Abstract
Background:
Diabetes is associated with endothelial dysfunction and impaired wound healing. The amino acid L-arginine is the only substrate for nitric oxide (NO) synthesis. The purpose of this study was to compare the topical versus systemic L-arginine treatment on total nitrite (NOx) and vascular endothelial growth factor (VEGF) concentrations in wound fluid and rate of wound healing in an acute incisional diabetic wound model.
Materials and Methods:
A total of 56 Sprague-Dawley rats were used of which 32 were rendered diabetic. Animals underwent a dorsal skin incision. Dm-sys-arg group (N = 8, diabetic) and Norm-sys-arg group (N = 8, normoglycemic) were gavaged with L-arginine. Dm-sys-control group (N = 8, diabetic) and Norm-sys-control group (N = 8, normoglycemic) were gavaged with water. Dm-top-arg group (N = 8, diabetic) and norm-top-arg group (N = 8, normoglycemic) received topical L-arginine gel. Dm-top-control group (N = 8, diabetic) received gel vehicle. On the day 5 the amount of NOx in wound fluid was measured by Griess reaction. VEGF/total protein in wound fluids was also measured on day 5 using enzyme-linked immunosorbent assay. All wound tissue specimens were fixed and stained to be evaluated for rate of healing. Data were analyzed using SPSS software (version 18.0, Chicago, IL, USA) through One-way analysis of variance test and Tukey's post-hoc.
Results:
In dm-sys-arg group, the level of NOx on day 5 was significantly more than dm-top-arg group (P < 0.05). VEGF content in L-arginine treated groups were significantly more than controls (P < 0.05). Rate of diabetic wound healing in dm-sys-arg group was significantly more than dm-top-arg group.
Conclusion:
Systemic L-arginine is more efficient than topical L-arginine in wound healing. This process is mediated at least in part, by increasing VEGF and NO in the wound fluid.
Keywords: L-arginine, nitric oxide, streptozotocin, vascular endothelial growth factor, wound healing
INTRODUCTION
The worldwide burden of diabetes is attributed to its various accompanying complications including impaired wound healing, which can ultimately result in amputation. Despite of demanding research and subsequent advances in diabetic wound care an effective treatment has yet to be determined. This complication has been observed even in case of glycemic control.[1] The diabetic wound shows several defects indicative of local endothelial dysfunction. Reduced wound NO expression, impaired healing as well as reduced collagen accumulation and wound breaking strengths have been demonstrated in experimental diabetic animal models of acute cutaneous wound healing.[2] It has been suggested that changes in nitric oxide (NO) production and metabolism may cause endothelial dysfunction, which is responsible for diabetic microvascular complications. Decreased NO production leads to impaired vasodilatation and neuropathy.[3] Reduced NO production by gene knockout impairs wound healing.[4] Gene therapy strategies have been effective in increasing cutaneous NO concentration and improving diabetic wound healing.[5] Furthermore, NO has a modulatory role in vascular endothelial growth factor (VEGF) mediated wound healing process. VEGF is a key angiogenic molecule with an important role in vascular permeability, which underlines the significance of VEGF in wound healing.[6] NO production and subsequent angiogenic activity of endothelial cells is dependent upon VEGF activity, and VEGF is synthesized through NO induction in some cell types including vascular smooth muscle cells, macrophages, and keratinocytes.[7] Since it has been shown that angiogenesis is impaired in diabetic wound healing, overexpression of VEGF can improve impaired angiogenesis in diabetes.[8] L-arginine as the main and only substrate for NO synthesis has been used in improving diabetic wound healing. Furthermore, L-arginine plays a key role in protein synthesis and urea cycle and is a precursor of polyamines, and proline.[9] Some studies have shown that L-arginine supplementation improves vascular endothelial dysfunction.[10] L-arginine has been administered in either systemic or topical manner to enhance wound healing. Because of undetectable levels of arginine at the wound site during the acute phase of healing, the topical route of administration could be efficient.[9,11] No study has been done yet about comparing systemic versus topical L-arginine in diabetic wound healing. In this study, systemic versus topical application of L-arginine on the rate of wound healing, NO and VEGF concentrations in wound fluid were compared in an acute incisional diabetic wound model.
MATERIALS AND METHODS
Animals and experimental protocol
The study protocol was approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran according to principles of laboratory animal care (http://grants1.nih.gov/grants/olaw/references/phspol.htm, accessed 18 May 2012). This was an experimental study that included 56 males Sprague-Dawley rats weighing 180-220 g were purchased from Razi Institute, Karaj, Iran. All of the animals were kept in 12 h light/dark cycle with 23°C temperature. The study was done in the physiology department of Isfahan Medical Faculty. The rats were rendered diabetic by injection of a single dose of 65 mg/kg streptozotocin (STZ; Sigma, USA), in saline-sodium citrate buffer (Sigma, Inc., St. Louis, MO., pH 4.5). Blood glucose levels of animals were measured 2 weeks after STZ injection and animals with blood glucose levels above 300 mg/dl were considered as diabetic.
After general anesthesia with sodium pentobarbital (80 mg/kg, intraperitoneal), hair on the back was shaved and then wiped with sterile water. A rectangular 1.5 cm × 1 cm wound site on the dorsum of the rats, was outlined using a template and the tissue excised to the level of the panniculus carnosus muscle by dissecting scissors and forceps. The wounds were covered by a layer of medical adhesive (Comfeel® Plus Transparent).
The animals were divided into seven groups of 8 animals each. Group 1, consisting of diabetic rats were gavaged with L-arginine (1 g/kg) twice daily (given as L-arginine hydrochloride in 1 ml water) (dm-sys-arg). Group 2 consisting of normoglycemic rats were gavaged with L-arginine (1 g/kg) twice daily (given as L-arginine hydrochloride in 1 ml water) (norm-sys-arg). Group 3 consisting of diabetic rats were gavaged with 1 ml water twice daily (dm-sys-control). Group 4 consisting of normoglycemic rats were gavaged with 1 ml water twice daily (norm-sys-control). Group 5, consisting of diabetic rats, received 1cc topical L-arginine 20% once daily (dm-top-arg). Group 6 consisting of normoglycemic rats received 1cc topical L-arginine 20% once daily (norm-top-arg). L-arginine 20% consisted of 20 g L-arginine per 100 g of gel; the vehicles were 0.2 g Nipastat sodium, 2.5 g Natrosol 250 HHX Pharm, and 78.4 ml purified water.[11] Group 7, consisting of diabetic rats, received 1cc gel vehicle once daily (dm-top-control).
Two samples of wound fluid were collected using sterile nitrate-free absorbent paper strips for measurements of VEGF and total nitrite (NOx) levels according to standard protocols that had been used in previous studies.[12,13,14]
Determination of total nitrite in wound fluid
On day 5 the amount of NOx in wound fluid was measured by Griess reaction according to the manufacturer's instruction (R&D Systems, USA).[15] Briefly, 50 µl aliquots of wound fluid were mixed with an equal volume of Griess reagent (a mixture of 0.1% naphthyl ethylenediamine dihydrochloride in water and 1% sulfanilamide in 5% phosphoric acid) and incubated for 10 min at room temperature. The absorbance was measured at dual wavelengths 570 nm/650 nm by the spectrophotometer. The NOx level of wound fluid was measured using the Griess assay after conversion of NO3 to NO2 with the NO reductase enzyme.
Determination of vascular endothelial growth factor/total protein in wound fluid
The amount of VEGF in wound fluid was measured using enzyme-linked immunosorbent assay using available reagents and recombinant standards (R&D Systems, Minneapolis, USA) according to manufacturer's instruction only on 5th-day samples. The VEGF assay has a minimum sensitivity of 30 pg/ml.
The concentration of total protein (TP) in wound fluid was determined by the Lowry's method using the commercial kit (Pars Azmoon, Iran).
The VEGF concentration of wound fluid was reported after correction by the TP level (VEGF/TP × 107).
Histological examination
On day 11, all animals were euthanized by pentobarbital overdose. All wound tissue specimens were fixed in 10% neutral-buffered formalin for at least 24 h at room temperature. After fixation, the specimens were dehydrated in graded ethanol, cleared in xylene, and embedded in paraffin. Five-micron-thick sections were prepared and mounted on glass slides, dewaxed, rehydrated to distilled water, and stained with hematoxylin and eosin. As part of the histological evaluation, all slides were examined by a pathologist, without knowledge of the previous treatment, under a microscope from ×10 to ×40 magnifications. Each slide was given a histological score ranging from 1 to 12: (1-3) None to minimal cell accumulation, no granulation tissue or epithelial travel; (4-6) thin, immature granulation, dominated by inflammatory cells with a few fibroblasts, capillaries, or collagen deposition, and minimal epithelial migration; (7-9) moderately thick granulation tissue, dominant inflammatory cells, more fibroblasts and collagen deposition, extensive neovascularization, and minimal to moderate migrating epithelium; (10-12), thick, vascular granulation tissue dominated by fibroblasts and extensive collagen deposition, and epithelium partially to completely covering the wound.[16]
Statistical analysis
We analyzed our data with SPSS software (version 18.0, Chicago IL, USA). All data are expressed as the mean plus or minus the standard deviation (mean ± SD). Data were analyzed by One-way analysis of variance test, followed by a Tukey's post-hoc multiple comparison. P < 0.05 was accepted as the measure for statistical significance.
RESULTS
Measurement of total nitrite on day 5 post wounding
The amount of NOx in wound fluid of dm-sys-arg group was significantly more than all other groups (P = 0.01). The difference of NOx between dm-top-arg, dm-top-control and norm-top-arg was not statistically significant. The concentration of NOx in norm-sys-arg group was not significantly more than norm-sys-control group while norm-sys-arg group had significantly more NOx level than dm-sys-control group (P < 0.05).
The amount of NOx in norm-sys-control group was significantly more than diabetic control groups (P < 0.05) [Figure 1].
Figure 1.
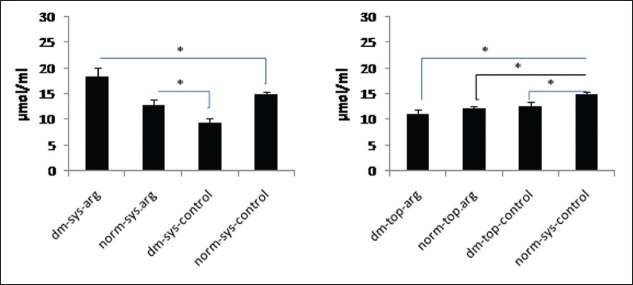
Wound fluid total nitrite concentration was measured by Griess reaction on day 5 post wounding. Data are reported in μmol/ml ± SDM (*P < 0.05)
Measurement of vascular endothelial growth factor/total protein on day 5 postwounding
Vascular endothelial growth factor/TP level in all arginine treated groups were significantly more than control groups (P = 0.001). There was no significant difference between dm-sys-arg and dm-top-arg groups. VEGF/TP level in dm-top-arg group was significantly more than norm-top-arg group (P = 0.001) [Figure 2]. VEGF/TP level in norm-sys-arg group was significantly more than norm-top-arg group (P = 0.005).
Figure 2.
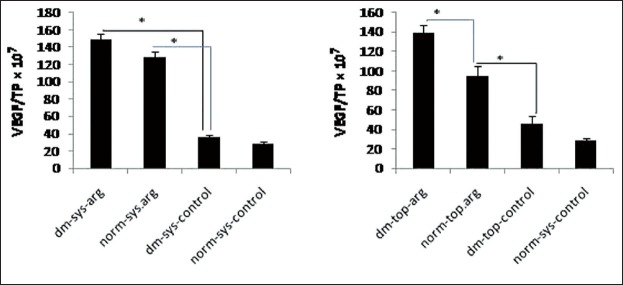
Wound fluid vascular endothelial growth factor (VEGF)/total protein (TP) was measured on day 5 post wounding. Date are reported in VEGF/TP × 107 (*P < 0.05)
Wound healing in systemic versus topical L-arginine treated groups on day 11
The rate of wound healing in all arginine treated groups, and norm-sys-control group was significantly more than diabetic control groups (P < 0.05). The rate of wound healing in dm-sys-arg and norm-top-arg groups was significantly more than dm-top-arg group (P = 0.02) [Figure 3].
Figure 3.
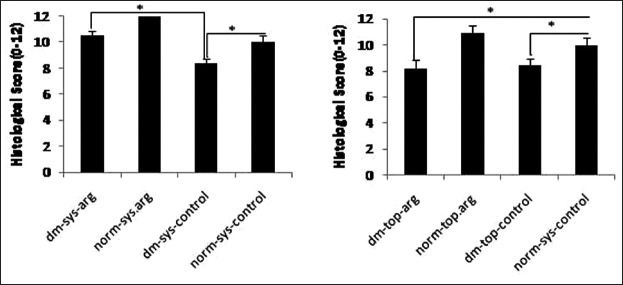
The histological scores of wound healing were examined by a pathologist, without knowledge of the prior treatment. Data are reported in mean scores ± SDM (*P < 0.05)
DISCUSSION
This study aimed to compare the effects of systemic versus topical application of L-arginine on wound levels of NOx, VEGF concentrations and wound healing in STZ-induced diabetic rats.
Our results showed that L-arginine treatment increased the wound fluid NOx and VEGF in the systemically treated diabetic animals more than control normoglycemic animals; however there is no difference between their pathological healing scores.
Macrophages are one of the important supply of NO synthesis in the wound site during wound healing process.[17] Furthermore, macrophages are one of the major sources of VEGF in wound sites. More NO and VEGF in L-arginine treated diabetic animals could be explained by increased numbers of macrophages causing more and sustained inflammation in diabetic wounds.[18] It has been shown that diabetic mice had increased levels of the pro-inflammatory cytokines such as tumor necrosis factor -α and IL-6 and decreased level of IL-10, an anti-inflammatory cytokine.[19]
Our results demonstrated that systemic L-arginine treatment was associated with more wound fluid levels of NOx expression and better wound healing compared to topical L-arginine treated diabetic animals.
Several lines of evidence can explain that why systemic L-arginine administration is more effective in diabetic conditions. First, Lower plasma arginine levels were reported in diabetic rats and patients. Dietary supplementation with L-arginine decreased plasma levels of homocysteine, fatty acids, and triglycerides, and improved insulin sensitivity in various animal models including STZ-induced diabetic rats, genetically obese Zucker diabetic fatty rats, and diet-induced obese rats.[20,21,22] Similar results have been reported for obese humans with type-II diabetes receiving oral or intravenous administration of arginine.[23] Furthermore, oral L-arginine supplementation decreased the advanced glycation end products and inflammatory protein production in diabetic rats, which have pro-oxidant effects and playing a substantial role in the development of diabetic complications.[24] In the same way, it has been shown that systemic treatment with L-arginine exerted an inhibitory effect of hemoglobin glycation and lipid peroxidation in vivo.[25] In another study, L-arginine improved insulin sensitivity index and increased adiponectin production whereas decreased IL-6 and monocyte chemoattractant protein-1contents.[26]
Second, oral administration of L-arginine can reverse systemic endothelial dysfunction.[22] It has been shown that oral L-arginine supplementation can increase the arginine/asymmetric dimethylarginine ratio and, therefore, increase endothelial NO synthesis and decrease superoxide production.[27]
The third mechanism by which the superior effect of systemic administration of L-arginine could be explained includes arginase/NO synthase competition. The Vmax of arginase for arginine exceeds that of NOS (1000 fold) even though the affinity of NOS for arginine is greater. Hence, arginase activity in the wound is likely to deplete the arginine and to reduce NO content. It has been demonstrated that increased arginase expression/activity contributes to endothelial dysfunction in diabetic rats. Since, arginase is expressed by different cells participating in wound healing process; it has been shown that arginase inhibitor could lead to increased arginine bioavailability in the wound environment.[28] This fact may explain more NOx in wound fluid of L-arginine treated diabetic animals compared to normoglycemic rats in our study.
Other studies suggested an important role for NO in regulating growth factor-mediated processes during wound repair. Therefore, we hypothesized that supplemental arginine might increase wound levels of NO with the subsequent increase in VEGF production.[7] Angiogenic effect of VEGF has been suggested to be dependent on NO pathway through several mechanisms. NO increased VEGF production through Akt pathway. Moreover, VEGF has a key role in the process of wound healing.[29]
It has been shown that neutralization of VEGF lowered the chemotactic activity of endothelial cells and impaired wound healing.[6] Topical administration of VEGF has been shown to improve diabetic wound healing by increasing epithelialization, matrix deposition, and cellular proliferation.[30]
Furthermore, supplemental L-arginine has been shown to increase VEGF concentration after physical exercise.[31] It has been shown that VEGF expression is dependent on NO expression by activation of inducible NO synthase.[7] Burns et al. have shown that, the change of VEGF expression was concomitant with NO concentration in wound healing process.[32]
The beneficial effects of L-arginine on wound healing could be explained through two metabolic pathways the first which occurs in the 1st 3 days, is accelerated by NO synthase, citrulline and NO as end products. Second pathway starts a few days later, in which arginine is converted to ornithine by arginase. Ornithine is an essential substance for the production of collagen. This is compatible with our findings in which systemic L-arginine treated group showed improved wound healing compared to diabetic control.[9]
Gosselink et al. demonstrated that topical L-arginine treatment increased healing in chronic anal fissure by reduction of anal resting pressure and normalizing anal blood flow. L-arginine treatment was effective even in patients that do not respond to other NO donors like isosorbide dinitrate.[11] However, in a study it has been shown that local L-Arginine caused excess production of NO without changing the amount of ornithine, polyamines and proline, which is indicative of arginase and ornithine decarboxylase inhibition.[33] It could explain lower efficiency of topical versus systemic L-arginine in our study.
CONCLUSIONS
Systemic application of L-arginine led to more expression of VEGF and NO that resulted in wound healing improvement. Future studies such as clinical trials are needed to realize whether similar findings are observed in human.
AUTHOR'S CONTRIBUTION
AZ and SHJ had substantial contributions to conception and design of the study, analysis of the data, drafting of the paper, approval of the final version of the manuscript and agreed for all aspects of the work. SS, EZ, SSB and AA had contributions to data collection and analysis. They also contributed to drafting of the paper, approval of the final version of the manuscript and agreed for all aspects of the work.
ACKNOWLEDGMENT
This study was supported by Isfahan University of Medical sciences, Isfahan, Iran (Grant # 384151).
Footnotes
Source of Support: This study was supported by Isfahan University of Medical sciences, Isfahan, Iran (Grant # 384151)
Conflict of Interest: All authors have read and approved the content of the manuscript. All authors disclose all potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of the manuscript.
REFERENCES
- 1.Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30:37–45. doi: 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 2.Schäffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, Barbul A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: A possible pathophysiologic correlation. Surgery. 1997;121:513–9. doi: 10.1016/s0039-6060(97)90105-7. [DOI] [PubMed] [Google Scholar]
- 3.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Most D, Efron DT, Shi HP, Tantry US, Barbul A. Characterization of incisional wound healing in inducible nitric oxide synthase knockout mice. Surgery. 2002;132:866–76. doi: 10.1067/msy.2002.127422. [DOI] [PubMed] [Google Scholar]
- 5.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation. 2004;110:2484–93. doi: 10.1161/01.CIR.0000137969.87365.05. [DOI] [PubMed] [Google Scholar]
- 6.Wilgus TA, DiPietro LA. Complex roles for VEGF in dermal wound healing. J Invest Dermatol. 2012;132:493–4. doi: 10.1038/jid.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howdieshell TR, Webb WL, Sathyanarayana, McNeil PL. Inhibition of inducible nitric oxide synthase results in reductions in wound vascular endothelial growth factor expression, granulation tissue formation, and local perfusion. Surgery. 2003;133:528–37. doi: 10.1067/msy.2003.128. [DOI] [PubMed] [Google Scholar]
- 8.Yan X, Chen B, Lin Y, Li Y, Xiao Z, Hou X, et al. Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract. 2010;90:66–72. doi: 10.1016/j.diabres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Vissers YL, Debats IB, Luiking YC, Jalan R, van der Hulst RR, Dejong CH, et al. Pros and cons of L-arginine supplementation in disease. Nutr Res Rev. 2004;17:193–210. doi: 10.1079/NRR200490. [DOI] [PubMed] [Google Scholar]
- 10.Nematbakhsh M, Haghjooyjavanmard S, Mahmoodi F, Monajemi AR. The prevention of endothelial dysfunction through endothelial cell apoptosis inhibition in a hypercholesterolemic rabbit model: The effect of L-arginine supplementation. Lipids Health Dis. 2008;7:27. doi: 10.1186/1476-511X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselink MP, Darby M, Zimmerman DD, Gruss HJ, Schouten WR. Treatment of chronic anal fissure by application of L-arginine gel: A phase II study in 15 patients. Dis Colon Rectum. 2005;48:832–7. doi: 10.1007/s10350-004-0858-7. [DOI] [PubMed] [Google Scholar]
- 12.Zandifar E, Sohrabi Beheshti S, Zandifar A, Haghjooy Javanmard S. The effect of captopril on impaired wound healing in experimental diabetes. Int J Endocrinol 2012. 2012:785247. doi: 10.1155/2012/785247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barro CD, Romanet JP, Fdili A, Guillot M, Morel F. Gelatinase concentration in tears of corneal-grafted patients. Curr Eye Res. 1998;17:174–82. doi: 10.1076/ceyr.17.2.174.5602. [DOI] [PubMed] [Google Scholar]
- 14.Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: The ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419–26. doi: 10.1111/j.1464-5491.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–91. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RH, Efron D, Tantry U, Barbul A. Nitric oxide in the healing wound: A time-course study. J Surg Res. 2001;101:104–8. doi: 10.1006/jsre.2001.6261. [DOI] [PubMed] [Google Scholar]
- 18.Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–53. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 19.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, et al. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–21. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 21.Kohli R, Meininger CJ, Haynes TE, Yan W, Self JT, Wu G. Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J Nutr. 2004;134:600–8. doi: 10.1093/jn/134.3.600. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, et al. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–5. doi: 10.1093/jn/137.12.2680. [DOI] [PubMed] [Google Scholar]
- 23.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291:E906–12. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 24.Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Götting C, et al. Effects of low-and high-advanced glycation endproduct meals on macro-and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–43. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 25.Méndez JD, Balderas FL. Inhibition by L-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed Pharmacother. 2006;60:26–31. doi: 10.1016/j.biopha.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, et al. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24:875–80. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 27.Böger GI, Rudolph TK, Maas R, Schwedhelm E, Dumbadze E, Bierend A, et al. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin: Effect of combination with oral L-arginine. J Am Coll Cardiol. 2007;49:2274–82. doi: 10.1016/j.jacc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 28.Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, et al. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol. 2012;302:H159–66. doi: 10.1152/ajpheart.00774.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulak J, Józkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: Facts and controversies. Antioxid Redox Signal. 2003;5:123–32. doi: 10.1089/152308603321223612. [DOI] [PubMed] [Google Scholar]
- 30.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorito C, Balestrieri ML, Crimi E, Giovane A, Grimaldi V, Minucci PB, et al. Effect of L-arginine on circulating endothelial progenitor cells and VEGF after moderate physical training in mice. Int J Cardiol. 2008;126:421–3. doi: 10.1016/j.ijcard.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Zhu KQ, Engrav LH, Armendariz R, Muangman P, Klein MB, Carrougher GJ, et al. Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig-further similarities between female, Duroc scar and human hypertrophic scar. Burns. 2005;31:5–10. doi: 10.1016/j.burns.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Heffernan D, Dudley B, McNeil PL, Howdieshell TR. Local arginine supplementation results in sustained wound nitric oxide production and reductions in vascular endothelial growth factor expression and granulation tissue formation. J Surg Res. 2006;133:46–54. doi: 10.1016/j.jss.2006.03.028. [DOI] [PubMed] [Google Scholar]


