efficacy in alcohol pharmacotherapy trials (Ray, Hutchison & Bryan 2006; Hartzler, Witkiewitz, Villarroel & Donovan 2011; Penberthy et al. 2011; Schaumberg et al. 2013). In an uncontrolled trial of olanzapine combined with a motivation-based intervention, Ray et al. 2006) found that self-efficacy for alcohol abstinence measured three times during the study did not predict drinking outcomes. Penberthy and colleagues (2011) found that, in a placebo-controlled trial of ondansetron in alcohol-dependent individuals who also received cognitive behavioral therapy (CBT), confidence to abstain from alcohol predicted greater reductions in the intensity of drinking. However, ondansetron did not affect this measure of self-efficacy. Schaumberg and colleagues (2013) examined attributions of change by problem drinkers who received a modified version of behavioral self-control therapy (MBSCT) combined with either naltrexone or placebo to reduce their drinking. Participants who believed that they received placebo reported greater self-efficacy for continued changes without medication than those in the other groups.
Not only are there few studies that have examined the role of self-efficacy in pharmacotherapy trials, the manner in which self-efficacy is commonly assessed often does not match how it is conceptualized theoretically. Although social learning theory suggests that self-efficacy is a dynamic construct that changes as a function of a variety of intra-individual and environmental demands (Bandura 1977, 1982), most alcohol treatment studies have considered it to be a relatively static construct assessed at discrete points at which subjects recall their global levels of efficacy (Loeber et al. 2006, Schaumberg et al. 2013). Self-efficacy to resist heavy drinking or abstain from alcohol may be best assessed in real-time with repeated assessments. Use of micro-longitudinal research designs [e.g., daily diary designs; ecological momentary assessment (EMA)] would provide information about momentary or daily state-like variation in self-efficacy and how it is associated with proximal drinking levels. This approach would also provide more accurate assessments of overall levels of self-efficacy during a given time period. For example, Kuerbis et al. (2013) used daily diary methods to explore the role of self-efficacy in predicting drinking during brief treatments aimed at moderating drinking. They found that average levels of self-efficacy assessed using a daily diary for the week prior to treatment and the final week of treatment were better predictors of end-of-treatment drinking than traditional retrospective one-time reports of self-efficacy for the same time periods.
The central aim of the present study was to use a micro-longitudinal research design to examine the role of overall levels and daily changes in self-efficacy that accompany topiramate treatment for heavy drinking. In recent studies, Kranzler and colleagues (2014a, 2014b) found that topiramate, resulted in moderate to large reductions in drinking, and that its effects were moderated by a single nucleotide polymorphism (SNP) in GRIK1, the gene encoding the GluK1 subunit of the kainate (glutamate) receptor. Specifically, results from the clinical trial (Kranzler et al. 2014a) showed that topiramate-treated individuals who were rs2832407*C-allele homozygotes had greater reductions in heavy drinking (reported using the Timeline Follow-back interview) than rs2832407*A-allele carriers. An analysis that was based on daily diary reports of drinking collected during the clinical trial using interactive voice response technology (IVR) replicated these findings (Kranzler et al. 2014b). The daily IVR also provided information on participants’ desire to drink and their positive and negative alcohol-outcome expectancies. This secondary analysis indicated that rs2832407*C-allele homozygotes treated with topiramate showed greater reductions in daily reports of desire to drink and positive alcohol expectancies. However, reduction in the desire to drink and expectancies did not mediate the effects of topiramate on drinking level for rs2832407*C-allele homozygotes (Kranzler et al. 2014b). Further analysis of the daily diary data (Kranzler et al., in press) showed that, for rs2832407*C-allele homozygotes, the effect of topiramate interacted with relative levels of daily positive expectancies (i.e., daily deviations from individual’s mean levels). The form of this interaction indicated that topiramate treatment in rs2832407*C-allele homozygotes exerted an effect on drinking level on all days except those characterized by extremely high levels of anticipated positive outcomes. Although these findings were not specifically predicted, they identified possible mechanisms at play in the effects of topiramate.
Building on our previous findings, in the present study we utilized the daily diary data examined by Kranzler and colleagues (2014b and in press) to gain a better understanding of the psychological mechanism underlying the effects of topiramate on drinking. Thus, we examined (a) whether topiramate treatment in rs2832407*C-allele homozygotes altered the within-person associations between daily levels of self-efficacy and proximal (later day) drinking levels and (b) whether mean levels of self-efficacy over the course of treatment mediated the effects of topiramate for rs2832407*C-allele homozygotes on mean drinking levels.
METHODS AND MATERIALS
Overview of Study Procedures
Data for the current study were drawn from our 12-week, parallel-group, placebo-controlled trial of topiramate in heavy drinkers (Kranzler et al. 2014a). The Institutional Review Board at each participating institution [the University of Connecticut Health Center (UCHC), where the study was initiated, and the University of Pennsylvania Perelman School of Medicine (Penn), where the study was completed] approved the study.
Following telephone screening of individuals who sought to reduce their alcohol consumption, we invited eligible participants for an in-person assessment, where they gave written, informed consent to participate in a treatment trial. After a baseline assessment, we randomly assigned participants to receive 12 weeks of treatment with topiramate or matched placebo, with weekly in-person visits for the first six weeks (during which we gradually increased the dosage of study medication), followed by three biweekly visits. All participants received medical management (Pettinati et al. 2004), which is a brief psychosocial intervention, at each of the nine treatment visits. In addition to periodic in-person assessments throughout the 12 weeks, participants were asked to complete IVR calls that elicited information each evening on drinking during the preceding 24 hours and a variety of subjective states (including confidence in resisting heavy drinking that night, a measure of self-efficacy).
Participants
One hundred thirty-eight participants were enrolled in the study based on the following inclusion criteria: 1) age 18–65; 2) average weekly consumption of ≥24 standard drinks for men and ≥18 standard drinks for women; 3) an explicit goal of reducing drinking to a safe level; 4) ability to read in English at a level ≥8th grade; 5) no gross evidence of cognitive impairment; 6) willingness to name a potential locator to ensure follow-up; and 7) willingness to provide written, informed consent to participate. Women of childbearing potential were required to: 1) be non-lactating and practicing a reliable method of birth control; and 2) have a negative serum pregnancy test at screening. Participants were excluded from the study if they were determined by the study physician and/or the nurse practitioner to have a current, clinically significant physical disease; a history of nephrolithiasis; or a serious psychiatric illness. Participants with a current DSM-IV diagnosis of drug (other than nicotine) dependence, and/or evidence that complete abstinence from alcohol was clinically warranted (i.e., due to current severe alcohol dependence, disorders exacerbated by heavy drinking [e.g., gastritis], self-reported inability to reduce drinking, current alcohol withdrawal symptoms, or a history of severe withdrawal symptoms, all of which were evaluated clinically clinically based on history and physical examination) were also excluded.
Study Treatments
Medical Management (MM)
The MM manual was modified to be consistent with a goal of non-hazardous drinking (Sanchez-Craig, Wilkinson, & Davila 1995). It promoted medication adherence and treatment participation through education and support. Men were advised to consume ≤ 3 standard drinks per day and ≤ 12 standard drinks per week and women were advised to consume ≤ 2 drinks per day and ≤ 8 drinks per week.
Medication (Topiramate or Placebo)
Placebo and topiramate were encapsulated and indistinguishable from one another. Treatment group assignment was double blind, with the participant and all study staff blind to medication condition throughout the trial. Treatment was initiated with a single daily dose of topiramate 25 mg (or a placebo capsule) and was increased weekly to a maximum of 200 mg/day of topiramate or placebo (in two divided doses) during the first six weeks of treatment. The maximal dosage of medication was achieved in week six.
Genotyping
We extracted DNA from whole blood with the PureGene kit (GentraSystems, Minneapolis, MN). Rs2832407 was genotyped using a TaqMan SNP genotyping assay (Life Technologies, Grand Island, NY). We repeated all genotypes with consistent results. Genotype frequencies in the European American subsample (n=122) were consistent with Hardy-Weinberg equilibrium expectations (χ2=0.61, df=2, p=0.74).
Measures
Daily Diary Survey Using IVR
Participants responded to daily survey questions via IVR by telephone (Kranzler et al. 2004, Kranzler et al. 2014a). Study staff provided initial training on the IVR system, gave participants a wallet-sized, follow-along guide detailing each question, and answered any questions regarding the process. Participants called daily between 5 and 8 PM to report their confidence to resist heavy drinking later that night (in addition to other subjective variables; see Kranzler et al. 2014b and Kranzler et al., in press) and their alcohol consumption since the prior night’s report by pressing the keys on the keypad, with responses entered automatically in a database. The IVR survey also captured all drinking during the preceding 24-hour period by asking the participants to report separately the number of standard alcoholic drinks that they consumed yesterday (in total), and any drinking during the current day, up until the time of the IVR report. Participants who failed to call in during the allotted time received a computerized reminder call and were given until 9 PM to complete the IVR report, after which the system denied them access until the next day. A staff person monitored calls daily to address problems and questions immediately (to enhance accuracy and adherence). Participants who missed a series of calls were contacted and reminded of the importance to the research of their adhering to the call-in schedule. Participants were paid $1 for each daily call completed, with a $3 bonus possible each week for completing all seven daily assessments.
Sociodemographics
A self-report, demographic questionnaire collected data on age, gender, race and ethnicity, marital status, educational and occupational information, medical history, and substance abuse treatment history.
Screening Assessments
Following a medical history and physical examination, laboratory assessments included urinalysis and urine toxicology testing, a complete blood count, γ-glutamyl transpeptidase concentration measurement, and a chemistry panel (including electrolytes, liver enzymes, and bilirubin). The Structured Clinical Interview for DSM-IV Axis I Disorders (First et al. 2001) was used to identify participants with serious DSM-IV psychiatric disorders. The Short Index of Problems (SIP), a 15-item self-report measure was used to quantify alcohol-related problems (Miller, Tonigan, & Longabaugh 1995) and the Beck Depression Inventory (Beck et al. 1961), a 21-item self-report measure, was used to quantify depressive symptoms at baseline.
Alcohol Use
The participant was asked to estimate the number of abstinent days and heavy drinking days during the 90-day pretreatment period, using the timeline follow-back method (Sobell & Sobell 1992). During treatment, the daily diary IVR survey assessed alcohol consumption by having participants report the number of standard drinks of beer, wine, and liquor that they consumed. We subtracted daytime drinking from total day drinking to arrive at nighttime drinking. This resulted in negative values on 4.9% of the days, because participants reported more daytime drinks than total drinks (as reported the next day). In these situations, the value for nighttime drinking was set to zero. We also winsorized the 0.2% of nighttime drinking values that exceeded 20 drinks to a maximum of 20.
Daily Self-efficacy to Resist Heavy Drinking
One item on the daily diary IVR survey assessed self-efficacy. Participants were asked “How confident are you that you can resist drinking heavily over the rest of the night?” Response options ranged from 0=not at all to 4=extremely. Because the goal of the treatment trial was to reduce heavy drinking, we asked participants about their confidence to resist heavy drinking rather than to abstain from alcohol.
Statistical Analysis
Because of the non-independence of daily assessments, we used multilevel modeling procedures (Raudenbush & Bryk 2002) to test our questions of interest. We first evaluated whether daily self-efficacy level was associated with later-day (nighttime) drinking and whether medication group and genotype and their interaction moderated the association. To evaluate the within-person association between daily self-efficacy and nighttime drinking (i.e., how drinking covaries with deviations from the individual’s mean level of self-efficacy), we person-mean centered daily self-efficacy by subtracting each person’s mean level from each daily value. We entered predictors in several blocks. Additive effects were examined in the first block, and the relevant 2- and 3-way interactions were entered into the model in subsequent blocks. All models included study day (coded 0-83), weekend (coded 0=Monday to Thursday, 1=Friday to Sunday) and earlier day drinking as control variables. We coded medication condition as 0=placebo and 1=topiramate, and genotype as 0=A-allele carrier and 1=C-allele homozygote.
We used all 12 weeks of data to examine the treatment effects. Although we used a maximum dosage of 200 mg/day of topiramate, the minimally efficacious dosage of the medication in alcohol treatment is not known. Thus, we did not want arbitrarily to exclude data from the analysis (e.g., by limiting the analysis to the last six weeks of treatment when participants were receiving the maximal topiramate dosage).
Next, we used similar models to examine whether topiramate’s effect on mean levels of nighttime drinking for rs2832407*C-allele homozygotes was mediated by mean levels of self-efficacy. We focused on nighttime drinking because our daily self-efficacy question focused on this time period and our previous results (Kranzler et al. 2014b) demonstrated a significant medication × genotype interaction in predicting overall levels of daily diary drinking. To evaluate the mediation effect, we first established whether there the medication × genotype interaction significantly predicted mean nighttime drinking levels (which would be shown in the models described above). We then estimated a model predicting daily self-efficacy from the medication and genotype contrasts and their interaction term. Because medication group and genotype are person-level (level-2) variables, significant effects reflected differences in mean levels of these outcomes over the treatment period. If both models showed significant interactions, we then included participants’ mean level of self-efficacy (i.e., person-means) into the model predicting nighttime drinking to determine whether it altered the size of the medication × genotype interaction. To summarize, descriptive evidence for the mediating role of mean self-efficacy would be evidenced by the following pattern: (a) a significant medication group × genotype interaction in predicting the nighttime drinking, (b) a significant medication group × genotype interaction in predicting levels of self-efficacy, and (c) a reduction in the size of the effect of the medication group × genotype interaction in predicting nighttime drinking when mean self-efficacy level was included in the predictive equation. Finally, we calculated an unbiased estimate and significance test of the indirect effect of the medication group × genotype interaction in predicting nighttime drinking level via self-efficacy (Preacher, Zyphur, & Zhang 2010). We used Mplus software (Muthén & Muthén 2007) to estimate a 2-1-1 multi-level structural equation model (MSEM) in which the self-efficacy and drinking were measured at level 1 (within subjects) and the medication group × genotype interaction was measured at level 2 (between subjects). The within-subject portion of the model also included daytime drinking and weekday as control variables. The indirect effect was the product of the between-subjects interaction effect on self-efficacy with the between-subjects self-efficacy effect on drinking. Although the MSEM estimates within- and between-person effects, our indirect effect can only occur at level 2 because the independent variables (medication group and genotype) are person-level variables.
The current analyses were limited to European American (EAs) to avoid a confounding effect on the pharmacogenetic analyses resulting from substantial differences in the population frequency of rs2832407 alleles. Allele frequencies are comparable among European-ancestry groups, with population stratification comparatively unlikely (Halder et al. 2009). Inclusion of the study site (UCHC vs. Penn) failed to influence any of the models, so it was removed from the analysis.
RESULTS
Participant Characteristics, IVR and Medication Adherence, and Overall Drinking Levels
The sample comprised 86 men (62.3%) and 52 women (37.7%), who were, on average, middle-aged (mean=51.1 yr, SD=8.2), married (60.9%), and employed (80.4%). Sixty-seven participants (48.6%) were randomly assigned treatment with topiramate and 71 participants (51.4%) received placebo. The majority of participants (n=122 or 88.4%) reported that they were of EA ancestry. Supplementary Figure 1 shows the progress through the phases of the trial for the EA subgroup, the focus of this report.
In EAs, the four groups resulting from the cross of medication group (topiramate vs. placebo) with genotype group (CC vs. A-carrier) yielded the following cell sizes: Topiramate/CC, n=21 (17.2% of the EA sample); Placebo/CC, n=30 (24.6%); Topiramate/A-carrier, n=35 (28.7%); Placebo/A-carrier, n=36 (29.5%). The medication groups did not differ by genotype frequency (χ2 =0.49, df=1, p=0.48). As shown in Table 1, the sample was mostly male and married, middle-aged, and educated. During the 90 days prior to study enrollment, participants drank on about 90% of days and drank heavily (>4 drinks in a day for men and >3 drinks in a day for women) on about two-thirds of days. There were no significant differences among the four groups on any of these measures.
Table 1.
Baseline Demographic & Clinical Characteristics (n=122 European-Americans)
| A-Allele Carriers | C-Allele Homozygotes | |||
|---|---|---|---|---|
| Topiramate (n=35) | Placebo (n=36) | Topiramate (n=21) | Placebo (n=30) | |
|
| ||||
| Demographic Features | ||||
| Sex (Male) | 62.9% | 66.7% | 71.4% | 46.7% |
| Agea | 50.1 ± 6.7 | 53.6 ± 8.0 | 51.7 ± 8.3 | 52.5 ± 6.4 |
| Married | 65.7% | 66.7% | 66.7% | 66.7% |
| Years of Educationa | 15.6 ± 2.1 | 15.3 ± 2.6 | 15.7 ± 2.4 | 15.0 ± 2.2 |
| Clinical Features | ||||
| 90-Day TLFB | ||||
| PHDD | 67.2 ± 28.8 | 61.1 ± 25.1 | 70.4 ± 25.0 | 72.5 ± 27.4 |
| Percent Days Abstinent* | 10.8 ± 15.1 | 14.4 ± 15.4 | 12.1 ± 14.7 | 6.8 ± 11.5 |
| SIP score | 15.2 ± 9.1 | 15.0 ± 6.8 | 14.5 ± 8.0 | 15.4 ± 6.5 |
| BDI score | 5.1 ± 3.7 | 6.8 ± 5.2 | 7.4 ± 5.1 | 6.8 ± 5.1 |
Mean (SD). BDI=Beck Depression Inventory: SIP=Short Index of Problems; TLFB= Timeline Follow-back Interview; PHDD=Percent Heavy Drinking Days
p=0.093 for genotype by medication interaction
Participants provided complete daily reports for analysis on 76.2% of days (SD=26.2%), with no difference by medication group [F(1, 118)=0.38, p=0.54], genotype group [F(1, 118)=1.75, p=0.19], or their interaction [F(1, 118)=1.07, p=0.30]. Using self-reports, verified by capsule counts, there was a high rate of medication adherence [placebo: mean=91.1% of daily doses (SD=24.7); topiramate: 89.4% of daily doses (SD=23.1)]. Among EAs, there was no difference in maximal dosage by genotype group or the interaction of genotype group with medication group.
Overall, participants consumed alcohol on 78.5% of days, with a mean of 4.5 drinks (SD=2.6) per drinking day. Participants reported drinking during the daytime (i.e., prior to the daily survey) on 47.2% of days [a mean of 3.7 drinks (SD=2.1) per daytime drinking period] and drinking at night (i.e., after the daily survey, captured the next day) on 56.7% of the days [a mean of 3.4 drinks (SD=2.3) per nighttime drinking period].
Interactive effects of Medication, Genotype and Daily Self-Efficacy in Predicting Nighttime Drinking
Table 2 presents results from the multilevel regression analysis examining the effects of relative levels of daily self-efficacy on nighttime drinking and whether medication group and genotype moderated the association. Results in block 1 indicated that the nighttime drinking level decreased across study days, was higher on weekends, and was negatively related to the number of drinks consumed earlier in the day. Nighttime drinking was also lower on days characterized by relatively higher daily self-efficacy levels (i.e., above mean levels). There was a marginally significant effect of medication group, with topiramate-treated individuals drinking less than placebo individuals, which was qualified by a significant medication group × genotype interaction (Table 2, Block 2 and Figure 1). Simple effects tests of this interaction reflected a significant medication effect for rs2832407*CC individuals (b= −1.25, p=0.001, 95% CI: −1.96 to −0.54), but not for rs2832407*A-carriers (b=0.13, p=0.67, 95% CI: −0.47 to 0.72). The 2-way and 3-way interactions examining the effect of the relative level of daily self-efficacy on nighttime drinking as a function of genotype (Block 2) or of genotype by medication group (Block 3) were not significant; these effects were not retained in the model.
Table 2.
Multilevel Regression Results
| Block | Predictor | Nighttime drinking | Self-efficacy | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| b | p | 95% CI | b | p | 95% CI | ||||
| LL | UL | LL | UL | ||||||
| 1 | Study Day | −.010 | <.001 | −.012 | −.008 | .007 | <.001 | .006 | .007 |
| Weekend | .479 | <.001 | .398 | .561 | −.124 | <.001 | −.166 | −.083 | |
| Daytime Drinks | −.290 | <.001 | −.312 | −.268 | −.085 | <.001 | −.096 | −.074 | |
| Daily Self-Efficacy | −.486 | <.001 | −.588 | −.384 | |||||
| Genotype | .071 | .769 | −.405 | .546 | −.059 | .732 | −.397 | .280 | |
| Medication Group | −.443 | .065 | −.914 | .028 | .500 | .004 | .165 | .836 | |
|
| |||||||||
| 2 | Medication Group × Genotype | −1.380 | .004 | −2.308 | −.452 | 1.007 | .002 | .410 | 1.722 |
| Medication Group × Daily Self-Efficacy | −.024 | .813 | −.229 | .180 | |||||
| Genotype × Daily Self-Efficacy | .105 | .315 | −.101 | .312 | |||||
|
| |||||||||
| 3 | Medication Group × Genotype × Daily Self-Efficacy | .176 | .404 | −.240 | .591 | ||||
Note. Values correspond to effects for predictors in each block.
Figure 1.
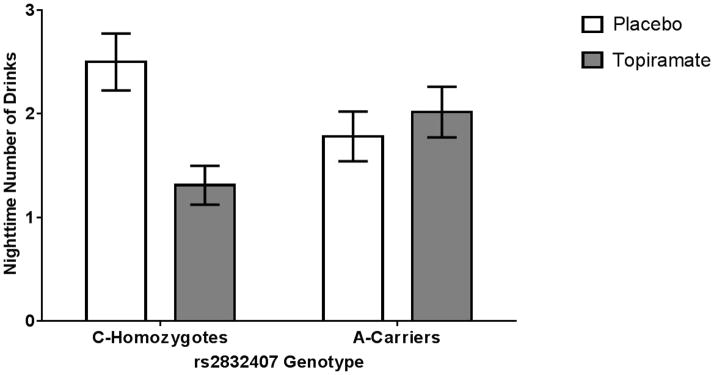
Mean nighttime drinking by medication and genotype groups. Among rs2832407*C homozygotes, the topiramate group reported significantly fewer nighttime drinks consumed than the placebo group (p=0.001). There was no medication effect on drinking level in the rs2832407*A-allele carrier group (p=0.67).
Mediating Effect of Self-efficacy
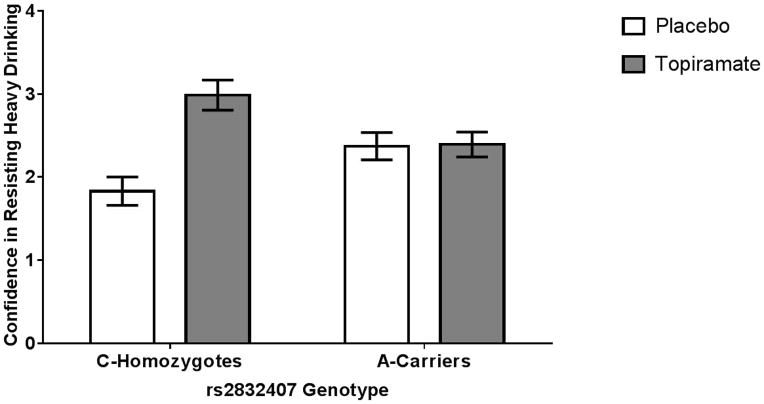
Also shown in Table 2 are the results from the multilevel models predicting daily self-efficacy levels. Results from Block 1 indicate that the daily self-efficacy level increased across study days, was lower on weekends, and was negatively related to the number of drinks consumed earlier in the day. A significant effect of medication was also evident, with individuals taking topiramate reporting higher mean levels of daily self-efficacy level. In Block 2, we found a significant medication group × genotype interaction, the form of which (see Figure 2) paralleled the findings for mean nighttime drinking levels. Specifically, we found that topiramate treatment resulted in higher mean levels of self-efficacy for rs2832407*CC genotype individuals (b=1.13, p<0.001, 95% CI: 9.62 to 1.63), but not for rs2832407*A-carriers (b=0.06, p=0.77, 95% CI: −0.36 to 0.48).
Figure 2.
Mean daily self-efficacy to resist heavy drinking by medication and genotype groups. Among rs2832407*C homozygotes, topiramate treatment was associated with significantly greater self-efficacy than those receiving placebo (p < 0.001). There was no medication effect on confidence to resist heavy drinking in the rs2832407*A-allele carrier group (p=0.77).
To explore whether the interactive effect of genotype and medication on nighttime drinking is mediated via self-efficacy levels, we entered the mean level of daily self-efficacy into the model containing the significant medication group × genotype interaction. Mean self-efficacy level was a significant predictor (b= −0.948, p < 0.001, 95% CI = −1.137 to −0.760), with higher mean self-efficacy levels associated with a lower overall nighttime drinking level. Inclusion of the mean self-efficacy level in the model rendered the formerly significant effect of the medication x genotype interaction (see Table 2) non-significant (b= −.368, p=0.31, 95% CI = −1.080 to 0.343), a pattern consistent with a mediating role of mean self-efficacy level in the effect of the medication group × genotype interaction, i.e., mediated moderation.
We also estimated the significance of the indirect effect of the medication group × genotype interaction in predicting nighttime drinking level through mean self-efficacy level using MSEM procedures. Results indicated a significant indirect effect of the medication group × genotype interaction on mean nighttime drinking level (b= −0.967, p=0.002, 95% CI: −1.571 to −0.362). The direct effect of the medication group × genotype interaction on mean nighttime drinking level was not significant (b= −0.431, p=0.28, 95% CI: −1.212 to 0.349).
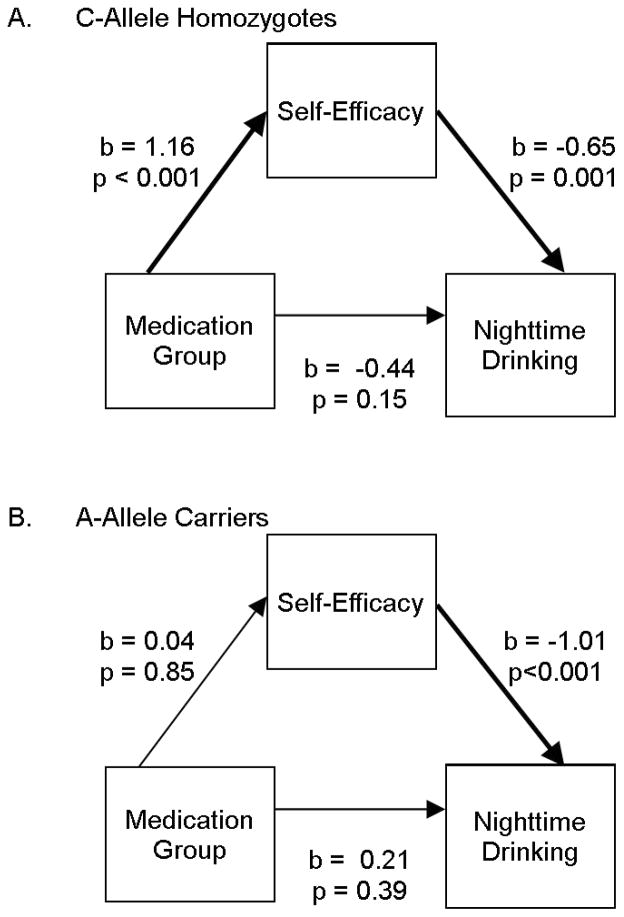
To further illustrate the indirect effect, we estimated a multi-group path model in Mplus (with genotype as the grouping variable), allowing all paths to vary by group. We specified the direct and indirect effects of medication group through mean daily self-efficacy on mean nighttime drinking. As can be seen in Figure 3A, among C-allele homozygotes, medication group was significantly related to self-efficacy, self-efficacy was significantly related to nighttime drinking, and the mediation effect was significant (b= −0.75, p=0.009). In contrast, for A-allele carriers (Figure 3B), medication group was not related to self-efficacy and thus there was no indirect effect (b= −0.04, p=0.85).
Figure 3.
Moderated mediation. A. The path coefficients for rs2832407*C homozygotes, which show significant effects of medication group on self-efficacy level and of self-efficacy level on nighttime drinking level, but no direct effect of medication group on nighttime drinking level (which prior to the inclusion of self-efficacy level was significant, consistent with mediation by self-efficacy level). B. The path coefficients for rs2832407*A-allele carriers, which show only a significant effect of self-efficacy level on nighttime drinking level, exclude the possibility of mediation by self-efficacy.
DISCUSSION
We found no evidence that topiramate treatment, rs2832407 genotype, or their interaction moderated the effect of relative levels of daily self-efficacy on later day drinking. We did find that topiramate and rs2832407 genotype interacted to predict mean levels of self-efficacy and nighttime drinking and that the effect on nighttime drinking was mediated by the level of self-efficacy. Specifically, among rs2832407*C homozygotes, topiramate treatment produced a higher mean level of self-efficacy to resist heavy drinking, which in turn was related to lower mean levels of nighttime drinking. There were no such effects of the medication among A-allele carriers.
Our findings are consistent with our prior observation of a pharmacogenetic effect of topiramate on heavy drinking, which was measured using both retrospective reports (Kranzler et al. 2014a) and daily reports (Kranzler et al. 2014b). The role of self-efficacy in predicting the effects of alcohol treatment on drinking is well documented (Hartzler et al. 2011, Kadden & Litt 2011, Penberthy et al. 2011, Schaumberg et al. 2013). The findings reported here contribute to the limited literature on mediators of the effects of pharmacotherapy on drinking (Kuerbis et al. 2013).
However, the mechanism by which topiramate enhanced self-efficacy in this group is not clear. Despite an absence of literature on glutamatergic (or other pharmacological) effects on measures of self-efficacy, it is possible that the pharmacological antagonism of GluK1-containing kainate receptors by topiramate enhanced genetically susceptible participants’ perceived control over drinking. This could be related to the joint actions of glutamate and dopamine, which are required for the activating effects of drugs (Birgner et al. 2010). A greater sense of control over drinking behavior could have facilitated an earlier cessation of drinking. However, we cannot rule out the possibility that the effect on self-efficacy was indirect, with greater perceived control over drinking resulting from the direct effects of topiramate on drinking behavior, which may have accumulated with repeated daily behavioral sampling. Although we controlled statistically for daytime drinking to avoid a confounding effect on evening reports of self-efficacy, we could not differentiate whether self-efficacy reduced drinking or reductions in drinking increased participants the rs2832407*CC/topiramate group’s confidence to resist heavy drinking. Further research using a variety of sophisticated methods will be required to elucidate the mechanism of the observed effects, potentially including molecular genetic studies to elucidate the effects of rs2832407 on kainate pharmacology and neuroimaging (e.g., proton magnetic resonance spectroscopy) to measure changes in brain neurotransmitter levels associated with ratings of self-efficacy and their modification by topiramate.
In both genotype groups, greater self-efficacy was associated with reduced nighttime drinking. Because topiramate does not appear to be efficacious in reducing drinking in rs2832407*A-allele carriers, other interventions could be used to enhance self-efficacy and thereby reduce drinking in this group. There is evidence that self-efficacy to resist drinking is one of the change mechanisms underlying the effects of different psychosocial interventions, including Alcoholics Anonymous (Finney et al. 1998, Kelly et al. 2012) and relapse prevention (Brown et al. 2002) or cognitive-behavioral skills training (Finney et al. 1998). Thus, a hypothesis to be tested in subsequent studies is whether genotyping for rs2832407 could be used to assign patients with a goal of reduced drinking (or for that matter, abstinence) to either topiramate treatment (for C-allele homozygotes) or a psychosocial intervention that enhances self-efficacy (for A-allele carriers).
We used a single item to measure self-efficacy: namely, participants’ reports of their overall confidence to avoid heavy drinking, which may not fully capture the variation in self-efficacy that occurs in different settings. Notably, however, a recent study showed that a single, general question about a person’s self-efficacy is a better predictor of alcohol treatment outcome than a questionnaire focusing on multiple settings [i.e., the Situational Confidence Questionnaire (Ludwig et al. 2013)]. To confirm the utility of this approach, subsequent studies could examine both the participants’ overall confidence to abstain from alcohol and their self-efficacy in a variety of environments. This could also provide a more detailed characterization of an individual’s self-efficacy, which may be of value in efforts to enhance the efficacy of topiramate by psychotherapeutic treatments that focus on increasing self-efficacy (e.g., cognitive-behavioral therapy). Further, self-efficacy was operationalized as participants’ overall confidence to avoid heavy drinking, which could be confounded with their intention to drink. Although our analysis of desire to drink, which would seemingly also be confounded with intention to drink, showed that it was not a mediator of the effects on drinking, we cannot rule out the possibility that intention to drink mediated the relation between self-efficacy and drinking levels. Analysis of intentions in subsequent studies may provide greater insight into the mechanism of the observed effects of topiramate. Because we framed the self-efficacy question in terms of confidence regarding nighttime drinking, we did not examine models involving daytime drinking.
Other limitations in the current study include the fact that the analysis was conducted only in EAs. Thus, in addition to requiring replication, the findings require extension to other populations. Further, we included only individuals whose expressed goal was to reduce drinking to non-hazardous levels. Extension of these findings to individuals with a goal of abstinence from alcohol would also broaden their potential utility. We also used a single daily assessment of drinking. Using multiple reports of drinking within days could provide greater precision in the assessment of drinking behavior. We did not examine models involving daytime drinking because we framed our evaluation of self-efficacy in terms of confidence regarding nighttime drinking. More frequent measurement of drinking and confidence in reducing it could help to characterize the mechanism of effects of daytime drinking, which in our study occurred frequently and was as intensive as evening drinking.
In summary, the findings reported here suggest that, in participants with the rs2832407*CC genotype, topiramate treatment enhances self-efficacy and reduces heavy drinking. Replication of these findings would make it possible to personalize the management of heavy drinking. Further research is needed to extend these findings to other groups and to examine whether psychosocial interventions could be used to augment the effects of topiramate on self-efficacy and heavy drinking and to intervene with rs2832407*A-allele carriers who appear not to be responsive to topiramate treatment.
Supplementary Material
Consort diagram showing the number of European-American participants randomized to each medication group, the study completion rate, and the reasons for early discontinuation of treatment.
Acknowledgments
Supported by NIH grants P60 AA03510 and K24 AA13736. Staff members of the Clinical Research and Evaluation Unit of the University of Connecticut Alcohol Research Center and the Center for Studies of Addiction of the University of Pennsylvania Perelman School of Medicine were instrumental in the conduct of the study.
Footnotes
FINANCIAL DISCLOSURES
SA, RW, RF, HT, JG, JC, and TP have no disclosures to make. HRK has been a consultant or advisory board member with Alkermes, Lilly, Lundbeck, Otsuka, Pfizer, and Roche. He is a member of the Alcohol Clinical Trials Initiative (ACTIVE) of the American Society of Clinical Psychopharmacology, which is supported by AbbVie, Ethypharm, Lilly, Lundbeck, and Pfizer.
References
- Annesi JJ. Relationship of initial self-regulatory ability with changes in self-regulation and associated fruit and vegetable consumption in severely obese women initiating an exercise and nutrition treatment: moderation of mood and self-efficacy. J Sports Sci Med. 2011;10:643–648. [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy–toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy mechanism in human agency. Am Psychol. 1982;37:122–147. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie A. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci USA. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG, Seraganian P, Tremblay J, Annis H. Process and outcome changes with relapse prevention versus 12-Step aftercare programs for substance abusers. Addiction. 2002;97:677–689. doi: 10.1046/j.1360-0443.2002.00101.x. [DOI] [PubMed] [Google Scholar]
- Brown LA, Wiley JF, Wolitzky-Taylor K, Roy-Byrne P, Sherbourne C, Stein MB, Sullivan G, Rose RD, Bystritsky A, Craske MG. Changes in self-efficacy and outcome expectancy as predictors of anxiety outcomes from the calm study. Depress Anxiety. 2014;31:678–689. doi: 10.1002/da.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JW, Noyes CA, Coutts AI, Moos RH. Evaluating substance abuse treatment process models: I. Changes on proximal outcome variables during 12-step and cognitive-behavioral treatment. J Stud Alcohol. 1998;59:371–380. doi: 10.15288/jsa.1998.59.371. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY Screen) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- Halder I, Yang BZ, Kranzler HR, Stein MB, Shriver MD, Gelernter J. Measurement of admixture proportions and description of admixture structure in different U.S. populations. Hum Mutat. 2009;30:1299–1309. doi: 10.1002/humu.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Witkiewitz K, Villarroel N, Donovan D. Self-efficacy change as a mediator of associations between therapeutic bond and one-year outcomes in treatments for alcohol dependence. Psychol Addict Behav. 2011;25:269–278. doi: 10.1037/a0022869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadden RM, Litt MD. The role of self-efficacy in the treatment of substance use disorders. Addict Behav. 2011;36:1120–1126. doi: 10.1016/j.addbeh.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Hoeppner B, Stout RL, Pagano M. Determining the relative importance of the mechanisms of behavior change within Alcoholics Anonymous: a multiple mediator analysis. Addiction. 2012;107:289–299. doi: 10.1111/j.1360-0443.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Abu-Hasaballah K, Tennen H, Feinn R, Young K. Using daily interactive voice response technology to measure drinking and related behaviors in a pharmacotherapy study. Alcohol Clin Exp Res. 2004;28:1060–1064. doi: 10.1097/01.alc.0000130806.12066.9c. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H, Gelernter J, Covault J. GRIK1 genotype moderates topiramate’s effects on daily drinking level, expectations of alcohol’s positive effects and desire to drink. Int J Neuropsychopharmacol. 2014b;17:1549–1556. doi: 10.1017/S1461145714000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Gelernter J, Covault J. GRIK1 genotype and daily expectations of alcohol’s positive effects moderate the reduction of heavy drinking by topiramate. Exp Clin Psychopharm. doi: 10.1037/a0038350. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014a;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbis A, Armeli S, Muench F, Morgenstern J. Motivation and self-efficacy in the context of moderated drinking: global self-report and ecological momentary assessment. Psychol Addict Behav. 2013;27:934–943. doi: 10.1037/a0031194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Croissant B, Heinz A, Mann K, Flor H. Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. Br J Clin Psychol. 2006;45:515–529. doi: 10.1348/014466505X82586. [DOI] [PubMed] [Google Scholar]
- Ludwig F, Tadayon-Manssuri E, Strik W, Moggi F. Self-efficacy as a predictor of outcome after residential treatment programs for alcohol dependence: simply ask the patient one question! Alcohol Clin Exp Res. 2013;37:663–667. doi: 10.1111/acer.12007. [DOI] [PubMed] [Google Scholar]
- Marceaux JC, Melville CL. Twelve-step facilitated versus mapping-enhanced cognitive-behavioral therapy for pathological gambling: a controlled study. J Gambl Stud. 2011;27:171–190. doi: 10.1007/s10899-010-9196-y. [DOI] [PubMed] [Google Scholar]
- Miller W, Tonigan J, Longabaugh R. Project MATCH Monograph Series. Vol. 4. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse. DHHS Publication No. 95-3911. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- Penberthy JK, Hook JN, Vaughan MD, Davis DE, Wagley JN, Diclemente CC, Johnson BA. Impact of motivational changes on drinking outcomes in pharmacobehavioral treatment for alcohol dependence. Alcohol Clin Exp Res. 2011;35:1694–1704. doi: 10.1111/j.1530-0277.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. DHHS Publication No. (NIH) 04-5289. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. COMBINE Monograph Series, Volume 2. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatmemt for Alcohol Dependence. [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–233. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Ray LA, Hutchison KE, Bryan A. Psychosocial predictors of treatment outcome, dropout, and change processes in a pharmacological clinical trial for alcohol dependence. Addict Disord Their Treat. 2006;5:179–190. [Google Scholar]
- Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health. 1995;85:823–828. doi: 10.2105/ajph.85.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg K, Kuerbis A, Morgenstern J, Muench F. Attributions of change and self-efficacy in a randomized controlled trial of medication and psychotherapy for problem drinking. Behav Ther. 2013;44:88–99. doi: 10.1016/j.beth.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. Humana Press; New Jersey: 1992. pp. 41–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort diagram showing the number of European-American participants randomized to each medication group, the study completion rate, and the reasons for early discontinuation of treatment.