Abstract
Introduction
The interaction between fatness, fitness, and C-reactive protein (CRP) in adolescents is not well characterized but may be important to prevent low grade inflammation. The purpose of this study was to assess the relationship between adiposity, different expressions of fitness, and CRP in late adolescence using direct measures of fitness and fatness.
Methods
Anthropometric measurements were taken on 245 eighteen-year-old participants (116 girls). Fasting CRP, glucose, and insulin were measured and homeostatic model assessment (HOMA) calculated. Body composition was estimated via dual energy X-ray absorptiometry. Fitness was assessed with maximal oxygen uptake (VO2max) during a treadmill test and also expressed relative to the fat-free mass (VO2maxFFM).
Results
Prevalence of overweight/obesity based on body mass index (BMI) was 20.7% and 25.6% among girls and boys, respectively (p = 0.407), but 42.5% and 58.1% when based on body fat percentage (%fat, p = 0.015). Higher proportion of boys (81.3%) than girls (54.5%) were highly fit (p<0.001), but the percentage of girls with high levels of CRP was greater (12.1% vs 6.2%, p = 0.028). Adiposity, indicated with BMI, waist circumference, fat mass, android fat mass (aFM), or %fat, was positively associated with CRP independent of VO2max (r = 0.13-0.18, p<0.05) and VO2maxFFM (r = 0.24-0.32, p<0.001). VO2max, was negatively associated with CRP independent only of BMI and waist circumference (r = -0.21, p = 0.001), but not %fat, fat mass or aFM (r = -0.08 to -0.12, p>0.05). VO2maxFFM was unrelated to CRP with (r = -0.07 to -0.11, p>0.05) or without (r = -0.10, p = 0.142) adjustment for adiposity. Additional adjustment for HOMA did not change any of the relationships, although the coefficients were attenuated.
Conclusions
Fatness has a greater association with CRP than fitness in late adolescence. However, VO2maxFFM, which is truly independent of adiposity, is unrelated to CRP, indicating that the effects of fitness might be mediated via the fatness component embedded in fitness expressed relative to body mass.
Introduction
Obesity has increased tremendously over the past decades and is one of the biggest health burdens in the world [1]. Iceland is no exception and studies have shown an increased rate of overweight and obesity in both children and adults [2,3]. Aerobic fitness (fitness) is another important health marker and is associated with lower rates of morbidity and mortality in adults [4,5] as well as various health outcomes in children and adolescents [6]. Moreover, adolescent fitness is negatively related to adult body fatness [7]. Unfortunately, some studies suggest that fitness among children and adolescents has declined over the last decades [8–10].
C-reactive protein (CRP) is a well-known marker of whole body systemic low-grade inflammation and data suggest that CRP is implicated in the development of cardiovascular disease [11]. Higher levels of CRP are positively associated with measures of adiposity [12–21] whereas better fitness is associated with lower CRP in children as well as adults [12–14,18–20,22,23]. The interactive effects of body fatness and fitness on CRP, however, have not been studied extensively. Some studies suggest that adiposity has a greater impact on CRP than fitness [12,14,19,23], whereas other suggest an independent association of fitness with CRP [13,17,22]. To further complicate this issue, the interactive effects may depend upon the age of the population studied as the independent relation of fitness to CRP appears less evident in children [12,14,16,17,23], which might be because CRP reflects chronic systematic low-grade inflammation that emerges over time [16]. Some intervention studies indicate that a decrease in CRP after an exercise intervention is dependent upon body mass or fat loss [21,24], others report that the effects of exercise are independent of changes in fatness and fitness [25], or even that changes in CRP are related to changes in physical activity and not body composition [26]. Not many intervention studies have investigated the fitness fatness interaction specifically and almost all of them have been conducted in middle-aged or older adults [20,27,28]. The results are conflicting, ranging from no relation between changes in CRP and changes in adiposity or fitness [20] to independent associations of changes in both fitness and fatness with changes in CRP [27]. In addition, many studies have used indirect measures or estimates of adiposity [12–14,16,22,26,27] and fitness [12–14,16,19,25–29], which may affect the nature of the relationship between fitness, fatness and CRP. Understanding the interrelationship of fitness and fatness may be the main way to develop preventive actions to attenuate systemic low grade inflammation [14] but precise measurements of the variables are needed [13].
The relation between fitness and CRP may be confounded or mediated by fatness, or fitness and fatness might share the same causal pathways [17]. In addition, fitness is traditionally expressed relative to body mass and is highly correlated with fatness leading to statistical problems due to multicolinearity, which can lead to wrong conclusions. Furthermore, since adiposity is a factor in both the value of fitness expressed relative to body mass and in value of the fatness measure controlled for (as is commonly done), the variables violate the statistical assumption of independence. Using a different approach by expressing fitness relative to the fat-free mass (FFM, fitnessFFM) enables researchers to estimate the effect of fitness adjusted for body composition on CRP. This expression of fitness (fitnessFFM) may be the best indirect size-independent estimate of the metabolic capacity of the muscle [30], and adiposity is not a component of fitnessFFM. In contrast, the traditional way of expressing fitness relative to body mass is heavily influenced by adiposity, and absolute values for fitness such as L/min are greatly impacted by body size.
The primary purpose of this study was to assess the relationship between adiposity measurements, fitness and CRP in late adolescence, using direct measures of fitness and fatness. We used two different methods to distinguish the association of fitness from the association of adiposity; a) control for adiposity and expressing fitness relative to body mass (for comparative purposes as this is the usual practice) and b) expressing fitness relative to FFM. To our knowledge, the relation of fitnessFFM to CRP has not been studied previously. A secondary objective was to determine whether fatness or fitness had a stronger association to CRP in this relatively understudied population. We hypothesized that 1) both fatness and fitness would be independently associated to CRP although the relation between fatness and CRP would be stronger, 2) fitnessFFM would be more strongly related to CRP than fitness, and 3) overweight adolescents with high fitness levels would have lower levels of CRP than overweight adolescents with low fitness levels.
Methods
Study design and participants
Participants in this cross-sectional study were 18 year-old (17.7–18.9 yr) high-school students. To ensure a diverse group of participants, three high-schools in metropolitan Reykjavik were selected that had different academic structures offering both traditional academic studies and vocational education. Approximately 67% of all high-school students in Iceland attend high schools in metropolitan Reykjavik. In an attempt to get a final sample of approximately 300 participants, a total of 383 students were randomly selected (computerized random number generator) from the class registers and invited to participate in the study. Of those, 275 participated (73% and 69% participation among students in traditional academic studies and vocational education, respectively) and 250 participants completed the blood draw and were included in the analysis for this study. Those 25 who elected not to undergo the blood draw, did not differ from those who did on any body composition or fitness variable. Prior to participating in the study, which was approved by the National Bioethics Committee in Iceland VSNb2007110010/03-1, all participants signed an informed consent form along with a parent or legal guardian for those students who had not yet reached 18 years of age.
Study protocol
Anthropometric measurements were taken in the morning at the participating schools. On another day (both mornings and afternoons), aerobic fitness was measured in the laboratory. The dual energy x-ray absorptiometry (DXA) (GE Lunar IDXA software 11.40.004; Little Chalfont, UK) measurements took place on the third day in the morning at the Icelandic Heart Association. Finally, on the fourth day in the morning, fasting blood samples were collected at the participating schools. Each participant finished all measurements in a span of 7–10 days.
Body composition
Height was measured to the nearest 0.1 cm (Seca 206; Hamburg, Germany) and body mass to the nearest 0.1 kg (Seca 799) with the participants lightly dressed (i.e. underwear), and body mass index (BMI) calculated. Waist circumference (WC) was measured to the nearest 0.1 cm at the narrowest place between the lowest rib and the iliac crest at the end of normal exhalation with a spring loaded inelastic measuring tape. Body composition was assessed via DXA, which returned values for whole body fat mass, FFM, trunk fat mass, android fat mass (aFM) and body fat percentage (%fat).
Aerobic fitness
Maximal oxygen uptake (VO2max) was assessed using open circuit spirometry (Parvomedics Trumax 2400; Sandy, UT, USA) with a graded exercise test protocol on a treadmill (Quasar med, HP Cosmos; Traunstein, Germany). Participants ran until exhaustion at a constant pace (8–13 km/hour depending upon fitness level) on the treadmill with a 2% grade increase every two minutes. Expired gases were sampled every 30 seconds, and heart rate (Polar heart rate monitor; Kempele, Finland) and rating of perceived exertion (Borg scale 6–20) were recorded every two minutes. A subject was considered to have reached VO2max if the oxygen uptake increased less than half of the estimated increase (based on the speed and grade [31]) between the last and next-to-last stage. If a participant did not reach VO2max based on that criterion, the test was still considered maximal if the subject reached at least two of the following three criteria: Respiratory exchange ratio ≥ 1.1; rating of perceived exertion ≥ 19; heart rate within 10 beats of age-dependent maximal heart rate (208–0.7*age) [32–35]. The maximal oxygen uptake relative to the FFM (VO2maxFFM) was also calculated.
Blood variables
Blood samples were drawn by venipuncture from the antecubital fossa of the non-dominant arm after an overnight fast (12 hours minimum) and after refraining from physical activity (other than ambulatory physical activity) for 12 hours. The blood was centrifuged and the serum drawn off and stored at -80°C until analysis. Serum high-sensitivity CRP, insulin, and glucose were assessed by the Landspitali University Hospital and homeostatic model assessment (HOMA) was determined by multiplying insulin with glucose and dividing by 22.5 [36].
Statistical analysis
Statistical analysis was performed using SPSS statistical software, version 17.0 for Windows. The data was inspected for normality and outliers. Five participants (four girls, one boy) with CRP values above 10.0 mg.L-1 were determined outliers and excluded from further analysis [37]. CRP, HOMA, body mass, BMI, WC, fat mass, trunk fat mass, aFM and %fat were positively skewed and were normalized by logarithmic transformations. Independent samples t-test was used to assess sex differences and chi-square to assess proportional sex difference in body compositional, fitness, or CRP classifications. Partial correlations controlled for sex and subsequently HOMA (because of the known relation of HOMA to CRP, fitness and adiposity [14]) were used to assess the relationship between a) CRP and adiposity measurements (WC, BMI, fat mass aFM, and %fat) while controlling for VO2max, VO2maxFFM; and b) CRP and VO2max or VO2maxFFM while controlling for the adiposity measures (one at a time).
Participants were classified based on VO2max into low (<42.2 and <35.5 ml/kg/min), average (42.2–45.69 and 35.5–39.49 ml/kg/min), or high (≥45.7 and ≥39.5 ml/kg/min) fitness categories for males and females, respectively [31]. The participants were also classified based on VO2maxFFM but because no empirical classifications for fitnessFFM exist, they were proportionally (within sex) classified in accordance with the VO2max classifications; i.e. the same percentage (within sex) as was within each VO2max category was classified into low (<56.3 and <55.1 ml/kgFFM/min), average (56.3–59.39 and 55.1–57.59 ml/kgFFM/min), or high (≥59.4 and ≥57.6 ml/kg/min) fitnessFFM categories for males and females, respectively. Additionally, participants were classified based on BMI into normal weight (<25 kg/m2) or overweight/obese (≥25 kg/m2) categories [38]. Likewise, participants were categorized according to Lohman et al. [39] on %fat into normal fat (<17.5% and <31.5%) and over-fat (≥17.5% and ≥31.5% body fat) categories for males and females, respectively. Based on these classifications, four fit-fat groups were created: low-fit/normal-weight, low-fit/overweight, high-fit/normal-weight, and high-fit/overweight (subjects classified in the average fitness/fitnessFFM category (n = 36) were omitted). Two-way ANOVA controlled for sex was used to examine the interaction between the fitness and fatness categorical classification. One-way ANOVA controlled for sex was used to detect differences between the four fit-fat groups with Post-Hoc comparisons using the modified Bonferroni adjustment to account for the family wise error rate. Group differences and relationships were determined significant at α-level < 0.05.
Results
A total of 245 students (116 girls, 129 boys) were included in the final analysis. Significant sex differences (p < 0.01) were observed for all variables except BMI, CRP, and HOMA. Girls had more fat based on all DXA measures of fatness and lower fitness levels, whereas boys were taller, heavier, had greater WC, and more FFM (Table 1).
Table 1. Subject characteristics.
| Girls | Boys | Total | |
|---|---|---|---|
| Anthropometry | n = 116 | n = 129 | n = 245 |
| Height (cm) | 168.1±5.6 | 182.2±6.5 † | 175.5±9.3 |
| Body mass (kg) | 63.7±9.9 | 77.7±14.3 † | 71.1±14.2 |
| BMI (kg/m2) | 22.6±3.5 | 23.4±4.2 | 23.0±3.9 |
| Waist circumference (cm) | 75.8±8.3 | 82.5±10.9 † | 79.3±10.3 |
| Body composition | n = 113 | n = 129 | n = 242 |
| Fat-free mass (kg) | 43.3±4.7 | 61.4±7.8 † | 53.0±11.1 |
| Fat mass (kg) | 19.9±6.5 | 16.7±9.7 † | 18.2±8.5 |
| Trunk fat mass (kg) | 8.7±3.5 | 7.9±5.6 † | 8.3±4.8 |
| Android fat mass (kg) | 1.4±0.7 | 1.3±1.2* | 1.4±1.0 |
| Body fat (%) | 31.0±5.8 | 20.4±7.7 † | 25.3±8.7 |
| Aerobic fitness | n = 110 | n = 128 | n = 238 |
| VO2max (ml/kg/min) | 40.3±5.5 | 51.5±7.4 † | 46.3±8.6 |
| VO2maxFFM (ml/kgFFM/min) | 58.9±5.6 a | 64.5±6.2 † | 61.9±6.5 b |
| Blood values | n = 116 | n = 129 | n = 245 |
| CRP (mg/L) | 1.4±1.7 | 1.0±1.2 | 1.2±1.5 |
| HOMA (mU/L) | 1.6±0.8 | 1.9±1.4 | 1.8±1.2 |
Values are mean±SD. BMI = body mass index, VO2max = maximal oxygen uptake, VO2maxFFM = maximal oxygen uptake relative to the fat-free mass, CRP = C-reactive protein. Significant sex differences
*p<0.01
†p<0.001
an = 109
bn = 237.
When classified based on BMI, 20.7% and 25.6% (p = 0.407) of the girls and boys, respectively, were categorized as overweight/obese, however when classified based on %fat, the proportions of over-fat girls and boys were 42.5% and 58.1% (p = 0.015), respectively. A higher percentage of boys (81.3%) were classified with high fitness than girls (54.5%, p < 0.001), whereas a greater proportion of girls (22.7%) were classified into the low fitness category compared to the boys (10.2%, p < 0.001). Most of the students had low CRP levels (<1 mg/l) or 58.6% of the girls and 75.2% of the boys, however, 12.1% of the girls and 6.2% of the boys (p = 0.028) had high levels (>3 mg/l) [40].
All adiposity measurements (WC, BMI, fat mass, aFM, and %fat) were highly correlated when adjusted for sex (r = 0.69–0.96, p < 0.001). Furthermore, all adiposity measurements, adjusted for sex, were positively related to CRP where %fat, fat mass, and aFM had the highest correlations (Table 2). The strength of these relationships held despite controlling for VO2max, or VO2maxFFM. VO2max, controlled for sex, showed negative associations with the adiposity measurements (r = -0.45 to -0.71, p < 0.001), as well as with CRP (Table 2). The latter relation was independent of BMI or WC but became non-significant when controlled for DXA measures of adiposity (Table 2). In contrast, VO2maxFFM was unrelated to the adiposity measures (r = -0.10 to 0.03, p = 0.139–0.735) and CRP after adjusting for sex (Table 2). Both measures of fitness were highly related (r = 0.74, p < 0.001) after controlling for sex, however. Additional adjustment for HOMA did not change any of the above mentioned relationships, although the partial correlation coefficients became somewhat attenuated (Table 2).
Table 2. Relationship between measures of adiposity, fitness and CRP.
| CRP | CRP | |||||
|---|---|---|---|---|---|---|
| Variable | Controlling for* | r | P | Controlling for † | r | P |
| WC | - | 0.26 | <0.001 | - | 0.19 | 0.004 |
| VO2max | 0.13 | 0.041 | VO2max | 0.13 | 0.054 | |
| VO2maxFFM | 0.24 | <0.001 | VO2maxFFM | 0.20 | 0.002 | |
| BMI | - | 0.25 | <0.001 | - | 0.19 | 0.003 |
| VO2max | 0.14 | 0.032 | VO2max | 0.13 | 0.050 | |
| VO2maxFFM | 0.24 | <0.001 | VO2maxFFM | 0.18 | 0.005 | |
| Fat mass | - | 0.31 | <0.001 | - | 0.24 | <0.001 |
| VO2max | 0.16 | 0.014 | VO2max | 0.14 | 0.027 | |
| VO2maxFFM | 0.30 | <0.001 | VO2maxFFM | 0.23 | <0.001 | |
| aFM | - | 0.31 | <0.001 | - | 0.24 | <0.001 |
| VO2max | 0.17 | 0.010 | VO2max | 0.15 | 0.020 | |
| VO2maxFFM | 0.30 | <0.001 | VO2maxFFM | 0.24 | <0.001 | |
| %fat | - | 0.33 | <0.001 | - | 0.26 | <0.001 |
| VO2max | 0.18 | 0.005 | VO2max | 0.17 | 0.010 | |
| VO2maxFFM | 0.32 | <0.001 | VO2maxFFM | 0.26 | <0.001 | |
| VO2max | - | -0.30 | <0.001 | - | -0.21 | 0.001 |
| WC | -0.21 | 0.001 | WC | -0.15 | 0.022 | |
| BMI | -0.21 | 0.001 | BMI | -0.16 | 0.016 | |
| Fat mass | -0.12 | 0.075 | Fat mass | -0.09 | 0.160 | |
| aFM | -0.12 | 0.062 | aFM | -0.10 | 0.143 | |
| %fat | -0.08 | 0.221 | %fat | -0.06 | 0.341 | |
| VO2maxFFM | - | -0.10 | 0.142 | - | -0.04 | 0.363 |
| WC | -0.10 | 0.112 | WC | -0.06 | 0.344 | |
| BMI | -0.11 | 0.103 | BMI | -0.07 | 0.317 | |
| Fat mass | -0.08 | 0.211 | Fat mass | -0.06 | 0.363 | |
| aFM | -0.08 | 0.204 | aFM | -0.06 | 0.359 | |
| %fat | -0.07 | 0.298 | %fat | -0.05 | 0.428 | |
*Sex controlled for in all correlations.
† = Sex and homeostatic model assessment controlled for in all correlations. WC = waist circumference, BMI = body mass index, aFM = android fat mass, %fat = body fat percentage, VO2max = maximal oxygen uptake, VO2maxFFM = maximal oxygen uptake relative to the fat-free mass. See Table 1 for units.
After adjustment for sex, overweight/obese participants had higher levels of CRP across both fitness classifications (VO2max p = 0.021; VO2maxFFM p = 0.001) than their normal weight peers. Similarly, over-fat participants had higher values of CRP across VO2maxFFM categories (p = 0.004), but interestingly, there was no such effect across VO2max categories (p = 0.117). Participants classified with high VO2max had lower CRP levels across BMI categories (p = 0.009) but not across %fat categories (p = 0.109), and no differences were found in CRP values between VO2maxFFM categories across both fatness classifications (BMI p = 0.058, %fat p = 0.196). No interaction effects were observed between the fitness and fatness classifications (p = 0.266–0.849).
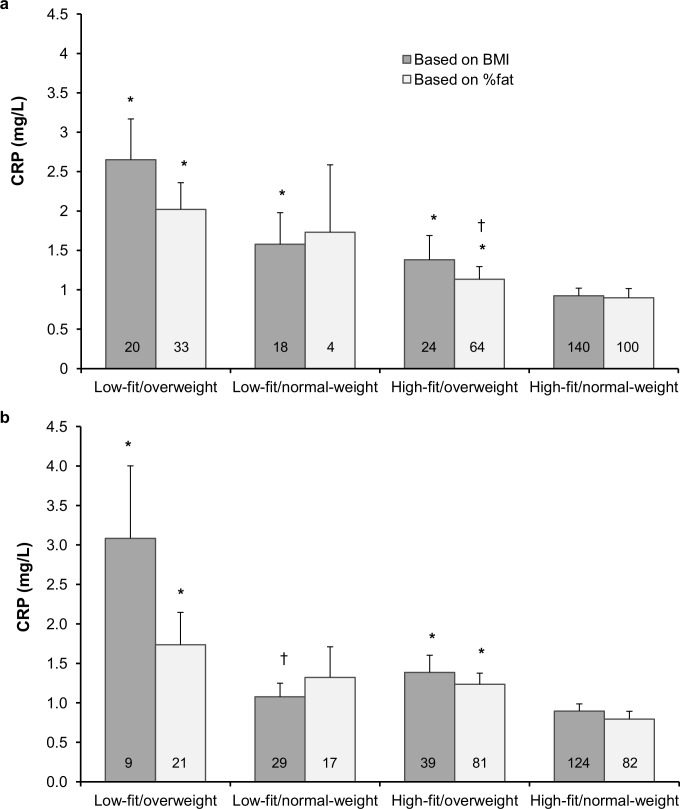
CRP values across fit-fat groups are compared in Fig 1. Adolescents classified as high-fit/normal-weight based on VO2max and BMI had significantly lower CRP values compared to the other fit-fat groups (Fig 1A). Likewise, those classified as high-fit/normal-weight based on VO2max and %fat had significantly lower CRP compared to those high-fit/overweight and low-fit/overweight. Similarly, using VO2maxFFM and either BMI or %fat, the high-fit/normal-weight had significantly lower CRP both from those classified as high-fit/overweight and low-fit/overweight (Fig 1B). Using %fat and VO2max, the low-fit/overweight adolescents had significantly higher CRP values than their high-fit/overweight counterparts (Fig 1A) but using VO2maxFFM and BMI the low-fit/overweight adolescents had significantly higher CRP than those low fit/normal weight (Fig 1B).
Fig 1. Comparison of CRP values across the fit-fat groups based on maximal oxygen uptake (a) and maximal oxygen uptake relative to the fat-free mass (b).
Values are means and SE. Numbers in bars represent the number of participants in each group. CRP = C-reactive protein, BMI = body mass index, %fat = body fat percentage, *Significantly different from the high-fit/normal-weight group, p < 0.05. †Significantly different from the low-fit/overweight group, p < 0.05.
Discussion
The main objective of the study was to assess the relationship between adiposity measurements, fitness and CRP in late adolescence using direct measures of fitness and fatness. Although the results indicate that both fatness and fitness may contribute to the level of CRP in late adolescence, the major finding, contrary to our hypothesis, was that fitness adjusted for body composition (VO2maxFFM) was unrelated to CRP. Our results suggest that previous findings of independent effects of fitness on CRP may have been blurred with multicolinearity due to the strong inverse relation between fitness and fatness. Furthermore, since fatness factors both in the value of fitness relative to body mass and in the value of the adiposity measure controlled for, the variables violate the statistical assumption of independence. To our knowledge, we present novel data obtained using objective methodology to study the relationships between fitness, fatness and CRP and add to the literature by analyzing the data in a manner not done previously.
Our results also suggest that the use of precise methods of body fat measurements such as DXA rather than relying on anthropometric adiposity indicators such as BMI or WC may be superior for studying the relation to CRP. Others have emphasized the importance of precise methods for estimating of both fitness and fatness [13,14].
Studies have previously examined the association of chronic low-level systematic inflammation, indicated by measures of CRP, with measures of fatness and fitness in various age-groups [12–16,18,21–23,29] although none of them have focused specifically on late adolescence as in our study. Some of the previous studies in children/adolescents have also been conducted across wide age range [13,14,22], undoubtedly affected by pubertal status, which was not always controlled for. Regardless, our results support the general agreement of these studies that increased fatness, whether indicated with BMI, WC, fat mass, %fat, or aFM, is related to higher levels of CRP.
Similarly, most of these studies [12–15,18,22,23,29] found increased unadjusted fitness, whether estimated from a submaximal test, predicted from a maximal test, or directly measured, linked to lower CRP as we found in this study. However, in many of the aforementioned studies [12–15,23] fitness and adiposity were strongly related leading to a potential problem of multicolinearity, which can both exaggerate and attenuate the independent effects of fitness. Among children and adolescents, the relations between fitness and cardiovascular risk factors were greatly influenced by the choice of expression (absolute, relative to body mass, relative to fat-free mass) of fitness [30]. FitnessFFM may be the best indirect estimate of the metabolic capacity of the muscle [30] and is a way to estimate the effects of fitness adjusted for body composition. FitnessFFM is also not subject to multicolinearity since it is unrelated to adiposity. In the present study, VO2maxFFM was not associated with CRP. To our knowledge, relating fitness expressed relative to the FFM to CRP has not been done previously, although such expression has lowered the relation between fitness and cardiovascular risk factors compared to expressing fitness relative to body mass [41]. The difference in the relations between VO2max vs. VO2maxFFM and CRP in the present study stems from the significant associations between adiposity and CRP. The use of a fitness index relative to body mass and adjusting it for adiposity is, therefore, highly questionable and may, along with indirect measures or estimates of fitness and fatness, factor in the reason for lack of agreement between studies about whether the impact of fitness on CRP is independent of fatness [12–14,16,17,23,29].
All adiposity measures retained a significant relationship with CRP when adjusted for VO2max although the relationship was reduced, which is similar to what others have found in adolescents [14] and adults [27,29]. However, such an adjustment is subject to the same concerns of multicolinearity and lack of independence as discussed above. Indeed, correcting the adiposity measures for VO2maxFFM had no effects on their relations to CRP. These findings confirm our hypothesis that fatness is more influential on CRP level than fitness and are supported by reports from children [12,19,23], adolescents [14], and adults [15,16,27,29]. Evidence from exercise intervention studies also suggest that fatness has a greater impact than fitness on CRP as only those exercisers who were in the highest tertile or quartile of body mass/fat loss had a significant reduction in CRP [21,24]. Similarly, exercise-induced reduction in CRP was not independent of changes in body composition [28]. This effect may depend on age, however, since among young women such an exercise-initiated decrease in CRP was independent of changes in body composition [25], and among children, the changes in CRP were not related to changes in body composition [26]. The interactive effects of fitness and fatness on CRP may also be more complicated as the effect appears to be larger among obese participants [25] and among more fit participants [28].
Fitness may indeed have important protective effects as those who were classified as high-fit/normal-weight based on empirical classifications [31,38,39] had significantly lower CRP values than the other fit-fat groups (Fig 1A). Moreover, high-fit/overweight individuals, when classified based on %fat, had significantly lower CRP values than their low-fit counterparts. This is similar to findings in adults [16,29] and children [23], although in two of these studies no difference between high-fit and low-fit overweight individuals was observed, possibly due to poor measures of fatness and fitness [16] or low number (n = 4) of high-fit/overweight participants [23]. Similarly, girls with the same baseline adiposity level had lower longitudinal increase in CRP if baseline fitness was higher [42]. Unfortunately, no empirical classifications for fitnessFFM exist. Using the same proportions, within sex, resulting from the empirical VO2max classification to classify participants into low-, average-, and high fitnessFFM categories along with empirical cut-offs for adiposity, really only distinguished between fatness classifications within fitness categories (Fig 1B).
Fitness and fatness can be linked to inflammation via several mechanisms. Adipocytes release proinflammatory cytokines [43], which stimulate the synthesis of CRP in the liver. High levels of free-fatty acids in the blood can also activate proinflammatory genes [44], a process modulated by exercise in animals [45]. The anti-inflammatory effects of exercise could also be mediated by increased insulin sensitivity and higher levels of high-density lipoproteins and adiponectin [43], and improvements in endothelial function may further play a role [17]. Inflammation and insulin resistance have been linked [14] and the impact of fitness and fatness on CRP might be indirect via insulin resistance. However, the present findings suggest that insulin resistance does not exert much influence on the relationship between adiposity, fitness and CRP as has been previously reported [14].
Our study was not without limitations. First and foremost is the cross-sectional design, which does not allow for the determination of a cause and effect relationships. Secondly, using young adult empirical cut-offs to classify 18 year-olds may be debatable. In addition, albeit significant, the variance independently explained in the correlational analysis was rather low (2–11%). Finally, dietary factors were not taken into account but dietary choices may contribute to inflammation. The Icelandic diet is generally high in protein (18%) and relatively low in carbohydrates (44%), whereas total fat consumption is moderate (36%), and 2% of the energy comes from alcohol [46]. Unpublished data from our research center on dietary intake of 18 year-olds reveals similar division of macronutrient but with slightly lower proportions of fat and alcohol in favour of carbohydrates (18% protein, 50% carbohydrates, 32% fat, 1% alcohol). We believe the strengths of the study overcome its limitations in that the cohort was relatively large, focused on a relatively healthy understudied population (late adolescence), and that empirical cut-offs were used to classify participants on BMI, %fat and VO2max. Additionally, we used precise laboratory measurements to assess fatness and fitness, which enabled us to demonstrate the association of fitness adjusted for body composition to CRP as well as the importance of considering multicolinearity when examining the interactive association of fitness and fatness to CRP levels. Neither has, to our knowledge, been done previously. Finally, the findings should have high generalizability as the cohort reasonably reflects 18 year-old adolescents in Iceland because it was recruited from metropolitan Reykjavik where 67% of all Icelandic high-school students are enrolled and all academic structures were represented.
In conclusion, our results support findings from other studies that fatness has a greater association with CRP than fitness. However, fitnessFFM, which is truly independent of adiposity, is not related to CRP, indicating that the previously reported independent effects of fitness might be mediated via the fatness component inherently embedded in fitness when it is expressed relative to body mass. Further studies, especially in different age groups, are needed to confirm or refute our findings. Finally, our study demonstrates the importance of using precise measurements of fitness and fatness when researching the interactive effects of the variables on CRP.
Supporting Information
(XLSX)
Acknowledgments
The authors would like to thank Andrés Eyjólfsson, Erlingur Birgir Richardsson and Gunnar Axel Davíðsson for invaluable help in data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Ministry of Education Sports Fund (no grant number) to SÁA ASÓ, (http://en.rannis.is/funding/youth-sport/the-icelandic-sport-fund/) and the University of Iceland Research Fund (no grant number) to SÁA ASÓ. (http://sjodir.hi.is/node/16131) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378: 815–825. 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 2. Johannsson E, Arngrimsson SA, Thorsdottir I, Sveinsson T. Tracking of overweight from early childhood to adolescence in cohorts born in 1988 and 1994: overweight in a high birth weight population. Int J Obes. 2006;30: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 3. Thorsson B, Aspelund T, Harris TB, Launer LJ, Gudnason V. [Trends in body weight and diabetes in forty years in Iceland]. Laeknabladid. 2009;95: 259–266. [PubMed] [Google Scholar]
- 4. Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA. 1989;262: 2395–2401. [DOI] [PubMed] [Google Scholar]
- 5. Barlow CE, Defina LF, Radford NB, Berry JD, Cooper KH, Haskell WL, et al. Cardiorespiratory fitness and long-term survival in "low-risk" adults. J Am Heart Assoc. 2012;1: e001354 10.1161/JAHA.112.001354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ortega FB, Ruiz JR, Castillo MJ, Sjostrom M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond). 2008;32: 1–11. 10.1038/sj.ijo.0803780 [DOI] [PubMed] [Google Scholar]
- 7. Eisenmann JC, Wickel EE, Welk GJ, Blair SN. Relationship between adolescent fitness and fatness and cardiovascular disease risk factors in adulthood: the Aerobics Center Longitudinal Study (ACLS). Am Heart J. 2005;149: 46–53. [DOI] [PubMed] [Google Scholar]
- 8. Huotari PR, Nupponen H, Laakso L, Kujala UM. Secular trends in aerobic fitness performance in 13-18-year-old adolescents from 1976 to 2001. Br J Sports Med. 2010;44: 968–972. 10.1136/bjsm.2008.055913 [DOI] [PubMed] [Google Scholar]
- 9. Dyrstad SM, Berg T, Tjelta LI. Secular trends in aerobic fitness performance in a cohort of Norwegian adolescents. Scand J Med Sci Sports. 2012;22: 822–827. 10.1111/j.1600-0838.2011.01315.x [DOI] [PubMed] [Google Scholar]
- 10. Ekblom OB, Bak EA, Ekblom BT. Cross-sectional trends in cardiovascular fitness in Swedish 16-year-olds between 1987 and 2007. Acta Paediatr. 2011;100: 565–569. 10.1111/j.1651-2227.2010.02135.x [DOI] [PubMed] [Google Scholar]
- 11. Rietzschel E, De Buyzere M. High-sensitive C-reactive protein: universal prognostic and causative biomarker in heart disease? Biomark Med. 2012;6: 19–34. 10.2217/bmm.11.108 [DOI] [PubMed] [Google Scholar]
- 12. Ruiz JR, Ortega FB, Warnberg J, Sjostrom M. Associations of low-grade inflammation with physical activity, fitness and fatness in prepubertal children; the European Youth Heart Study. Int J Obes (Lond). 2007;31: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 13. Martinez-Gomez D, Gomez-Martinez S, Ruiz JR, Diaz LE, Ortega FB, Widhalm K, et al. Objectively-measured and self-reported physical activity and fitness in relation to inflammatory markers in European adolescents: the HELENA Study. Atherosclerosis. 2012;221: 260–267. 10.1016/j.atherosclerosis.2011.12.032 [DOI] [PubMed] [Google Scholar]
- 14. Martinez-Gomez D, Eisenmann JC, Warnberg J, Gomez-Martinez S, Veses A, Veiga OL, et al. Associations of physical activity, cardiorespiratory fitness and fatness with low-grade inflammation in adolescents: the AFINOS Study. Int J Obes (Lond). 2010;34: 1501–1507. 10.1038/ijo.2010.114 [DOI] [PubMed] [Google Scholar]
- 15. Valentine RJ, Vieira VJ, Woods JA, Evans EM. Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause. 2009;16: 84–89. 10.1097/gme.0b013e31817fcb8f [DOI] [PubMed] [Google Scholar]
- 16. Hamer M, Steptoe A. Prospective study of physical fitness, adiposity, and inflammatory markers in healthy middle-aged men and women. Am J Clin Nutr. 2009;89: 85–89. 10.3945/ajcn.2008.26779 [DOI] [PubMed] [Google Scholar]
- 17. Hamer M. The relative influences of fitness and fatness on inflammatory factors. Preventive Medicine. 2007;44: 3–11. [DOI] [PubMed] [Google Scholar]
- 18. McVean JJ, Carrel AL, Eickhoff JC, Allen DB. Fitness level and body composition are associated with inflammation in non-obese children. J Pediatr Endocrinol Metab. 2009;22: 153–159. [DOI] [PubMed] [Google Scholar]
- 19. Gutin B, Harris RA, Howe CA, Johnson MH, Zhu H, Dong Y. Cardiometabolic biomarkers in young black girls: relations to body fatness and aerobic fitness, and effects of a randomized physical activity trial. Int J Pediatr. 2011;2011: 219268 10.1155/2011/219268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campbell KL, Campbell PT, Ulrich CM, Wener M, Alfano CM, Foster-Schubert K, et al. No reduction in C-reactive protein following a 12-month randomized controlled trial of exercise in men and women. Cancer Epidemiol Biomarkers Prev. 2008;17: 1714–1718. 10.1158/1055-9965.EPI-08-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stewart LK, Earnest CP, Blair SN, Church TS. Effects of different doses of physical activity on C-reactive protein among women. Med Sci Sports Exerc. 2010;42: 701–707. 10.1249/MSS.0b013e3181c03a2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isasi CR, Deckelbaum RJ, Tracy RP, Starc TJ, Berglund L, Shea S. Physical fitness and C-reactive protein level in children and young adults: the Columbia University BioMarkers Study. Pediatrics. 2003;111: 332–338. [DOI] [PubMed] [Google Scholar]
- 23. Parrett AL, Valentine RJ, Arngrimsson SA, Castelli DM, Evans EM. Adiposity, activity, fitness, and C-reactive protein in children. Medicine and Science in Sports and Exercise. 2010;42: 1981–1986. 10.1249/MSS.0b013e3181e0355e [DOI] [PubMed] [Google Scholar]
- 24. Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, et al. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42: 708–716. 10.1249/MSS.0b013e3181c03a43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arikawa AY, Thomas W, Schmitz KH, Kurzer MS. Sixteen weeks of exercise reduces C-reactive protein levels in young women. Med Sci Sports Exerc. 2011;43: 1002–1009. 10.1249/MSS.0b013e3182059eda [DOI] [PubMed] [Google Scholar]
- 26. Nemet D, Oren S, Pantanowitz M, Eliakim A. Effects of a multidisciplinary childhood obesity treatment intervention on adipocytokines, inflammatory and growth mediators. Horm Res Paediatr. 2013;79: 325–332. 10.1159/000348732 [DOI] [PubMed] [Google Scholar]
- 27. Belalcazar LM, Reboussin DM, Haffner SM, Hoogeveen RC, Kriska AM, Schwenke DC, et al. A 1-year lifestyle intervention for weight loss in individuals with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change: from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33: 2297–2303. 10.2337/dc10-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedenreich CM, Neilson HK, Woolcott CG, Wang Q, Stanczyk FZ, McTiernan A, et al. Inflammatory marker changes in a yearlong randomized exercise intervention trial among postmenopausal women. Cancer Prev Res. 2012;5: 98–108. 10.1158/1940-6207.CAPR-11-0369 [DOI] [PubMed] [Google Scholar]
- 29. Arsenault BJ, Cartier A, Cote M, Lemieux I, Tremblay A, Bouchard C, et al. Body composition, cardiorespiratory fitness, and low-grade inflammation in middle-aged men and women. Am J Cardiol. 2009;104: 240–246. 10.1016/j.amjcard.2009.03.027 [DOI] [PubMed] [Google Scholar]
- 30. McMurray RG, Hosick PA, Bugge A. Importance of proper scaling of aerobic power when relating to cardiometabolic risk factors in children. Ann Hum Biol. 2011;38: 647–654. 10.3109/03014460.2011.598561 [DOI] [PubMed] [Google Scholar]
- 31. Thompson WR, editor ACSM´s Guidelines for Exercise Testing and Prescription 8 ed. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 32. McConnell TR. Practical considerations in the testing of VO2max in runners. Sports Med. 1988;5: 57–68. [DOI] [PubMed] [Google Scholar]
- 33. Lucia A, Rabadan M, Hoyos J, Hernandez-Capilla M, Perez M, San Juan AF, et al. Frequency of the VO2max plateau phenomenon in world-class cyclists. Int J Sports Med. 2006;27: 984–992. [DOI] [PubMed] [Google Scholar]
- 34. Duncan GE, Howley ET, Johnson BN. Applicability of VO2max criteria: discontinuous versus continuous protocols. Med Sci Sports Exerc. 1997;29: 273–278. [DOI] [PubMed] [Google Scholar]
- 35. Skinner JS, Gaskill SE, Rankinen T, Leon AS, Rao DC, Wilmore JH, et al. Heart rate versus %VO2max: age, sex, race, initial fitness, and training response—HERITAGE. Med Sci Sports Exerc. 2003;35: 1908–1913. [DOI] [PubMed] [Google Scholar]
- 36. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23: 57–63. [DOI] [PubMed] [Google Scholar]
- 37. Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2007;34: 18–22. [DOI] [PubMed] [Google Scholar]
- 38. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363: 157–163. [DOI] [PubMed] [Google Scholar]
- 39. Lohman TG, Houtkooper LB, Going SB. Body fat measurements goes hi-tech: Not all are created equal. ACSM's Health Fitness J. 1997;1: 30–35. [Google Scholar]
- 40. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107: 363–369. [DOI] [PubMed] [Google Scholar]
- 41. Ondrak KS, McMurray RG, Bangdiwala SI, Harrell JS. Influence of aerobic power and percent body fat on cardiovascular disease risk in youth. J Adolesc Health. 2007;41: 146–152. [DOI] [PubMed] [Google Scholar]
- 42. Puder JJ, Schindler C, Zahner L, Kriemler S. Adiposity, fitness and metabolic risk in children: a cross-sectional and longitudinal study. Int J Pediatr Obes. 2011;6: e297–306. 10.3109/17477166.2010.533774 [DOI] [PubMed] [Google Scholar]
- 43. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83: 461s–465s. [DOI] [PubMed] [Google Scholar]
- 44. Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52: 2882–2887. [DOI] [PubMed] [Google Scholar]
- 45. Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, et al. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18: 749–750. [DOI] [PubMed] [Google Scholar]
- 46. Steingrimsdottir L, Valgeirsdottir H, Halldorsson TI, Gunnarsdottir I, Gisladottir E, Thorgeirsdottir H, et al. [National nutrition surveys and dietary changes in Iceland. Economic differences in healthy eating]. Laeknabladid. 2014;100: 659–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.