Abstract
Context
Gastroenteritis remains a leading cause of childhood morbidity.
Objective
Because prior reviews have focused on isolated symptoms and studies conducted in developing countries, this study focused on interventions commonly considered for use in developed countries. Intervention specific, patient-centered outcomes were selected.
Data Sources
MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, trial registries, grey literature, and scientific meetings.
Study Selection
Randomized controlled trials, conducted in developed countries, of children aged <18 years, with gastroenteritis, performed in emergency department or outpatient settings which evaluated oral rehydration therapy (ORT), antiemetics, probiotics or intravenous fluid administration rate.
Data Extraction
The study was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and the PRISMA guidelines. Data were independently extracted by multiple investigators. Analyses employed random effects models.
Results
31 trials (4,444 patients) were included. ORT: Compared with intravenous rehydration, hospitalization (RR 0.80, 95%CI 0.24, 2.71) and emergency department return visits (RR 0.86, 95%CI 0.39, 1.89) were similar. Antiemetics: Fewer children administered an antiemetic required intravenous rehydration (RR 0.40, 95%CI 0.26, 0.60) While the data could not be meta-analyzed, three studies reported that ondansetron administration does increase the frequency of diarrhea. Probiotics: No studies reported on the primary outcome, three studies evaluated hospitalization within 7 days (RR 0.87, 95%CI 0.25, 2.98). Rehydration: No difference in length of stay was identified for rapid vs. standard intravenous or nasogastric rehydration. A single study found that 5% dextrose in normal saline reduced hospitalizations compared with normal saline alone (RR 0.70, 95% CI 0.53, 0.92).
Conclusions
There is a paucity of patient-centered outcome evidence to support many interventions. Since ORT is a low-cost, non-invasive intervention, it should continue to be used. Routine probiotic use cannot be endorsed at this time in outpatient children with gastroenteritis. Despite some evidence that ondansetron administration increases diarrhea frequency, emergency department use leads to reductions in intravenous rehydration and hospitalization. No benefits were associated with ondansetron use following emergency department discharge.
Introduction
Gastroenteritis results in nearly 2 million pediatric emergency department (ED) visits in the United States annually.[1] Although rotavirus vaccination has altered the epidemiology of acute gastroenteritis (AGE),[2] emerging pathogens such as norovirus[3] continue to result in symptoms prompting medical evaluation.[4]
While systematic reviews (SR) have evaluated treatment options,[5–11] they are often inconclusive or discordant. Recent guidelines published by European Society for Pediatric Gastroenterology, Hepatology and Nutrition (EPGHAN) and the European Society of Pediatric Infectious Diseases (ESPID)[12] and those issued by the National Institute for Health and Clinical Excellence (NICE)[13] are vague regarding their recommendation regarding the use of antiemetics, a therapy endorsed by other position papers and meta-analyses.[10,11,14] The aforementioned guidelines also have differing recommendations regarding probiotics (Table 1).[15] The most recent guidelines endorsed by the American Academy of Pediatrics were published over a decade ago.[16]
Table 1. Summary of differing recommendations of prominent gastroenteritis guidelines.
| Antiemetics | Probiotics | |
|---|---|---|
| Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H European society for pediatric gastroenterology, hepatology, and nutrition/european society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in europe: update 2014. J Pediatr Gastroenterol Nutr 2014, 59:132–152. | Ondansetron, at the dosages used in the available studies and administered orally or intravenously, may be effective in young children with vomiting related to AGE. Before a final recommendation is made, a clearance on safety in children is, however, needed (II, B) (strong recommendation, low-quality evidence). | Administration of effective probiotic strains reduce the duration of hospital stay and may be considered in children admitted with AGE (II, B) (strong recommendation, low quality evidence). Active treatment with probiotics, in adjunct to ORS, is effective in reducing the duration and intensity of symptoms of gastroenteritis. Selected probiotics can be used in children with AGE (I, A) (strong recommendation, moderate-quality evidence). New evidence has confirmed that probiotics are effective in reducing the duration of symptoms in children with AGE (I, A) (strong recommendation, moderate-quality evidence). The use of the following probiotics should be considered in the management of children with AGE as an adjunct to rehydration therapy: L rhamnosus GG and S boulardii (I, A) (strong recommendation, low-quality evidence). |
| National Collaborating Centre for Women's and Children's Health Diarrhoea and vomiting caused by gastroenteritis: diagnosis, assessment and management in children younger than 5 years. Commissioned by the National Institute for Health and Clinical Excellence; Available at: http://www.nice.org.uk/guidance/cg84/resources/cg84-diarrhoea-and-vomiting-in-children-under-5-full-guideline2. Accessed October 15, 2014. | The guideline development group (GDG) considered that evidence from randomised controlled trials indicated that oral ondansetron could increase the success rate with oral rehydration therapy. The GDG was concerned that ondansetron might have adverse effects such as worsening diarrhoea. There was no evidence to support other agents, including metoclopramide and dexamethasone. The GDG concluded that administration of anti-emetics could not currently be recommended. | There was evidence from a high-quality systematic review suggesting that probiotic treatment had a beneficial effect–shortening the duration of diarrhoea and reducing the stool frequency. However, the available studies varied in quality, in the specific probiotics studied, in the treatment regimens used and in the outcomes examined. Therefore, despite some evidence of possible clinical benefit, the GDG did not consider it appropriate to recommend the use of a probiotic at this time. |
| Cheng A, Canadian Paediatric Society—Acute Care Committee Emergency department use of oral ondansetron for acute gastroenteritis-related vomtiing in infants and children. Paediatr Child Health 2011, 16:177–179. | Oral ondansetron therapy, as a single dose, should be considered for infants and children six months to 12 years of age who present to the ED with vomiting related to suspected acute gastroenteritis, and who have mild to moderate dehydration or who have failed oral rehydration therapy. | Not applicable. |
| Piescik-Lech M, Shamir R, Guarino A, Szajewska H Review article: the management of acute gastroenteritis in children. Aliment Pharmacol Ther 2013, 37:289–303. | New evidence indicates that ondansetron, at the dosages used in the studies and administered orally or intravenously, may be considered for use in young children with vomiting related to AGE. However, before a final recommendation is made, a clearance on safety in children is needed. | New evidence has confirmed that the probiotics currently supported by ESPGHAN/ESPID–Lactobacillus GG and S. boulardii–are effective in reducing the duration of diarrhoea. Current evidence clearly indicates that these are not the only effective probiotic microorganisms; however, these are the most studied. Probiotic effects are strain-specific, so the efficacy and safety of each should be established. |
| King CK, Glass R, Bresee JS, Duggan C Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003, 52:1–16. | No clear recommendation in report. | Ondansetron, a serotonin antagonist, either by the oral or IV route, can be effective in decreasing vomiting and limiting hospital admission. However, reliance on pharmacologic agents shifts the therapeutic focus away from appropriate fluid, electrolyte, and nutritional therapy, can result in adverse events, and can add unnecessarily to the economic cost of illness. Because acute diarrhea is a common illness, cost-effective analyses should be undertaken before routine pharmacologic therapy is recommended. |
Participant eligibility criteria in 'gastroenteritis' studies can vary significantly between studies. Since the diagnosis is made on clinical grounds, the precision is debatable. Most clinical trials employ clearly defined clinical features to determine eligibility. As these studies reflect pragmatic considerations and employ randomization, they retain internal validity. However, without careful consideration, their grouping together in SR and meta-analyses can be problematic. Consequently, significant variation has been documented in the management of AGE in developed countries at institutional,[17] national,[18] and international levels.[19]. This is in part explained by heterogeneity–population, setting, etiologic agents, and nutritional status. Studies from low and middle income countries include more severe cases, organisms rarely seen in developed nations, and malnourished children.[20,21] Outcome selection is increasingly a concern with most SRs focusing on diarrhea duration–a single symptom which in addition to being heterogeneous itself, also overlooks other key symptoms (e.g. vomiting). An analysis of 138 pediatric AGE randomized clinical trials (RCT) identified 64 unique definitions of diarrhea, and 69 of “resolution.”[22]
Driven by recent evidence and uncertainties in practice, the efficacy of oral rehydration therapy (ORT), antiemetics, probiotics and intravenous rehydration in developed countries was evaluated.
Methods
A protocol (S1 File) was established a priori and followed standard SR procedures.[23] The planned approach involved initially conducting a comprehensive search to identify all relevant SRs performed to date to ensure that previously identified relevant studies were included in the review. This was followed by a thorough search for additional studies. Lastly, the evidence focusing on studies of outpatient children in developed countries was re-examined.
Information Sources and Searches
A medical librarian (A.M.) developed the search strategy in collaboration with the research team to identify previous SRs of: (1) ORT; (2) antiemetics; (3) probiotics; and (4) intravenous fluid therapy (IVT). The following sources were searched: (a) MEDLINE (2000 to April 2012), EMBASE (2000 to April 2012), and the Cochrane Database of Systematic Reviews (2005 to April 2012) via the OvidSP platform; (b) appropriate journals and major, relevant scientific meetings; (c) reference lists of relevant reviews; and (d) primary authors were contacted. The search was not restricted by language or publication status. All studies contained in previous relevant SRs were screened for inclusion. This approach is an economically efficient method of identifying all prior relevant studies dating back to the origins of the search engines.
The librarian then searched the literature to identify trials published since the dates included in the earliest SR identified. The search included electronic databases (i.e. MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials; S2 File) and the grey literature. The latter search included clinical trials registries (clinicaltrials.gov, the World Health Organization trials registry, and the Current Controlled Trials registry) and conference proceedings (Society for Pediatric Research, American Academy of Pediatrics, Canadian Pediatric Society, International Conference on Emergency Medicine; 2010–2012). Reference lists were screened and experts contacted. No language restrictions were employed. The search was re-run in September 2014 to identify any recently published studies.
Inclusion Criteria
Search results were screened independently by two reviewers to identify potentially relevant citations. The full text of potentially relevant citations was assessed for inclusion by two independent reviewers using predefined criteria. Disagreements were resolved by consensus. Eligible RCTs involved children <18 years of age with AGE and evaluated: (1) any ORT regimen vs. intravenous or nasogastric rehydration; (2) any antiemetic medication vs. placebo or alternative; (3) any probiotic agent vs. placebo or alternative; and (4) different rates and compositions of intravenous fluid rehydration protocols. Studies were included if the condition evaluated was consistent with AGE and the location was an ED or similar outpatient setting in a developed nation as defined by the United Nations (i.e. Australia, Canada, European countries, Japan, New Zealand, and the United States).[24]
Outcomes
The interventions and outcome measures were identified by clinician authors (SF, KB, EF, SG) and knowledge users (DJ, FB, BH, MJ, TK) a priori based on clinical relevance incorporating recommendations to employ outcomes of interest to parents, clinicians, and health systems (Table 2).[25]
Table 2. Interventions and their specific outcomes evaluated.
| Primary Outcome | Secondary Outcomes |
|---|---|
| Oral Rehydration Therapy | |
| Hospitalization | Length of Stay |
| Return Visits | |
| Adverse Effects | |
| Antiemetic Agents | |
| Administration of Intravenous Rehydration | Hospitalization |
| Length of Stay | |
| Return Visits | |
| Adverse Effects | |
| Probiotic Agents | |
| Any Subsequent Healthcare Visit (7 days) | Administration of Intravenous Rehydration |
| Hospitalization | |
| Adverse Effects | |
| Intravenous Fluid Therapy | |
| Length of Stay | Hospitalization |
| Return Visits | |
| Dysnatremia* | |
*The term dysnatremia refers to the presence of a serum sodium value outside of the age accepted range of normal values.
Data Extraction
As is commonly performed, one reviewer extracted data using a structured form with verification performed by a second reviewer.[26–29] Items extracted were: study characteristics, participants, interventions and comparisons, outcomes, funding source, and results. Data were entered into Microsoft Excel (Microsoft, Redmond, WA). Disagreements were resolved by consensus, or by a third reviewer.
Risk of Bias Assessment
The Cochrane Risk of Bias (RoB) tool[23] was applied, independently by two reviewers to assess internal validity (S1 Table). Discrepancies were resolved by consensus or by involving a third reviewer.
Grading the Body of Evidence
The quality of evidence was assessed using methods developed by the GRADE Working Group.[30] For each comparison and outcome, the following were assessed: risk of bias, consistency, directness, and precision. Overall quality was graded as high, moderate, low, or very low by two reviewers with discrepancies resolved through discussion.
Statistical Analysis
Evidence tables to describe the studies were developed. A quantitative analysis synthesized numerically the effectiveness of each intervention and investigated heterogeneity. Data was reported for continuous outcomes as mean differences which were combined, where appropriate, using a weighted mean difference and inverse-variance methods.[31] Data for dichotomous outcomes are reported employing risk ratios (RR) or risk differences. The latter was employed when there were zero events in one of the treatment arms of an individual study which contributed data to the meta-analysis (e.g. adverse events). Results are reported with 95% confidence intervals. The primary analysis was based on a random effects model due to anticipated clinical variability between studies.[32] Sensitivity analyses were conducted using a fixed effects model and no differences were identified in the results. Heterogeneity was quantified using the I-squared statistic.[33,34] When heterogeneity was substantial (I2 ≥75%), pooling of studies was not performed. Due to insufficient numbers pre-planned sensitivity analyses based on risk of bias, intention-to-treat analysis, and funding source could not be conducted. Testing for publication bias was not performed due to insufficient numbers. Analyses were conducted using Review Manager 5.0 (The Cochrane Collaboration, Copenhagen, Denmark).
Results
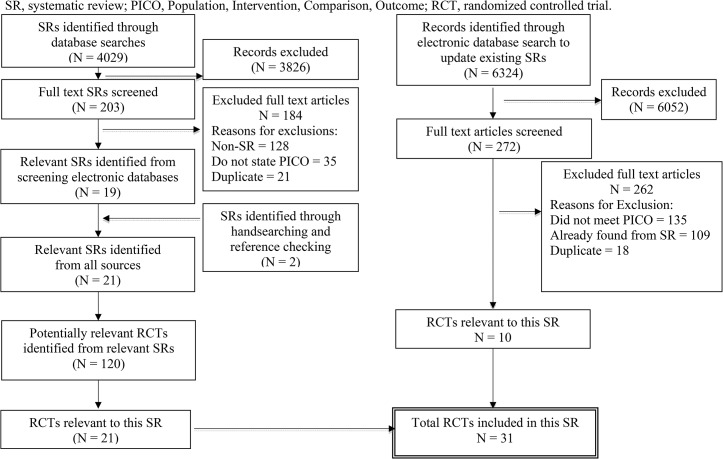
Sixty-six RCTS were relevant; 35 did not report any of the a priori identified outcomes of interest, therefore, 31 RCTs involving 4,444 patients were included (Fig 1; Table 3). Four antiemetic agents were studied along with 11 different probiotic strains. Overall risk of bias was low for 23% of trials (7/31), unclear for 74% (23/31), and high for 3% (1/31); S1 Table. Industry funding was identified in the following studies: ORT – 5 (50%); antiemetics – 3 (38%); probiotics – 2 (33%); IVT – 2 (33%).
Fig 1. Flow diagram of study selection.
SR, systematic review; PICO, Population, Intervention, Comparison, Outcome; RCT, randomized controlled trial.
Table 3. Overview of studies included in systematic review.
| Comparison | Number of studies (Number of patients) | Number of studies providing data for primary outcome (Number of patients) | Number of studies providing data for secondary outcomes (Number of patients) | Years of publication, median (range) | Countries of study (Numer of studies) | Risk of bias |
|---|---|---|---|---|---|---|
| Intravenous Therapy vs. Oral Rehydration Therapy | 10 (599) | 3 (136) | 10 (599) | 1992 (1985–2005) | Australia (1), Canada (1), Finland (1), USA (7) | 9 unclear, 1 low |
| Any Antiemetic vs. Placebo | 9 (1691) | 7 (1043) | 9 (1691) | 2008 (2002–2014) | Australia (1), Canada (1), Germany (1), Saudi Arabia (1), USA (5) | 7 unclear, 2 low |
| Any Probiotic vs. Placebo | 6 (1170) | 1 (155) | 6 (1170) | 2009 (2007–2012) | Australia (1), Italy (2),Ukraine (2), USA (1) | 4 unclear, 2 low |
| Intravenous Fluid Rates & Compositions | 6 (984) | 2 (305) | 4(644) | 2011 (2006–2014) | Australia (2), Canada (1), USA (1) | 3 unclear, 2 low, 1 high |
Oral Rehydration Therapy (ORT)
Ten studies involving 599 patients compared ORT with IVT (S2 Table). Sample sizes ranged from 24 to 111 (median 47, inter-quartile range 35 to 91). Five studies did not report dehydration severity; the remainder, with one exception,[35] included primarily children with mild dehydration.
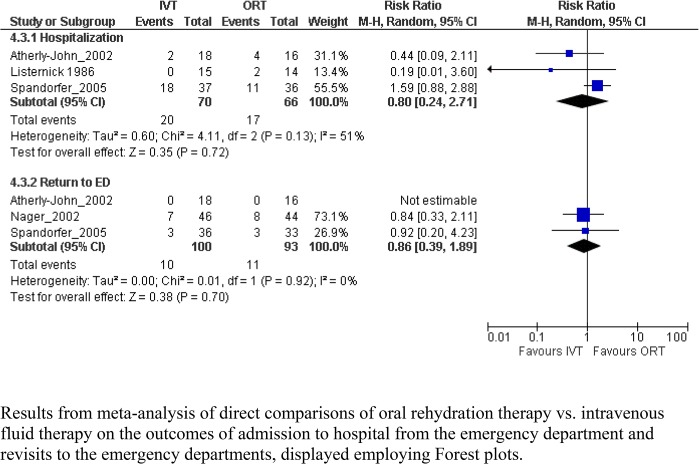
Three studies provided data on the effect for the primary outcome of hospitalization, which in meta-analysis, showed no significant difference between groups (RR 0.80, 95% CI 0.24, 2.71, I2 = 51%; Table 4; Fig 2). The quality of evidence was low owing to inconsistencies in effect estimates across studies and imprecision in the pooled result. No difference was observed in the secondary outcome of return to the ED. Six studies provided data on ED length of stay. However, there was substantial heterogeneity across studies and results could not be pooled (I2 = 91%). Quality of the evidence was very low. The mean difference in length of stay reported by individual studies, including time spent in hospital, ranged from 1.20 days less for ORT (95% CI -2.16, -0.24) to 0.92 days longer (95% CI 0.31, 1.53). Five studies reported on adverse effects (Table 5); no significant differences were identified.
Table 4. Results for Primary and Secondary Outcomes.
| Comparison | Outcome | Number of studies (Number of patients) | Risk Ratio (95% CI) | I2 (%) | Quality of evidence based on GRADE |
|---|---|---|---|---|---|
| Oral Rehydration Therapy | |||||
| IVT vs. ORT | |||||
| Primary | Hospitalization | 3 (136) | 0.80 (0.24, 2.71) | 51 | Low |
| Secondary | Length of ED Stay | 6 (308) | Not pooled due to substantial heterogeneity | 91 | very low |
| Return to ED | 3 (193) | 0.86 (0.39, 1.89) | 0 | moderate | |
| Antiemetics | |||||
| Any antiemetic vs. placebo | |||||
| Primary | IV Fluid Administration | 5 (733) | 0.40 (0.26, 0.60)† | 30 | High |
| Secondary | Hospitalization | 7 (1043) | 0.44 (0.23, 0.82)† | 27 | High |
| Return to ED | 8 (1074) | 1.31 (0.73, 2.35) | 52 | moderate | |
| Length of ED Stay | 5 (991) | Not pooled due to substantial heterogeneity | 75 | moderate | |
| Dimenhydrinate vs. placebo | |||||
| Primary | IV Fluid Administration | 1 (144) | 0.74 (0.29, 1.87) | NA | low |
| Secondary | Hospitalization | 2 (368) | 0.72 (0.34, 1.53) | 0 | moderate |
| Return to ED | 2 (343) | 0.61 (0.34, 1.12) | 0 | moderate | |
| Ondansetron vs. dexamethasone | |||||
| Primary | Hospitalization | 1 (93) | 0.29 (0.06, 1.33) | NA | low |
| Secondary | Return to ED | 1 (56) | 4.30 (1.00, 18.47)† | NA | low |
| Ondansetron vs. placebo | |||||
| Primary | IV Fluid Administration | 3 (433) | 0.38 (0.27, 0.54)† | 0 | High |
| Secondary | Hospitalization | 5 (613) | 0.32 (0.18, 0.57)† | 2 | moderate |
| Return to ED | 5 (609) | 1.57 (0.70, 3.52) | 36 | Low | |
| Length of ED Stay | 4 (826) | Not pooled due to substantial heterogeneity | NA | moderate | |
| Granisetron vs. placebo | |||||
| Primary | IV Fluid Administration | 1 (156) | 0.05 (0.00, 0.78) | NA | Very low |
| Secondary | Hospitalization | 1 (165) | 3.04 (0.13, 73.46) | NA | Very low |
| Return to ED | 1 (122) | 3.09 (1.19, 8.05) | NA | Very low | |
| Length of ED Stay | 1 (165) | -0.65 (-1.29, -0.01) | NA | Very low | |
| Probiotics | |||||
| Probiotic (Any) vs. Placebo | |||||
| Primary | Return to ED | 1 (155) | 0.78 (0.36, 1.67) | NA | Low |
| Secondary | Hospitalization | 3 (833) | 0.53 (0.26, 1.07) | 20 | Low |
| IV Fluid Administration | 1 (64) | 1.13 (0.81, 1.57) | NA | Low | |
| Probiotic (Combo) vs. Placebo | |||||
| Secondary | Hospitalization | 1 (189) | 0.47 (0.09, 2.53) | NA | very low |
| B. clausii vs. Placebo | |||||
| Secondary | Hospitalization | 1 (192) | 0.92 (0.24, 3.57) | NA | very low |
| E. faecium vs. Placebo | |||||
| Secondary | Hospitalization | 1 (183) | 1.01 (0.26, 3.92) | NA | very low |
| L. casei vs. Placebo | |||||
| Secondary | IV Fluid Administration | 1 (64) | 1.13 (0.81, 1.57) | NA | Low |
| L. paracasei vs. Placebo | |||||
| Secondary | Hospitalization | 1 (107) | 0.37 (0.18, 0.75)† | NA | Low |
| L. rhamnosus vs. Placebo | |||||
| Primary | Return to ED | 1 (155) | 0.78 (0.36, 1.67) | NA | very low |
| Secondary | Hospitalization | 2 (347) | 0.65 (0.08, 5.45) | 43 | very low |
| S. boulardii vs. Placebo | |||||
| Secondary | Hospitalization | 1 (183) | 1.01 (0.26, 3.92) | NA | very low |
| IV Fluids | |||||
| Rapid IV vs. Standard IV | |||||
| Primary | Length of ED Stay >6 hours | 1 (226) | 1.06 (0.74, 1.53) | NA | low |
| Secondary | Hospitalization | 2 (318) | 1.03 (0.44, 2.39) | 23 | low |
| Return to ED | 2 (311) | 0.88 (0.52, 1.48) | 0 | low | |
| Rapid NG vs. Standard NG | |||||
| Primary | Length of ED Stay* | 1 (228) | -1.90 (-9.11, 5.31) | NA | very low |
| Secondary | Hospitalization | 1 (228) | 0.69 (0.49, 0.97)† | NA | low |
| Isotonic IV Fluid vs. Hypotonic IV Fluid | |||||
| Secondary | Dysnatremia | 1 (44) | -0.23 (-0.41, -0.04)† | NA | very low |
| 5% Dextrose in Normal Saline vs. Normal Saline | |||||
| Primary | Length of ED Stay | 1 (188) | 0.13 (-0.27, 0.53) | NA | Very low |
| Secondary | Hospitalization | 1 (114) | 0.70 (0.53, 0.92) | NA | Very low |
| Return visits | 1 (80) | 0.64 (0.30, 1.36) | NA | Very low | |
| Plasma-Lyte A vs. 0.9% Sodium Chloride | |||||
| Secondary | Hospitalization | 1 (54) | 1.09 (0.59, 2.03) | NA | Very low |
IVT, Intravenous Therapy; ORT, Oral Rehydration Therapy; ED, Emergency Department; GRADE, Grading of Recommendations Assessment, Development and Evaluation; IV, intravenous; NA, Not Applicable; NG, nasogastric; vs, versus.
† Statistically significant effect.
*Length of stay, as a continuous variable, is reported as mean difference (95% CI).
Fig 2. Meta-graph comparing oral rehydration therapy vs. intravenous fluid therapy.
Results from meta-analysis of direct comparisons of oral rehydration therapy vs. intravenous fluid therapy on the outcomes of admission to hospital from the emergency department and revisits to the emergency departments, displayed employing Forest plots.
Table 5. Adverse Events.
| Adverse Event | Total number of patients | Number of events/total (%) | Risk Difference (95% CI) | Risk Ratio* (95% CI) | I2 (%)‡ |
|---|---|---|---|---|---|
| IVT vs ORT | |||||
| Periorbital Edema | 219 | IVT 6/99 (6); ORT 8/120 (7) | 0.03 (-0.07, 0.13) | 1.30 (0.29, 5.87) | 54 |
| Hyponatremia | 104 | IVT 3/52 (6); ORT 4/52 (8) | 0.02 (-0.08,0.12) | 1.33 (0.31, 5.67) | NA |
| Seizure | 152 | IVT 1/67 (1); ORT 1/85 (1) | 0.00 (-0.07, 0.08) | 0.70 (0.04, 11.94) | 31 |
| Phlebitis | 52 | IVT 0/17 (0); ORT 0/35 (0) | 0.00 (-0.08, 0.08) | - | NA |
| Antiemetics | |||||
| Headache | 137 | Dimenhydrinate 3/69 (4); Placebo 1/68 (2) | 0.03 (-0.03, 0.08) | 2.96 (0.32, 27.72) | NA |
| Rash | 137 | Dimenhydrinate 4/69 (6); Placebo 0/68 (0) | 0.06 (0.00, 0.12) | - | NA |
| Hyperactivity | 137 | Dimenhydrinate 4/69 (6); Placebo 0/68 (0) | 0.06 (0.00, 0.12) | - | NA |
| GI Upset | 137 | Dimenhydrinate 3/69 (4); Placebo 3/68 (3) | 0.00 (-0.07, 0.07) | 0.99 (0.21, 4.71) | NA |
| Sedation | 208 | Dimenhydrinate 22/106 (22); Placebo 18/102 (19) | 0.03 (-0.08, 0.14) | 1.18 (0.67, 2.06) | NA |
| Exanthem | 208 | Dimenhydrinate 1/106 (1); Placebo 1/102 (1) | 0.00 (-0.03, 0.03) | 0.96 (0.06, 15.18) | NA |
| Drowsiness | 137 | Dimenhydrinate 29/69 (46); Placebo 25/68 (37) | 0.01 (-0.02, 0.22) | 1.14 (0.75, 1.73) | 0 |
| Urticaria | 214 | Ondansetron 0/107 (0); Placebo 1/107 (1) | -0.01 (-0.03, 0.02) | - | NA |
| Probiotics | |||||
| Rhinitis | 113 | Escherichia coli Nissle 1/55 (2); Placebo 0/58 (0) | 0.02 (-0.03, 0.07) | - | NA |
| Otitis Media | 113 | Escherichia coli Nissle 1/55 (2); Placebo 0/58 (0) | 0.02 (-0.03, 0.07) | - | NA |
| Abdominal Pain | 261 | Escherichia coli Nissle 2/130 (1); Placebo 4/131 (3) | 0.01 (-0.07, 0.05) | 0.67 (0.06, 7.97) | 60 |
| Hypersensitivity | 151 | Escherichia coli Nissle 1/75 (1); Placebo 0/76 (0) | 0.01 (-0.02, 0.05) | - | NA |
| Isotonic IV Fluids vs Hypotonic IV Fluids | |||||
| Dysnatremia | 44 | Isotonic solution 0/20 (0); Hypotonic solution 5/22 (23) | -0.23 (-0.41, -0.04)† | - | NA |
| Plasma-Lyte A vs. 0.9% Sodium Chloride | |||||
| Dysnatremia | 75 | Plasma-Lyte A 1/38 (3)0.9% Sodium Chloride 1/37 (3) | 0.00 (-0.07, 0.07) | 0.97 (0.06, 15.00) | NA |
IVT, Intravenous Therapy; ORT, Oral Rehydration Therapy; GI, Gastrointestinal; IV, Intravenous; NA, Not Applicable.
* Risk ratio calculated where there was at least one incidence in each group
† Statistically significant difference between groups
‡ Based on risk difference
Antiemetics
Nine studies involving 1,691 patients evaluated three antiemetic agents: ondansetron (N = 6), dimenhydrinate (N = 2), and granisetron (N = 1). Eight RCTs compared the antiemetic agent with placebo; one RCT compared ondansetron with dexamethasone (Table 3; Fig 3; S3 Table).
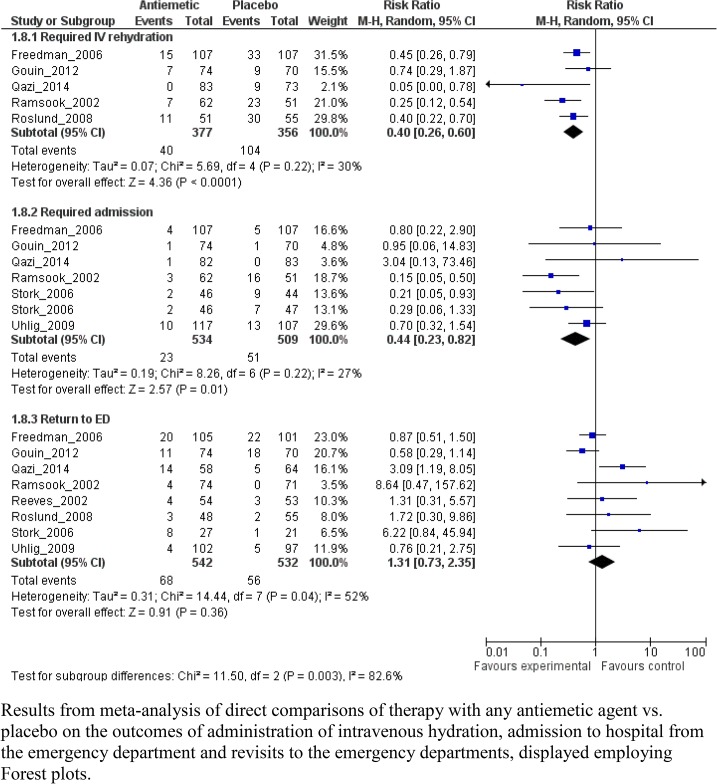
Fig 3. Meta-graph comparing any antiemetic therapy vs. placebo.
Results from meta-analysis of direct comparisons of therapy with any antiemetic agent vs. placebo on the outcomes of administration of intravenous hydration, admission to hospital from the emergency department and revisits to the emergency departments, displayed employing Forest plots.
All five studies demonstrated a reduction in the primary outcome of intravenous rehydration usage amongst children administered an antiemetic agent (Fig 3; RR 0.40, 95% CI 0.26, 0.60, I2 = 30%; N = 733). Quality of evidence for this outcome was high. Pooled results demonstrated that patients receiving an antiemetic agent were hospitalized less often (RR 0.44, 95% CI 0.23, 0.82, I2 = 27%; N = 1043). There was substantial heterogeneity for length of stay results across studies (I2 = 75%); therefore, data were not pooled. Mean length of stay reported in individual studies ranged from 0.23 hours (95% CI -0.49, 0.03) to 1.0 hours (95% CI -1.34, -0.66) less for antiemetics. There was no difference between groups in the proportions of children experiencing ED revisits.
Findings were similar when only those studies involving ondansetron were analyzed. No significant differences were identified when studies involving dimenhydrinate were evaluated. Three studies evaluating dimenhydrinate reported specific adverse events—drowsiness, headache, rash, hyperactivity, gastrointestinal upset, and sedation; no differences were noted compared with placebo (Table 5).
Diarrhea frequency was evaluated in several studies but due to the varying methods of reporting the findings, the results could not be combined. Following a single dose of ondansetron or placebo, during ED ORT one study (N = 215) reported that ondansetron administration resulted in a statistically significant increase in diarrhea frequency (1.4 vs. 0.5 stools; group (P < 0.001).[36] Another study, which similarly evaluated single oral dose ondansetron vs. placebo, collected post-discharge diarrhea frequency information. In their cohort (N = 106), the median number of episodes of diarrhea post-discharge was 0 in both groups; 93% of children administered placebo and 80% of those administered ondansetron had < 3 episodes of diarrhea after discharge and the mean was 1.8 vs. 0.5 episodes of diarrhea respectively (no test of significance provided).[37] A multi-dose ondansetron vs. placebo study (N = 145) reported no difference in stool frequency while in the ED (mean 0.70 vs. 0.61 episodes respectively; P = 0.62); however, following discharge there was a significant increase in stool frequency amongst those administered ondansetron at both 24 (4.7 vs. 1.4; P = 0.002) and 48-hour (3.0 vs. 1.0; P = 0.02) outcome time points.[38] A study evaluating single dose, intravenous ondansetron (N = 107), reported that compared with placebo, there were no significant differences in the proportion (41%—ondansetron; 40%—placebo; P = 0.93), frequency (median of 5 in both groups; P = 0.87) or duration (60 hours in the ondansetron vs. 49 hours in placebo groups; P = 0.72) of diarrheal episodes following the intervention.[39] Lastly, a multi-dose study of granisetron (N = 165) reported a similar odds (OR 1.29, 95% CI 0.64, 2.60) and frequency of diarrhea following medication administration (6.5 ± 6.1 vs. 5.8 ± 7.6; P = 0.51).[40]
Probiotics
Six studies, involving 1170 patients, examined different probiotics (Table 4; S4 Table); five compared individual probiotic agents with placebo, one compared multiple and combination products with placebo.
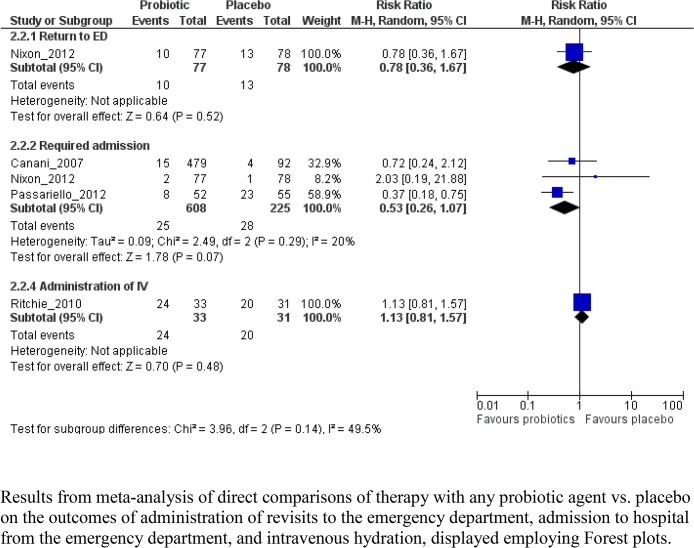
No studies reported findings related to any subsequent healthcare provider visits. One study reported no difference between groups in terms of return for additional ED care (Fig 4 and Table 4). Pooled results from 3 studies showed no difference between groups for hospitalization within 7 days (RR 0.53, 95% CI 0.26, 1.07, I2 = 20%; N = 571). Based on one study, no difference was observed between probiotic and placebo groups in the need to administer intravenous rehydration within 7 days.
Fig 4. Meta-graph comparing any probiotic therapy vs. placebo.
Results from meta-analysis of direct comparisons of therapy with any probiotic agent vs. placebo on the outcomes of administration of revisits to the emergency department, admission to hospital from the emergency department, and intravenous hydration, displayed employing Forest plots.
When analyzed by individual probiotic product, most comparisons included a single RCT and reported no significant differences between groups. The quality of evidence for all comparisons was low or very low. Specific adverse events were reported in only one study; no differences were found between groups (Table 5).
Intravenous Fluid Therapy (IVT)
Six studies involving 984 patients compared different rates or compositions of intravenous fluids (S5 Table). Two studies (N = 318) compared rapid (60 ml/kg and 50 ml/kg over 1 hour) vs standard rehydration rates (20 ml/kg over 1 hour and 50 ml/kg over 3 hours, respectively); one study compared rapid polyelectrolyte with rapid nasogastric (NG) rehydration (N = 254); one study compared isotonic with hypotonic intravenous solutions (N = 124); one study compared 5% dextrose in normal saline solution with normal saline solution alone (N = 188); and, one study compared Plasma-Lyte A with 0.9% sodium chloride (N = 100).
No difference in length of stay was identified for rapid vs. standard IVT or rapid vs. standard NG rehydration (Table 4). Two studies comparing rapid vs. standard IVT also identified no difference between groups in admissions or ED revisits. Significantly fewer admissions occurred with rapid compared with standard NG rehydration. The study comparing isotonic vs. hypotonic IVT did not report on any of the outcomes of interest except for dysnatremia; significantly fewer cases occurred with isotonic intravenous hydration. A reduction in hospitalizations (RR 0.70, 95% CI 0.53, 0.92) was identified when 5% dextrose in normal saline was compared with normal saline; however, no differences were found for length of ED stay or return visits. Similarly, no differences were found for time to rehydration, hospitalization, or incidence of dysnatremia in the study comparing Plasma-Lyte A with 0.9% sodium chloride. Quality of evidence for all outcomes and comparisons was low or very low.
Discussion
Key treatment decisions were examined—route of rehydration, use of antiemetics and probiotics, and methods of intravenous and nasogastric rehydration—in a single SR, with a unique focus on children in developed countries in order to provide information needed by clinician and knowledge users. Although, the use of antiemetics confers short term benefits in outpatients by reducing intravenous rehydration administration and hospitalization, no difference was identified in terms of ED revisits. Aside from individual studies which documented positive results, no other interventions evaluated were found to result in improved outcomes. This must be interpreted with caution because most often there were insufficient numbers of eligible studies evaluating the outcomes of interest.
The treatment of AGE was approached from a perspective which yielded a paucity of studies from developed countries that reported on the a priori identified outcome measures. Consequently the findings contradict those of prior reviews which endorse the use of probiotics based on their ability to reduce the mean duration of diarrhea (by 25 hours), the likelihood of diarrhea lasting ≥4 days (risk ratio 0.41; 0.32 to 0.53), and stool frequency on day #2 (mean difference 0.80 stools).[8] However, prior reviews included heterogeneous groups of children and the importance of the outcomes evaluated has been questioned.[22] Because of the methodological limitations of many of the trials included in prior reviews of probiotics, it is suggested that the evidence be viewed with caution.[41]
To minimize heterogeneity and maximize relevance the study focused on a well-defined population, key interventions, and clinically important outcomes. This differs from other reviews that “summarise the more recent data” and search only MEDLINE and The Cochrane Database of Systematic Reviews.[15] Although a recent overview of reviews reported similar findings, the methodologies, which differed significantly, resulted in the inclusion of different studies.[42] Other prior meta-analyses have not restricted their populations to developed regions and have struggled with the inclusion of studies with varying outcome measure definitions (e.g. duration of diarrhea).[22] These two characteristics differentiate this review and underlie the differences in the studies included compared with others.
Oral Rehydration Therapy
Although ORT is the most fundamental and accepted treatment,[16] studies comparing ORT to IVT generally provided inadequate descriptions of the severity of dehydration, which drives treatment decisions.[16] The trials identified were small, often single-centre, and rarely reported sample size calculations.[43] Children with mild dehydration were the typical target population, reflecting the overuse of IVT in North America.[44] Although no difference in the primary outcome of hospitalization was identified, in the context of the comparison evaluated (ORT vs. IVT), this supports the use of ORT.
Although oral rehydration solution (ORS) use per se (i.e. we did not focus on the solution used) was not evaluated, the conceptual approach of ORT as opposed to IVT was evaluated. Since ORS remains the cornerstone of AGE management and is considered to be the one of the top medical discoveries of the 20th century,[45] its effectiveness in children with moderate dehydration was not questioned. Given the paucity of studies identified in this review, to further reduce IVT use in children with moderate dehydration in developed countries, quality improvement studies documenting the keys to successful knowledge translation in the target environments, are needed.[46] Such studies have been called for to enhance the ability to translate research findings into clinical practice to maximize the use of evidence-based therapies.[47]
Antiemetics
Despite limited endorsement in most practice guidelines,[16] this intervention included the largest number of children of the interventions included in this study. While the results favoured the use of antiemetics as it relates to short-term outcomes, no difference was identified in ED revisits. This finding is in keeping with the expectations of single dose use of a medication with relatively short half-life. Although prior reviews have been hesitant to recommend ondansetron use in light of concerns related to its arrhythmogenic potential,[15] recent evidence has reduced concerns related to single oral dose use in otherwise healthy children.[48] Although we could not meta-analyze the available data on the impact of antiemetics on diarrhea frequency, the data we report does seem to indicate that ondansetron administration does increase the frequency of diarrhea. The clinical relevance of this increase (range: 0.1–0.9 stools while undergoing ORT) in the ED is minimal as reflected by the clinically relevant outcomes of intravenous rehydration and admission which are both reduced amongst children administered ondansetron relative to placebo. Following discharge, there similarly appears to be an increase in the number of diarrheal episodes and this appears to be most pronounced with multi-dose therapy. Given the lack of benefit seen with multi-dose therapy and the increased risk of diarrhea, such regimens are not recommended,[49]
Probiotics
European guidelines state that probiotics “should be considered in the management of children with AGE as an adjunct to rehydration therapy.”[12] Use in North America remains limited, and has been reported to be as low as 1% amongst inpatients in large U.S. academic pediatric centres.[50] Although over 60 studies have been conducted,[8] the current analysis raises concerns as it relates to outcomes evaluated. No studies evaluated the primary outcome identified as most important by our knowledge users—subsequent healthcare provider visits. This outcome was deemed to reflect a clinically significant benefit to the child and family and extends beyond simply measuring the absolute number of stools or time to last stool. Furthermore, the quality of the evidence included was 'very low' or 'low' and a disproportionate number of studies (4 out of 6) emerged from Italy and Ukraine.
This review grouped all probiotic products into a single intervention for analytical purposes. While not ideal, as not all probiotic preparations are equally effective,[51] it was necessary given the paucity of studies performed with each individual strain. Additionally, a prebiotic (nondigestible food that beneficially affects the host by selectively stimulating the growth/activity of colonic bacteria in the colon) plus probiotic (xilooligosaccharides plus arabinogalactan and Lactobacillus paracasei B21060)[52] study was included in the analyses. Sensitivity analyses, which were conducted when a minimum of two studies employing the same probiotic were identified, did not produce any changes in the conclusions.
A trend towards reductions in future hospitalizations was detected; although this did not achieve significance, one could interpret this as evidence of a possible clinical benefit associated with probiotic use. However, it is challenging to generalize findings from probiotic clinical trials with the most frequently studied strain (Lactobacillus GG) having its benefits confined to studies conducted outside North America.[41] The only North American outpatient study employing Lactobacillus GG found no difference in the time to normal stool or the number of diarrheal stools.[53]
Intravenous Fluid Therapy
Few studies evaluating IVT were identified, and limited evidence supporting the use of rapid rehydration therapy was found. The limited evidence of benefit may relate to the inaccuracy of dehydration assessment[54] or a delay in the timing between intravascular rehydration and clinical improvement. As it relates to choice of maintenance IV hydration solution, a single, low quality study, reported that dysnatremias are more frequent amongst children administered hypotonic fluids.
Overall
Investigators should conduct more sophisticated studies that answer clinically relevant questions employing outcomes of importance to end-users. Pragmatic, comparative effectiveness trials using factorial or non-inferiority designs and valid outcome measures[55] answering key questions such as the success of ORT in children with moderate dehydration would significantly enhance the uptake of ORT. Such work is needed to confirm and convince knowledge-users (if positive) of the utility of interventions such as probiotics. Although multiple meta-analyses have identified some benefits to be associated with the latter,[8,41] their uptake has been limited. Antiemetics have been investigated employing clinically important outcome measures and consequently uptake has occurred rapidly.[56] The use of patient-centered outcomes and well defined patient populations to minimize heterogeneity and maximize clinical applicability resulted in the exclusion of many reports which highlights the need for further investigations employing outcomes established as important to parents and children.[22] While diarrhea remains a concern with ondansetron administration the clinical impact appears to be negligible with single dose regimens, however when multiple doses are administered this becomes more of a concern.
This review has adhered to the latest methodological standards. Relevant evidence was searched for extensively and this review included all studies regardless of language of publication. Although not all possible interventions were considered, this review focused on common clinical intervention options and represents a comprehensive synthesis incorporating two key perspectives: 1) patient-centered outcomes, and 2) developed countries.
This review has several limitations. It is limited by the challenges of synthesizing unrelated outcomes which resulted in a limited body of evidence. Since this review focused on outpatient studies, the results cannot be applied to the care of children managed at home by caregivers for whom very limited data exists, or to the care of hospitalized children. The latter group, which represents a fraction of children with AGE, has been the focus of most clinical trials. While the ED setting may differ significantly from other outpatient settings and this might be a source of heterogeneity, within each therapy the location was very consistent (e.g. ondansetron studied in ED; probiotics studied in primary care). Since participant ages varied across the studies, planned sub-analyses (i.e. < 5 years vs. ≥ 5 years) could not be conducted. Additionally, the only interventions evaluated are those currently being considered for routine use in developed countries; interventions that are primarily considered for use only in developing nations (e.g. antibiotics, zinc) were not evaluated. Although "exp Diarrhea/” was included in the original search strategy (S2 File) data related to the duration and frequency of diarrhea was not abstracted originally as these were not included in the a priori defined outcome measures. However, in the context of antiemetic evaluation, they were deemed to be important and thus were included in the current version of the SR. Lastly, despite attempts to minimize heterogeneity amongst different studies, it could not be completely eliminated as studies included almost certainly varied in terms of infectious etiology, seasonality, and local factors influencing clinical decision making. Nonetheless, the knowledge gaps identified can serve to guide future research efforts.
Conclusions
Although some clinical practice guidelines endorse probiotic use, there is a paucity of supporting evidence for their use in developed countries. Routine probiotic use appears unjustified at present and future studies employing patient-centered outcomes are needed. While further evidence supporting ORT is needed to expand its use, such studies may be challenging to justify as expert opinion overwhelmingly supports its use as first-line therapy in children with AGE. Ondansetron has a strong evidence base supporting use and the key will be ensuring that administration is directed at the populations included in the RCTs. It should be noted that ondansetron use is not associated with reduction in ED revisits and it has the potential to increase diarrheal episodes. Moving forward, studies focusing on important outcomes and patient populations are needed to build a stronger evidence base to guide therapy for this extremely common condition.
Supporting Information
(PDF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(PDF)
Acknowledgments
Data Access
Drs. Stephen Freedman and Lisa Hartling had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributorship
We thank the following for their contributions to this project: Drs. Bob Hilliard, Francois Belanger, Mona Jabbour and Terry Klassen for their contributions as knowledge users. We thank David Johnson for his assistance with protocol development and critical review of the manuscript. We thank the following staff from the Alberta Research Centre for Health Evidence: Melanie Muise for assistance with data extraction and quality assessment, Jennifer Seida for assistance grading the quality of evidence, Ben Vandermeer for advice on the statistical analysis, and Robin Featherstone for re-running the search.
Members of the Pediatric Emergency Research Canada Gastroenteritis Study Group: Stephen Freedman (Lead), David Johnson, Karen Black, Robert Robert, Gary Joubert, Serge Gouin, Quynh Doan, Janie Williamson, Lynell Aucoin, Eleanor Fitzpatrick, Mona Jabbour, Terry Klassen.
Data sharing
The authors are prepared to provide the data on which the manuscript is based for examination by the editors or their assignees if requested.
Meetings
Presented at 2014 Pediatric Academic Society Meeting in Vancouver, Canada, May 5, 2014.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Canadian Institutes of Health Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Freedman SB, Steiner MJ, Chan KJ Oral ondansetron administration in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med 2010, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics 2009, 124:465–471. [DOI] [PubMed] [Google Scholar]
- 3. Lee BE, Pang XL New strains of norovirus and the mystery of viral gastroenteritis epidemics. CMAJ 2013, 185:1381–1382. 10.1503/cmaj.130426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013, 368:1121–1130. 10.1056/NEJMsa1206589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szajewska H, Mrukowicz JZ Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 2001, 33 Suppl 2:S17–25. [DOI] [PubMed] [Google Scholar]
- 6. Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig Dis Sci 2002, 47:2625–2634. [DOI] [PubMed] [Google Scholar]
- 7. Van Niel CW, Feudtner C, Garrison MM, Christakis DA Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002, 109:678–684. [DOI] [PubMed] [Google Scholar]
- 8. Allen SJ, Martinez EG, Gregorio GV, Dans LF Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2010, 11:CD003048 10.1002/14651858.CD003048.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev 2006, 3:CD004390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeCamp LR, Byerley JS, Doshi N, Steiner MJ Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med 2008, 162:858–865. 10.1001/archpedi.162.9.858 [DOI] [PubMed] [Google Scholar]
- 11. Carter B, Fedorowicz Z Antiemetic treatment for acute gastroenteritis in children: an updated Cochrane systematic review with meta-analysis and mixed treatment comparison in a Bayesian framework. BMJ Open 2012, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H European society for pediatric gastroenterology, hepatology, and nutrition/european society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in europe: update 2014. J Pediatr Gastroenterol Nutr 2014, 59:132–152. 10.1097/MPG.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 13.National Collaborating Centre for Women's and Children's Health Diarrhoea and vomiting caused by gastroenteritis: diagnosis, assessment and management in children younger than 5 years. Commissioned by the National Institute for Health and Clinical Excellence; Available: http://www.nice.org.uk/guidance/cg84/resources/cg84-diarrhoea-and-vomiting-in-children-under-5-full-guideline2. Accessed 2014 October 15.
- 14. Cheng A, Canadian Paediatric Society—Acute Care Committee Emergency department use of oral ondansetron for acute gastroenteritis-related vomtiing in infants and children. Paediatr Child Health 2011, 16:177–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piescik-Lech M, Shamir R, Guarino A, Szajewska H Review article: the management of acute gastroenteritis in children. Aliment Pharmacol Ther 2013, 37:289–303. 10.1111/apt.12163 [DOI] [PubMed] [Google Scholar]
- 16. King CK, Glass R, Bresee JS, Duggan C Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003, 52:1–16. [PubMed] [Google Scholar]
- 17. Powell EC, Hampers LC Physician variation in test ordering in the management of gastroenteritis in children. Arch Pediatr Adolesc Med 2003, 157:978–983. [DOI] [PubMed] [Google Scholar]
- 18. Freedman SB, Gouin S, Bhatt M, Black KJ, Johnson D, Guimont C, et al. Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics 2011, 127:e287–295. 10.1542/peds.2010-2214 [DOI] [PubMed] [Google Scholar]
- 19. Freedman SB, Sivabalasundaram V, Bohn V, Powell EC, Johnson DW, Boutis K The treatment of pediatric gastroenteritis: a comparative analysis of pediatric emergency physicians' practice patterns. Acad Emerg Med 2011, 18:38–45. 10.1111/j.1553-2712.2010.00960.x [DOI] [PubMed] [Google Scholar]
- 20. Opintan JA, Newman MJ, Ayeh-Kumi PF, Affrim R, Gepi-Attee R, Sevilleja JE, et al. Pediatric diarrhea in southern Ghana: etiology and association with intestinal inflammation and malnutrition. Am J Trop Med Hyg 2010, 83:936–943. 10.4269/ajtmh.2010.09-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al Jarousha AM, El Jarou MA, El Qouqa IA Bacterial enteropathogens and risk factors associated with childhood diarrhea. Indian J Pediatr 2011, 78:165–170. 10.1007/s12098-010-0249-0 [DOI] [PubMed] [Google Scholar]
- 22. Johnston BC, Shamseer L, da Costa BR, Tsuyuki RT, Vohra S Measurement issues in trials of pediatric acute diarrheal diseases: a systematic review. Pediatrics 2010, 126:e222–231. 10.1542/peds.2009-3667 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S (2009) Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available: www.cochrane-handbook.org.
- 24.(revised February 17, 2011) Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings (footnote c). United Nations Statistics Division.
- 25.(2014) U.S. Department of Health & Human Services. Child Health Care Quality Toolbox: Established Child Health Care Quality Measures–AHRQ Quality Indicators—Gastrointestinal Diseases. Rockville: Agency for Healthcare Research and Quality.
- 26. Seida JC, LeBlanc C, Schouten JR, Mousavi SS, Hartling L, Vandermeer B, et al. Systematic review: nonoperative and operative treatments for rotator cuff tears. Ann Intern Med 2010, 153:246–255. 10.7326/0003-4819-153-4-201008170-00263 [DOI] [PubMed] [Google Scholar]
- 27. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol 2006, 59:697–703. [DOI] [PubMed] [Google Scholar]
- 28. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 2013, 159:123–129. 10.7326/0003-4819-159-2-201307160-00661 [DOI] [PubMed] [Google Scholar]
- 29. Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ 2011, 342:d1714 10.1136/bmj.d1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The GRADE Working Group GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 ed. 2009; Schünemann H, Brozek J, Oxman A, editors.
- 31. Deeks JJ, Altman DG, Bradburn MJ (2001) Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publication Group. [Google Scholar]
- 32. Petitti DB Approaches to heterogeneity in meta-analysis. Stat Med 2001, 20:3625–3633. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG Quantifying heterogeneity in a meta-analysis. Stat Med 2002, 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 34. Higgins JP, Thompson SG, Deeks JJ, Altman DG Measuring inconsistency in meta-analyses. BMJ 2003, 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamer AM, Friedman LB, Maxwell SR, Cynamon HA, Perez HN, Cleveland WW Oral rehydration of infants in a large urban U.S. medical center. J Pediatr 1985, 107:14–19. [DOI] [PubMed] [Google Scholar]
- 36. Freedman SB, Adler M, Seshadri R, Powell EC Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med 2006, 354:1698–1705. [DOI] [PubMed] [Google Scholar]
- 37. Roslund G, Hepps TS, McQuillen KK The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med 2008, 52:22–29 e26. [DOI] [PubMed] [Google Scholar]
- 38. Ramsook C, Sahagun-Carreon I, Kozinetz CA, Moro-Sutherland D A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med 2002, 39:397–403. [DOI] [PubMed] [Google Scholar]
- 39. Reeves JJ, Shannon MW, Fleisher GR Ondansetron decreases vomiting associated with acute gastroenteritis: a randomized, controlled trial. Pediatrics 2002, 109:e62 [DOI] [PubMed] [Google Scholar]
- 40. Qazi K, BinSalleeh HM, Shah UH, AlGhamedi N, Tamim H, Mubasher M, et al. Effectiveness of granisetron in controlling pediatric gastroenteritis-related vomiting after discharge from the ED. Am J Emerg Med 2014, 32:1046–1050. 10.1016/j.ajem.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 41. Szajewska H, Skorka A, Ruszczynski M, Gieruszczak-Bialek D Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children—updated analysis of randomised controlled trials. Aliment Pharmacol Ther 2013, 38:467–476. 10.1111/apt.12403 [DOI] [PubMed] [Google Scholar]
- 42. Freedman SB, Ali S, Oleszczuk M, Gouin S, Hartling L Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries. Evid Based Child Health 2013, 8:1123–1137. 10.1002/ebch.1932 [DOI] [PubMed] [Google Scholar]
- 43. Campbell H, Surry SA, Royle EM A review of randomised controlled trials published in Archives of Disease in Childhood from 1982–96. Arch Dis Child 1998, 79:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Freedman SB, Thull-Freedman JD, Rumantir M, Atenafu EG, Stephens D Emergency department revisits in children with gastroenteritis. J Pediatr Gastroenterol Nutr 2013, 57:612–618. 10.1097/MPG.0b013e3182a1dd93 [DOI] [PubMed] [Google Scholar]
- 45. Fontaine O, Garner P, Bhan MK Oral rehydration therapy: the simple solution for saving lives. BMJ 2007, 334 Suppl 1:s14 [DOI] [PubMed] [Google Scholar]
- 46. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci 2011, 6:133 10.1186/1748-5908-6-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keren R Ondansetron for acute gastroenteritis: a failure of knowledge translation. JAMA Pediatr 2014, 168:308–309. 10.1001/jamapediatrics.2013.5378 [DOI] [PubMed] [Google Scholar]
- 48.Freedman SB, Uleryk E, Rumantir M, Finkelstein Y Ondansetron and the Risk of Cardiac Arrhythmias: A Systematic Review and Postmarketing Analysis. Ann Emerg Med (Epub Ahead of Print) 2013. [DOI] [PubMed]
- 49. Eltorki M Letters to the editor. Paediatr Child Health 2014, 19:500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parker MW, Schaffzin JK, Lo Vecchio A, Yau C, Vonderhaar K, Guiot A, et al. Rapid adoption of Lactobacillus rhamnosus GG for acute gastroenteritis. Pediatrics 2013, 131 Suppl 1:S96–102. 10.1542/peds.2012-1427l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 2007, 335:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Passariello A, Terrin G, Cecere G, Micillo M, De Marco G, Di Costanzo M, et al. Randomised clinical trial: efficacy of a new synbiotic formulation containing Lactobacillus paracasei B21060 plus arabinogalactan and xilooligosaccharides in children with acute diarrhoea. Aliment Pharmacol Ther 2012, 35:782–788. 10.1111/j.1365-2036.2012.05015.x [DOI] [PubMed] [Google Scholar]
- 53. Nixon AF, Cunningham SJ, Cohen HW, Crain EF The effect of Lactobacillus GG on acute diarrheal illness in the pediatric emergency department. Pediatr Emerg Care 2012, 28:1048–1051. 10.1097/PEC.0b013e31826cad9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pringle K, Shah SP, Umulisa I, Mark Munyaneza RB, Dushimiyimana JM, Stegmann K, et al. Comparing the accuracy of the three popular clinical dehydration scales in children with diarrhea. Int J Emerg Med 2011, 4:58 10.1186/1865-1380-4-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA 2009, 302:1437–1443. 10.1001/jama.2009.1409 [DOI] [PubMed] [Google Scholar]
- 56. Kharbanda AB, Hall M, Shah SS, Freedman SB, Mistry RD, Macias CG, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr 2013, 163:230–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.