Abstract
Primary defects in mitochondrial function are implicated in over 100 diseases, and the list continues to grow. Yet the first mitochondrial defect--a myopathy--was demonstrated only 35 years ago. The field's dramatic expansion reflects growth of knowledge in three areas: (i) characterization of mitochondrial structure and function, (ii) elucidation of the steps involved in mitochondrial biosynthesis, and (iii) discovery of specific mitochondrial DNA. Many mitochondrial diseases are accompanied by mutations in this DNA. Inheritance is by maternal transmission. The metabolic defects encompass the electron transport complexes, intermediates of the tricarboxylic acid cycle, and substrate transport. The clinical manifestations are protean, most often involving skeletal muscle and the central nervous system. In addition to being a primary cause of disease, mitochondrial DNA mutations and impaired oxidation have now been found to occur as secondary phenomena in aging as well as in age-related degenerative diseases such as Parkinson, Alzheimer, and Huntington diseases, amyotrophic lateral sclerosis and cardiomyopathies, atherosclerosis, and diabetes mellitus. Manifestations of both the primary and secondary mitochondrial diseases are thought to result from the production of oxygen free radicals. With increased understanding of the mechanisms underlying the mitochondrial dysfunctions has come the beginnings of therapeutic strategies, based mostly on the administration of antioxidants, replacement of cofactors, and provision of nutrients. At the present accelerating pace of development of what may be called mitochondrial medicine, much more is likely to be achieved within the next few years.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Endogenous DNA damage as related to cancer and aging. Mutat Res. 1989 Sep;214(1):41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Profet M., Gold L. S. Dietary pesticides (99.99% all natural). Proc Natl Acad Sci U S A. 1990 Oct;87(19):7777–7781. doi: 10.1073/pnas.87.19.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K. Oxidants are a major contributor to aging. Ann N Y Acad Sci. 1992 Nov 21;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Arnaudo E., Dalakas M., Shanske S., Moraes C. T., DiMauro S., Schon E. A. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. 1991 Mar 2;337(8740):508–510. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- Attardi G., Chomyn A., King M. P., Kruse B., Polosa P. L., Murdter N. N. Regulation of mitochondrial gene expression in mammalian cells. Biochem Soc Trans. 1990 Aug;18(4):509–513. doi: 10.1042/bst0180509. [DOI] [PubMed] [Google Scholar]
- Ballard P. A., Tetrud J. W., Langston J. W. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985 Jul;35(7):949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Shoffner J. M., Hedaya E. V., Trounce I., Polak M. A., Koontz D. A., Wallace D. C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992 Apr;1(1):11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Bazzett T. J., Becker J. B., Kaatz K. W., Albin R. L. Chronic intrastriatal dialytic administration of quinolinic acid produces selective neural degeneration. Exp Neurol. 1993 Apr;120(2):177–185. doi: 10.1006/exnr.1993.1053. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Kowall N. W., Swartz K. J., Ferrante R. J., Martin J. B. Systemic approaches to modifying quinolinic acid striatal lesions in rats. J Neurosci. 1988 Oct;8(10):3901–3908. doi: 10.1523/JNEUROSCI.08-10-03901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko B. A., Swift M. X-linked dilated cardiomyopathy. N Engl J Med. 1987 May 7;316(19):1186–1191. doi: 10.1056/NEJM198705073161904. [DOI] [PubMed] [Google Scholar]
- Beyer R. E., Burnett B. A., Cartwright K. J., Edington D. W., Falzon M. J., Kreitman K. R., Kuhn T. W., Ramp B. J., Rhee S. Y., Rosenwasser M. J. Tissue coenzyme Q (ubiquinone) and protein concentrations over the life span of the laboratory rat. Mech Ageing Dev. 1985 Nov;32(2-3):267–281. doi: 10.1016/0047-6374(85)90085-5. [DOI] [PubMed] [Google Scholar]
- Bindoff L. A., Birch-Machin M. A., Cartlidge N. E., Parker W. D., Jr, Turnbull D. M. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson's disease. J Neurol Sci. 1991 Aug;104(2):203–208. doi: 10.1016/0022-510x(91)90311-t. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Avigan J., Uhlendorf B. W. A defect in pyruvate decarboxylase in a child with an intermittent movement disorder. J Clin Invest. 1970 Mar;49(3):423–432. doi: 10.1172/JCI106251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E., Schotland D. L., Di Mauro S., Lee C-P Luft's disease: an electron cytochemical study. J Ultrastruct Res. 1977 Jan;58(1):1–9. doi: 10.1016/s0022-5320(77)80002-6. [DOI] [PubMed] [Google Scholar]
- Brennan W. A., Jr, Bird E. D., Aprille J. R. Regional mitochondrial respiratory activity in Huntington's disease brain. J Neurochem. 1985 Jun;44(6):1948–1950. doi: 10.1111/j.1471-4159.1985.tb07192.x. [DOI] [PubMed] [Google Scholar]
- Brouillet E., Jenkins B. G., Hyman B. T., Ferrante R. J., Kowall N. W., Srivastava R., Roy D. S., Rosen B. R., Beal M. F. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem. 1993 Jan;60(1):356–359. doi: 10.1111/j.1471-4159.1993.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., Torroni A., Yang C. C., Wallace D. C. Mitochondrial DNA complex I and III mutations associated with Leber's hereditary optic neuropathy. Genetics. 1992 Jan;130(1):163–173. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Cohen G., Spina M. B. Deprenyl suppresses the oxidant stress associated with increased dopamine turnover. Ann Neurol. 1989 Nov;26(5):689–690. doi: 10.1002/ana.410260518. [DOI] [PubMed] [Google Scholar]
- Cooper J. M., Mann V. M., Schapira A. H. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992 Nov;113(1):91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Stepien G., Shoffner J. M., Lott M. T., Kanter K., Wallace D. C. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. JAMA. 1991 Oct 2;266(13):1812–1816. [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
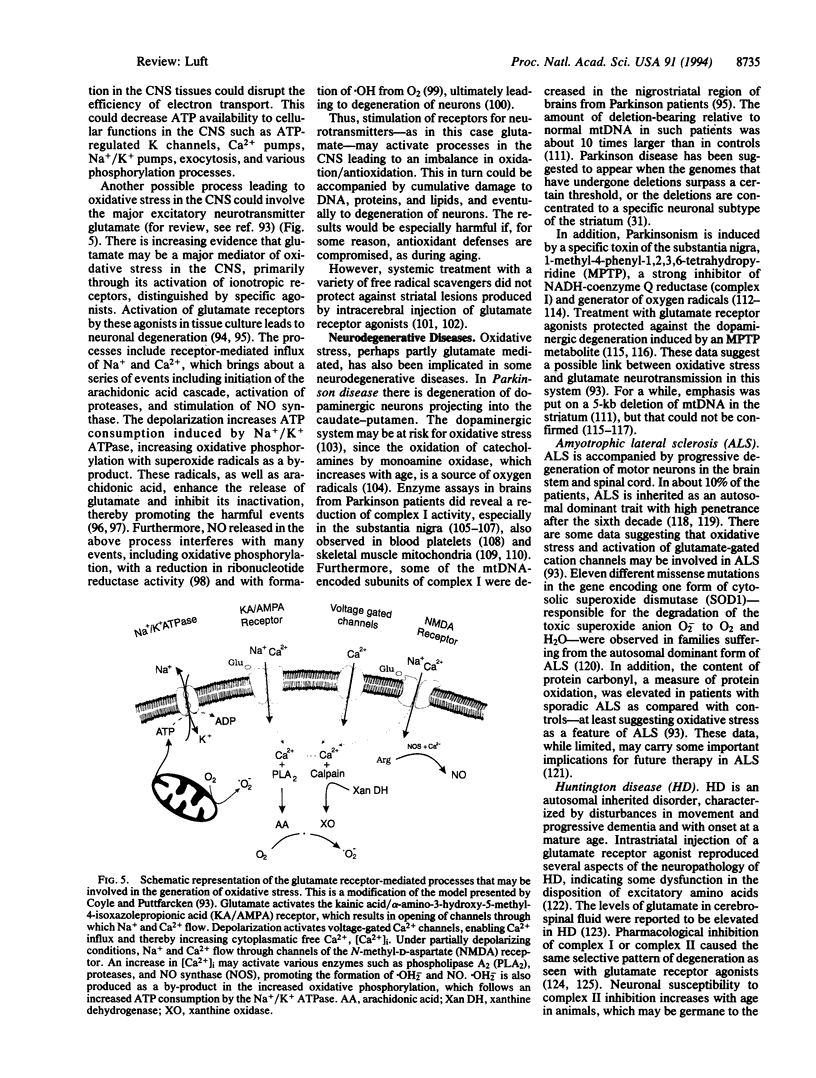
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature. 1976 Sep 16;263(5574):244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L., Snyder S. H. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992 Sep;32(3):297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Lee C. P., Schotland D. L., Scarpa A., Conn H., Jr, Chance B. Luft's disease. Further biochemical and ultrastructural studies of skeletal muscle in the second case. J Neurol Sci. 1976 Feb;27(2):217–232. doi: 10.1016/0022-510x(76)90063-0. [DOI] [PubMed] [Google Scholar]
- DiMauro S., DiMauro P. M. Muscle carnitine palmityltransferase deficiency and myoglobinuria. Science. 1973 Nov 20;182(4115):929–931. doi: 10.1126/science.182.4115.929. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S., Ahnström G., Welsh M. Exposure of pancreatic islets to different alkylating agents decreases mitochondrial DNA content but only streptozotocin induces long-lasting functional impairment of B-cells. Biochem Pharmacol. 1991 Nov 27;42(12):2275–2282. doi: 10.1016/0006-2952(91)90230-3. [DOI] [PubMed] [Google Scholar]
- Eleff S., Kennaway N. G., Buist N. R., Darley-Usmar V. M., Capaldi R. A., Bank W. J., Chance B. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3529–3533. doi: 10.1073/pnas.81.11.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. B., Chase H. P. Prevention or delay of type 1 (insulin-dependent) diabetes mellitus in children using nicotinamide. Diabetologia. 1991 May;34(5):362–365. doi: 10.1007/BF00405010. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science. 1973 Mar 2;179(4076):899–902. doi: 10.1126/science.179.4076.899. [DOI] [PubMed] [Google Scholar]
- Enstrom J. E., Kanim L. E., Klein M. A. Vitamin C intake and mortality among a sample of the United States population. Epidemiology. 1992 May;3(3):194–202. doi: 10.1097/00001648-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Enter C., Müller-Höcker J., Zierz S., Kurlemann G., Pongratz D., Förster C., Obermaier-Kusser B., Gerbitz K. D. A specific point mutation in the mitochondrial genome of Caucasians with MELAS. Hum Genet. 1991 Dec;88(2):233–236. doi: 10.1007/BF00206080. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Wäg G., Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull. 1993 Jul;49(3):566–576. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- Fahn S., Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992 Dec;32(6):804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fernandez-Silva P., Petruzzella V., Fracasso F., Gadaleta M. N., Cantatore P. Reduced synthesis of mtRNA in isolated mitochondria of senescent rat brain. Biochem Biophys Res Commun. 1991 Apr 30;176(2):645–653. doi: 10.1016/s0006-291x(05)80233-5. [DOI] [PubMed] [Google Scholar]
- French J. H., Sherard E. S., Lubell H., Brotz M., Moore C. L. Trichopoliodystrophy. I. Report of a case and biochemical studies. Arch Neurol. 1972 Mar;26(3):229–244. doi: 10.1001/archneur.1972.00490090055004. [DOI] [PubMed] [Google Scholar]
- Gadaleta M. N., Petruzzella V., Renis M., Fracasso F., Cantatore P. Reduced transcription of mitochondrial DNA in the senescent rat. Tissue dependence and effect of L-carnitine. Eur J Biochem. 1990 Feb 14;187(3):501–506. doi: 10.1111/j.1432-1033.1990.tb15331.x. [DOI] [PubMed] [Google Scholar]
- Gaziano J. M., Manson J. E., Buring J. E., Hennekens C. H. Dietary antioxidants and cardiovascular disease. Ann N Y Acad Sci. 1992 Sep 30;669:249–259. doi: 10.1111/j.1749-6632.1992.tb17104.x. [DOI] [PubMed] [Google Scholar]
- Gerbitz K. D. Does the mitochondrial DNA play a role in the pathogenesis of diabetes? Diabetologia. 1992 Dec;35(12):1181–1186. doi: 10.1007/BF00401375. [DOI] [PubMed] [Google Scholar]
- Gey K. F., Puska P. Plasma vitamins E and A inversely correlated to mortality from ischemic heart disease in cross-cultural epidemiology. Ann N Y Acad Sci. 1989;570:268–282. doi: 10.1111/j.1749-6632.1989.tb14926.x. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Stern B. R., Manley N. B., Ames B. N. Rodent carcinogens: setting priorities. Science. 1992 Oct 9;258(5080):261–265. doi: 10.1126/science.1411524. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of low-density lipoprotein receptors: implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation. 1987 Sep;76(3):504–507. doi: 10.1161/01.cir.76.3.504. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Grossman L. I. Mitochondrial DNA in sickness and in health. Am J Hum Genet. 1990 Mar;46(3):415–417. [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Ogawa T., Kondo T., Mochizuki M., Tanaka M., Sugiyama S., Ito T., Satake T., Ozawa T. Cardiomyopathy with mitochondrial DNA mutations. Am Heart J. 1991 Sep;122(3 Pt 1):866–869. doi: 10.1016/0002-8703(91)90542-p. [DOI] [PubMed] [Google Scholar]
- Hattori K., Tanaka M., Sugiyama S., Obayashi T., Ito T., Satake T., Hanaki Y., Asai J., Nagano M., Ozawa T. Age-dependent increase in deleted mitochondrial DNA in the human heart: possible contributory factor to presbycardia. Am Heart J. 1991 Jun;121(6 Pt 1):1735–1742. doi: 10.1016/0002-8703(91)90020-i. [DOI] [PubMed] [Google Scholar]
- Heineke E. W., Johnson M. B., Dillberger J. E., Robinson K. M. Antioxidant MDL 29,311 prevents diabetes in nonobese diabetic and multiple low-dose STZ-injected mice. Diabetes. 1993 Dec;42(12):1721–1730. doi: 10.2337/diab.42.12.1721. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Parisi M. A., Bennett J. L., Clayton D. A. Impairment of mitochondrial transcription termination by a point mutation associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1991 May 16;351(6323):236–239. doi: 10.1038/351236a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Horton W. A., Eldridge R., Brody J. A. Familial motor neuron disease. Evidence for at least three different types. Neurology. 1976 May;26(5):460–465. doi: 10.1212/wnl.26.5.460. [DOI] [PubMed] [Google Scholar]
- Jialal I., Grundy S. M. Effect of dietary supplementation with alpha-tocopherol on the oxidative modification of low density lipoprotein. J Lipid Res. 1992 Jun;33(6):899–906. [PubMed] [Google Scholar]
- Jinnai K., Yamada H., Kanda F., Masui Y., Tanaka M., Ozawa T., Fujita T. A case of mitochondrial myopathy, encephalopathy and lactic acidosis due to cytochrome c oxidase deficiency with neurogenic muscular changes. Eur Neurol. 1990;30(1):56–60. doi: 10.1159/000116641. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Drachman D. B., Hurko O. Identical mitochondrial DNA deletion in blood and muscle. Lancet. 1989 Feb 18;1(8634):393–394. doi: 10.1016/s0140-6736(89)91779-0. [DOI] [PubMed] [Google Scholar]
- Kalén A., Appelkvist E. L., Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989 Jul;24(7):579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- Kamikawa T., Kobayashi A., Yamashita T., Hayashi H., Yamazaki N. Effects of coenzyme Q10 on exercise tolerance in chronic stable angina pectoris. Am J Cardiol. 1985 Aug 1;56(4):247–251. doi: 10.1016/0002-9149(85)90843-4. [DOI] [PubMed] [Google Scholar]
- Kasai H., Okada Y., Nishimura S., Rao M. S., Reddy J. K. Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following long-term exposure to a peroxisome proliferator. Cancer Res. 1989 May 15;49(10):2603–2605. [PubMed] [Google Scholar]
- Katagiri H., Asano T., Ishihara H., Inukai K., Anai M., Yamanouchi T., Tsukuda K., Kikuchi M., Kitaoka H., Ohsawa N. Mitochondrial diabetes mellitus: prevalence and clinical characterization of diabetes due to mitochondrial tRNA(Leu(UUR)) gene mutation in Japanese patients. Diabetologia. 1994 May;37(5):504–510. doi: 10.1007/s001250050139. [DOI] [PubMed] [Google Scholar]
- Katayama M., Tanaka M., Yamamoto H., Ohbayashi T., Nimura Y., Ozawa T. Deleted mitochondrial DNA in the skeletal muscle of aged individuals. Biochem Int. 1991 Sep;25(1):47–56. [PubMed] [Google Scholar]
- Kato K., Puttfarcken P. S., Lyons W. E., Coyle J. T. Developmental time course and ionic dependence of kainate-mediated toxicity in rat cerebellar granule cell cultures. J Pharmacol Exp Ther. 1991 Jan;256(1):402–411. [PubMed] [Google Scholar]
- Klinkhammer C., Popowa P., Gleichmann H. Specific immunity to streptozocin. Cellular requirements for induction of lymphoproliferation. Diabetes. 1988 Jan;37(1):74–80. doi: 10.2337/diab.37.1.74. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- LAKIN M., LOCKE S. Progressive ocular myopathy with ovarian insufficiency and diabetes mellitus. Report of a case. Diabetes. 1961 May-Jun;10:228–231. doi: 10.2337/diab.10.3.228. [DOI] [PubMed] [Google Scholar]
- LUFT R., IKKOS D., PALMIERI G., ERNSTER L., AFZELIUS B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962 Sep;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Lodish H. Mitochondrial diseases: gene mapping and gene therapy. Cell. 1990 Jun 15;61(6):925–926. doi: 10.1016/0092-8674(90)90055-j. [DOI] [PubMed] [Google Scholar]
- Langsjoen P. H., Langsjoen P. H., Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1990 Feb 15;65(7):521–523. doi: 10.1016/0002-9149(90)90824-k. [DOI] [PubMed] [Google Scholar]
- Langsjoen P. H., Vadhanavikit S., Folkers K. Effective treatment with coenzyme Q10 of patients with chronic myocardial disease. Drugs Exp Clin Res. 1985;11(8):577–579. [PubMed] [Google Scholar]
- Langsjoen P. H., Vadhanavikit S., Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4240–4244. doi: 10.1073/pnas.82.12.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Holme E., Kristiansson B., Oldfors A., Tulinius M. Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res. 1990 Aug;28(2):131–136. doi: 10.1203/00006450-199008000-00011. [DOI] [PubMed] [Google Scholar]
- Law R. H., Farrell L. B., Nero D., Devenish R. J., Nagley P. Studies on the import into mitochondria of yeast ATP synthase subunits 8 and 9 encoded by artificial nuclear genes. FEBS Lett. 1988 Aug 29;236(2):501–505. doi: 10.1016/0014-5793(88)80086-3. [DOI] [PubMed] [Google Scholar]
- LeDoux S. P., Hall C. R., Forbes P. M., Patton N. J., Wilson G. L. Mechanisms of nicotinamide and thymidine protection from alloxan and streptozocin toxicity. Diabetes. 1988 Aug;37(8):1015–1019. doi: 10.2337/diab.37.8.1015. [DOI] [PubMed] [Google Scholar]
- Lestienne P., Nelson J., Riederer P., Jellinger K., Reichmann H. Normal mitochondrial genome in brain from patients with Parkinson's disease and complex I defect. J Neurochem. 1990 Nov;55(5):1810–1812. doi: 10.1111/j.1471-4159.1990.tb04973.x. [DOI] [PubMed] [Google Scholar]
- Lestienne P., Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet. 1988 Apr 16;1(8590):885–885. doi: 10.1016/s0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Lin R., Wisniewski H. M., Hwang Y. W., Grundke-Iqbal I., Healy-Louie G., Iqbal K. Detection of point mutations in codon 331 of mitochondrial NADH dehydrogenase subunit 2 in Alzheimer's brains. Biochem Biophys Res Commun. 1992 Jan 15;182(1):238–246. doi: 10.1016/s0006-291x(05)80136-6. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Marzuki S., Ozawa T., Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989 Mar 25;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Loveland B., Wang C. R., Yonekawa H., Hermel E., Lindahl K. F. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990 Mar 23;60(6):971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- Michels V. V., Moll P. P., Miller F. A., Tajik A. J., Chu J. S., Driscoll D. J., Burnett J. C., Rodeheffer R. J., Chesebro J. H., Tazelaar H. D. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992 Jan 9;326(2):77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Coyle J. T. Idebenone attenuates neuronal degeneration induced by intrastriatal injection of excitotoxins. Exp Neurol. 1990 Apr;108(1):38–45. doi: 10.1016/0014-4886(90)90005-d. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., Oya H., Ozawa T., Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Shanske S., Tritschler H. J., Aprille J. R., Andreetta F., Bonilla E., Schon E. A., DiMauro S. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991 Mar;48(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- Mortensen S. A., Vadhanavikit S., Baandrup U., Folkers K. Long-term coenzyme Q10 therapy: a major advance in the management of resistant myocardial failure. Drugs Exp Clin Res. 1985;11(8):581–593. [PubMed] [Google Scholar]
- Mulder D. W., Kurland L. T., Offord K. P., Beard C. M. Familial adult motor neuron disease: amyotrophic lateral sclerosis. Neurology. 1986 Apr;36(4):511–517. doi: 10.1212/wnl.36.4.511. [DOI] [PubMed] [Google Scholar]
- Müller-Höcker J. Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J Neurol Sci. 1990 Dec;100(1-2):14–21. doi: 10.1016/0022-510x(90)90006-9. [DOI] [PubMed] [Google Scholar]
- NASS S., NASS M. M. INTRAMITOCHONDRIAL FIBERS WITH DNA CHARACTERISTICS. II. ENZYMATIC AND OTHER HYDROLYTIC TREATMENTS. J Cell Biol. 1963 Dec;19:613–629. doi: 10.1083/jcb.19.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas W. J., Vyas I., Heikkila R. E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985 Jul 1;36(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Noer A. S., Marzuki S., Trounce I., Byrne E. Mitochondrial DNA deletion in encephalomyopathy. Lancet. 1988 Nov 26;2(8622):1253–1254. doi: 10.1016/s0140-6736(88)90847-1. [DOI] [PubMed] [Google Scholar]
- Nomikos I. N., Wang Y., Lafferty K. J. Involvement of O2 radicals in 'autoimmune' diabetes. Immunol Cell Biol. 1989 Feb;67(Pt 1):85–87. doi: 10.1038/icb.1989.12. [DOI] [PubMed] [Google Scholar]
- Novelli A., Reilly J. A., Lysko P. G., Henneberry R. C. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988 Jun 7;451(1-2):205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Ogasahara S., Nishikawa Y., Yorifuji S., Soga F., Nakamura Y., Takahashi M., Hashimoto S., Kono N., Tarui S. Treatment of Kearns-Sayre syndrome with coenzyme Q10. Neurology. 1986 Jan;36(1):45–53. doi: 10.1212/wnl.36.1.45. [DOI] [PubMed] [Google Scholar]
- Ohhara H., Kanaide H., Yoshimura R., Okada M., Nakamura M. A protective effect of coenzyme Q10 on ischemia and reperfusion of the isolated perfused rat heart. J Mol Cell Cardiol. 1981 Jan;13(1):65–74. doi: 10.1016/0022-2828(81)90229-7. [DOI] [PubMed] [Google Scholar]
- Oka Y., Katagiri H., Yazaki Y., Murase T., Kobayashi T. Mitochondrial gene mutation in islet-cell-antibody-positive patients who were initially non-insulin-dependent diabetics. Lancet. 1993 Aug 28;342(8870):527–528. doi: 10.1016/0140-6736(93)91649-7. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Ikebe S., Ohno K., Kondo T., Mizuno Y. Quantitative determination of deleted mitochondrial DNA relative to normal DNA in parkinsonian striatum by a kinetic PCR analysis. Biochem Biophys Res Commun. 1990 Oct 30;172(2):483–489. doi: 10.1016/0006-291x(90)90698-m. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Hattori K., Ito T., Ohno K., Takahashi A., Sato W., Takada G., Mayumi B. Multiple mitochondrial DNA deletions exist in cardiomyocytes of patients with hypertrophic or dilated cardiomyopathy. Biochem Biophys Res Commun. 1990 Jul 31;170(2):830–836. doi: 10.1016/0006-291x(90)92166-w. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Ino H., Ohno K., Hattori K., Ohbayashi T., Ito T., Deguchi H., Kawamura K. Patients with idiopathic cardiomyopathy belong to the same mitochondrial DNA gene family of Parkinson's disease and mitochondrial encephalomyopathy. Biochem Biophys Res Commun. 1991 May 31;177(1):518–525. doi: 10.1016/0006-291x(91)92014-b. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Boyson S. J., Parks J. K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989 Dec;26(6):719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Filley C. M., Parks J. K. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990 Aug;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Partridge T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve. 1991 Mar;14(3):197–212. doi: 10.1002/mus.880140302. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E., Cherici G., Alesiani M., Carlà V., Moroni F. Excitatory amino acid release from rat hippocampal slices as a consequence of free-radical formation. J Neurochem. 1988 Dec;51(6):1960–1963. doi: 10.1111/j.1471-4159.1988.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Hansen S. What excitotoxin kills striatal neurons in Huntington's disease? Clues from neurochemical studies. Neurology. 1990 Jan;40(1):20–24. doi: 10.1212/wnl.40.1.20. [DOI] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Gardiner R. M. Duplications of mitochondrial DNA in mitochondrial myopathy. Lancet. 1989 Feb 4;1(8632):236–240. doi: 10.1016/s0140-6736(89)91256-7. [DOI] [PubMed] [Google Scholar]
- Poulton J. Mitochondrial DNA and genetic disease. Bioessays. 1992 Nov;14(11):763–768. doi: 10.1002/bies.950141108. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Suarez W. L., Thomas P. D., Strynadka K., Simpson I. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia. 1992 May;35(5):409–413. doi: 10.1007/BF02342435. [DOI] [PubMed] [Google Scholar]
- Reardon W., Ross R. J., Sweeney M. G., Luxon L. M., Pembrey M. E., Harding A. E., Trembath R. C. Diabetes mellitus associated with a pathogenic point mutation in mitochondrial DNA. Lancet. 1992 Dec 5;340(8832):1376–1379. doi: 10.1016/0140-6736(92)92560-3. [DOI] [PubMed] [Google Scholar]
- Richter C. Do mitochondrial DNA fragments promote cancer and aging? FEBS Lett. 1988 Dec 5;241(1-2):1–5. doi: 10.1016/0014-5793(88)81018-4. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma R. A., Wood D. A., Macintyre C. C., Elton R., Gey K. F., Oliver M. F. Low plasma vitamins E and C. Increased risk of angina in Scottish men. Ann N Y Acad Sci. 1989;570:291–295. doi: 10.1111/j.1749-6632.1989.tb14928.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rötig A., Bessis J. L., Romero N., Cormier V., Saudubray J. M., Narcy P., Lenoir G., Rustin P., Munnich A. Maternally inherited duplication of the mitochondrial genome in a syndrome of proximal tubulopathy, diabetes mellitus, and cerebellar ataxia. Am J Hum Genet. 1992 Feb;50(2):364–370. [PMC free article] [PubMed] [Google Scholar]
- Sai K., Takagi A., Umemura T., Hasegawa R., Kurokawa Y. Changes of 8-hydroxydeoxyguanosine levels in rat organ DNA during the aging process. J Environ Pathol Toxicol Oncol. 1992 May-Jun;11(3):139–143. [PubMed] [Google Scholar]
- Salonen J. T., Nyyssönen K., Korpela H., Tuomilehto J., Seppänen R., Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992 Sep;86(3):803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990 Mar;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989 Jun 3;1(8649):1269–1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Holt I. J., Sweeney M., Harding A. E., Jenner P., Marsden C. D. Mitochondrial DNA analysis in Parkinson's disease. Mov Disord. 1990;5(4):294–297. doi: 10.1002/mds.870050406. [DOI] [PubMed] [Google Scholar]
- Scholte H. R. The biochemical basis of mitochondrial diseases. J Bioenerg Biomembr. 1988 Apr;20(2):161–191. doi: 10.1007/BF00768393. [DOI] [PubMed] [Google Scholar]
- Sheu K. F., Kim Y. T., Blass J. P., Weksler M. E. An immunochemical study of the pyruvate dehydrogenase deficit in Alzheimer's disease brain. Ann Neurol. 1985 May;17(5):444–449. doi: 10.1002/ana.410170505. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., 4th, Wallace D. C. Oxidative phosphorylation diseases. Disorders of two genomes. Adv Hum Genet. 1990;19:267–330. [PubMed] [Google Scholar]
- Shoffner J. M., 4th, Wallace D. C. Oxidative phosphorylation diseases. Disorders of two genomes. Adv Hum Genet. 1990;19:267–330. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., Wallace D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991 Sep;30(3):332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Shoulson I. Antioxidative therapeutic strategies for Parkinson's disease. Ann N Y Acad Sci. 1992 May 11;648:37–41. doi: 10.1111/j.1749-6632.1992.tb24522.x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Pathophysiology and treatment of focal cerebral ischemia. Part II: Mechanisms of damage and treatment. J Neurosurg. 1992 Sep;77(3):337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- Sims N. R., Finegan J. M., Blass J. P., Bowen D. M., Neary D. Mitochondrial function in brain tissue in primary degenerative dementia. Brain Res. 1987 Dec 8;436(1):30–38. doi: 10.1016/0006-8993(87)91553-8. [DOI] [PubMed] [Google Scholar]
- Spiro A. J., Moore C. L., Prineas J. W., Strasberg P. M., Rapin I. A cytochrome-related inherited disorder of the nervous system and muscle. Arch Neurol. 1970 Aug;23(2):103–112. doi: 10.1001/archneur.1970.00480260009002. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Antioxidants in the prevention of human atherosclerosis. Summary of the proceedings of a National Heart, Lung, and Blood Institute Workshop: September 5-6, 1991, Bethesda, Maryland. Circulation. 1992 Jun;85(6):2337–2344. doi: 10.1161/01.cir.85.6.2337. [DOI] [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunamori M., Tanaka H., Maruyama T., Sultan I., Sakamoto T., Suzuki A. Clinical experience of coenzyme Q10 to enhance intraoperative myocardial protection in coronary artery revascularization. Cardiovasc Drugs Ther. 1991 Mar;5 (Suppl 2):297–300. doi: 10.1007/BF00054751. [DOI] [PubMed] [Google Scholar]
- Suomalainen A., Majander A., Haltia M., Somer H., Lönnqvist J., Savontaus M. L., Peltonen L. Multiple deletions of mitochondrial DNA in several tissues of a patient with severe retarded depression and familial progressive external ophthalmoplegia. J Clin Invest. 1992 Jul;90(1):61–66. doi: 10.1172/JCI115856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A., Paetau A., Leinonen H., Majander A., Peltonen L., Somer H. Inherited idiopathic dilated cardiomyopathy with multiple deletions of mitochondrial DNA. Lancet. 1992 Nov 28;340(8831):1319–1320. doi: 10.1016/0140-6736(92)92496-3. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Miyamoto S., Kinoshita Y., Yamada K., Sasaki N., Makino E., Nakajima H. Diabetes mellitus in Kearns-Sayre syndrome. Eur Neurol. 1988;28(1):34–38. doi: 10.1159/000116225. [DOI] [PubMed] [Google Scholar]
- Tellez-Nagel I., Johnson A. B., Terry R. D. Studies on brain biopsies of patients with Huntington's chorea. J Neuropathol Exp Neurol. 1974 Apr;33(2):308–332. doi: 10.1097/00005072-197404000-00008. [DOI] [PubMed] [Google Scholar]
- Trounce I., Byrne E., Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989 Mar 25;1(8639):637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Turski L., Bressler K., Rettig K. J., Löschmann P. A., Wachtel H. Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991 Jan 31;349(6308):414–418. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA sequence variation in human evolution and disease. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8739–8746. doi: 10.1073/pnas.91.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C. Report of the committee on human mitochondrial DNA. Cytogenet Cell Genet. 1989;51(1-4):612–621. doi: 10.1159/000132809. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- Yen T. C., Chen Y. S., King K. L., Yeh S. H., Wei Y. H. Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun. 1989 Dec 29;165(3):944–1003. doi: 10.1016/0006-291x(89)92701-0. [DOI] [PubMed] [Google Scholar]
- Yuzaki M., Ohkoshi N., Kanazawa I., Kagawa Y., Ohta S. Multiple deletions in mitochondrial DNA at direct repeats of non-D-loop regions in cases of familial mitochondrial myopathy. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1352–1357. doi: 10.1016/0006-291x(89)91818-4. [DOI] [PubMed] [Google Scholar]
- Zeviani M., DiDonato S. Neurological disorders due to mutations of the mitochondrial genome. Neuromuscul Disord. 1991;1(3):165–172. doi: 10.1016/0960-8966(91)90020-s. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- Zhang J. R., Sevanian A. Effect of vitamin E on arachidonic acid peroxidation and its binding to Chinese hamster V79 cell DNA. Biochim Biophys Acta. 1991 Sep 11;1085(2):159–166. doi: 10.1016/0005-2760(91)90090-5. [DOI] [PubMed] [Google Scholar]
- van den Ouweland J. M., Lemkes H., Maassen J. A. A rare mitochondrial DNA BstNI polymorphism in a family with type II diabetes. Nucleic Acids Res. 1991 Apr 25;19(8):1962–1962. doi: 10.1093/nar/19.8.1962-a. [DOI] [PMC free article] [PubMed] [Google Scholar]