Abstract
Background:
The objective of this study was to investigate the fatigue status and related factors in patients with early-stage non-small cell lung cancer (NSCLC) 1-5 years after surgery.
Materials and Methods:
This cross-sectional study included 254 patients with stage IA or IB NSCLC, who had undergone surgery. They completed several surveys, including the Brief Fatigue Inventory, Karnofsky Performance Scale, Physical Activity Questionnaire, Baseline Dyspnea Index, Hospital Anxiety and Depression Scale. The association between fatigue and functional status was assessed using Chi-square analysis. Spearman rank correlation and multivariate logistic regression analysis were used to assess the correlation between fatigue and various other factors.
Results:
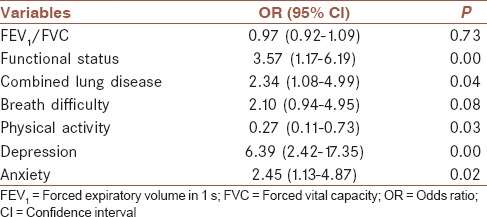
The overall incidence of postoperative fatigue was 59.8%. Among patients with moderate to severe fatigue, 21.1% had obvious dysfunction, whereas only 9.6% of patients with mild or no fatigue (χ2 = 5.369; P = 0.02) showed obvious dysfunction. Multivariate logistic regression analysis showed that functional status (odds ratio [OR]: 3.57; 95% confidence interval [CI]: 1.17-6.19), concurrent lung disease (OR: 2.34; 95% CI: 1.08-4.99), depression (OR: 6.39; 95% CI: 2.42-17.35), and anxiety (OR: 2.45; 95% CI: 1.13-4.87) were independent risk factors for fatigue, whereas physical activity (OR: 0.27; 95% CI: 0.11-0.73) could prevent fatigue.
Conclusion:
More than half of the patients with early-stage NSCLC experienced fatigue 1-5 years after surgery, and moderate to severe fatigue was often associated with obvious dysfunction. The strong association of fatigue with anxiety, depression, and lung complications suggests that fatigue and other symptoms constitute a symptom cluster. Therefore, comprehensive treatment methods may achieve better therapeutic results.
Keywords: Early-stage, fatigue, non-small cell lung cancer
INTRODUCTION
Lung cancer is the most common malignant disease leading to death.[1] Non-small cell lung cancer (NSCLC) is the most common type of lung cancer.[1] In recent years, there has been substantial progress in the treatment of lung cancer, however, the long-term survival after surgical resection remains low due to the complex biological characteristics and high recurrence and metastasis of lung cancer,[2] and the 5-year survival rates of patients with stage IA and IB NSCLC has increased to 73% and 58%, respectively.[3] Therefore, assessment of their quality-of-life has taken precedence.
Fatigue, which is a nonspecific, multidimensional condition with psychological, social, and physiological aspects, is increasingly recognized as the most common side effect in cancer during treatment (surgery, radiation, and/or chemotherapy). Hartvig et al. reported that fatigue was more commonly seen in lung cancer patients than in other cancer patients,[4] which could have serious adverse effects on clinical treatment, rehabilitation, and quality-of-life. For NSCLC survivors, the prevalence of fatigue was reported as high as 75% 4 months after thoracotomy,[5] and a number of studies showed complete resolution of fatigue by 3-6 months after lung cancer surgery.[6,7]
Fatigue was reported to be associated with anxiety, depression, as well as functional dependency. Therefore, examining the prevalence, severity, and association of fatigue with other health consequences is important for understanding quality of life among lung cancer survivors.[8] To our knowledge, only a few studies to date have examined fatigue in lung cancer survivors, especially in early-stage NSCLC patients.[9,10] Therefore, this study enrolled patients who had undergone surgery for early-stage NSCLC 1-5 years previously. The incidence and impact of various factors associated with fatigue were investigated to provide a theoretical basis for improving therapeutic effectiveness and quality of life.
MATERIALS AND METHODS
Subjects
A total of 290 NSCLC patients, who were diagnosed and treated at the Department of Cardiothoracic Surgery in the Second Affiliated Hospital of Wenzhou Medical College between June 2005 and June 2010 were enrolled in this study. The inclusion criteria were as follows:
Stage IA or IB NSCLC;
Radical surgery, negative postoperative pathologic margins, and no tumor recurrence during data collection;
Disease-free and no chemotherapy or radiotherapy;
Surgery 1-5 years previously with no significant mental, psychological, or cognitive disorders.
This study was approved by the Institutional Review Board of our hospital, and informed consent was obtained from all patients prior to study commencement.
Patient data
Data were collected when patients returned to the hospital for routine postoperative evaluation; general information was obtained from their medical records. Sex, age, and the ratio of forced expiratory volume in 1 s and forced vital capacity (FEV1/FVC) were recorded. The type of surgery performed included wedge resection, lung resection, lobectomy, dual lobectomy, and pneumonectomy. The surgical approaches used included thoracoscopy and thoracotomy. Observed complications included those involving the heart and lungs, such as coronary heart disease and chronic obstructive pulmonary disease (COPD).
Fatigue status assessment
Fatigue was assessed using the Brief Fatigue Inventory (BFI),[11] which is a fatigue self-assessment tool with proven reliability and validity. It can not only assess fatigue status and that for the past 2 weeks, but also the severity of fatigue and the resulting interference with daily functions, and has been widely used for the assessment of cancer patients.[12] The scale ranges from 0 to 10, where 0 indicates no fatigue, 1-3 indicates mild fatigue, 4-6 indicates moderate fatigue, and 7-10 indicates severe fatigue.
Functional status assessment
The ability to care for themselves and carry out normal activities was assessed for each patient using the Karnofsky performance scale[13] to give a measure of their functional status. The scale ranges from 0 to 100, where 100 indicates normal, 70-90 indicates mild dysfunction (able to care for themselves), 40-60 indicates moderate dysfunction (some assistance needed), and <40 indicates severe dysfunction (total dependency). Moderate to severe dysfunction indicates significant dysfunction that affects the quality-of-life.
Physical activity assessment
Physical activity was assessed using the International Physical Activity Questionnaire (Chinese Version),[14] which has proven validity and reliability. Patients were assessed as to whether they met the international guidelines for the recommended amount of physical activity, which is no <150 min of moderate-intensity physical activity per week.[15]
Dyspnea assessment
The baseline dyspnea index is a detailed scoring method designed by Mahler et al.[16] for assessment of functional impairment, magnitude of the task, and degree of effort. Its scale ranges from 0 to 12, where a score ≤9 suggests dyspnea.
Psychological assessment
The patients’ psychological condition was assessed using the Hospital Anxiety and Depression Scale,[17] which is composed of two subscales, for anxiety and depression. Both the anxiety and depression scales range from 0 to 21, where a score of 8 or above suggests anxiety or depression. This measure is commonly used in patient population and produces subscale scores for both anxiety (α = 0.93) and depressive symptoms (α = 0.90).
Statistical analysis
Relationship between the categorical responses was made using the Chi-square (χ2) test. Spearman rank correlation analysis was used to assess the relationship between severity of fatigue and other numerical variables. To determine the most closely associated risk factor for fatigue, the incidence of moderate to severe fatigue was used as the dependent variable, and the fatigue-associated factors from the univariate analysis were used as independent variables for multivariate logistic regression analysis. The SPSS 16.0 software was used for statistical analysis. All tests were two-sided, and a value of P < 0.05 indicated a significant difference.
RESULT
Characteristics of the participants
A total of 290 patients were initially recruited to this study; of these, 36 did not meet the eligibility criteria. The reasons for exclusion were tumor recurrence (14 cases), death (5 cases), and refusal to participate in the investigation (17 cases); the reasons for nonparticipation were either passive refusal (12 cases) or because patients could not be contacted (5 cases). Finally, 254 patients participated in the study, a response rate of 87.6%, and male patients accounted for 65% (165/254). The mean age of the patients was 65.6 ± 9.6 years, the mean time from surgery to evaluation was 2.9 ± 1.5 years, and patient with stage IA disease accounted for 57.1% (145/254) of the study patients. Before surgery, 28.7% (73/254) of the patients had normal lung function (FEV1/FVC >70%), 59.8% (152/254) showed a slight decline in lung function (FEV1/FVC 61-70%), and 11.5% (29/254) showed a significant decrease in lung function (FEV1/FVC ≤60%). Of all the patients, 31.9% (81/254) had coronary heart disease, hypertension, rheumatic heart disease, or other cardiovascular diseases, whereas 43.3% (110/254) had chronic bronchitis, COPD, asthma, tuberculosis, or other lung diseases. The percentage of patients who underwent wedge resection, lung resection, lobectomy, double lobectomy, and pneumonectomy were 9.1% (23/254), 9.8% (25/254), 77.2% (196/254), 3.1% (8/254), and 0.8% (2/254), respectively; thoracoscopic surgery was used in 15% (38/254) of patients.
Results of each scale
As shown in Table 1, 59.8% of participants reported various degrees of fatigue, among these 37.4% had mild fatigue (BFI = 1-3) and 22.4% had moderate to severe fatigue (BFI ≥4). The percentages of patients with mild, moderate, and severe dysfunction were 70.1%, 11.4%, and 0.8%, respectively. In the 57 patients with moderate to severe fatigue, 21.1% (12/57) showed obvious dysfunction, whereas only 9.6% (19/197) of patients with no fatigue or mild fatigue showed obvious dysfunction (χ2 = 5.369; P = 0.02). There were 26.0% of participants who met the guidelines for physical activity, 57.9% who experienced difficulty breathing, and 18.5% and 10.2% who displayed symptoms of anxiety and depression, respectively.
Table 1.
Results from each scale used to assess fatigue and other variables in survivors
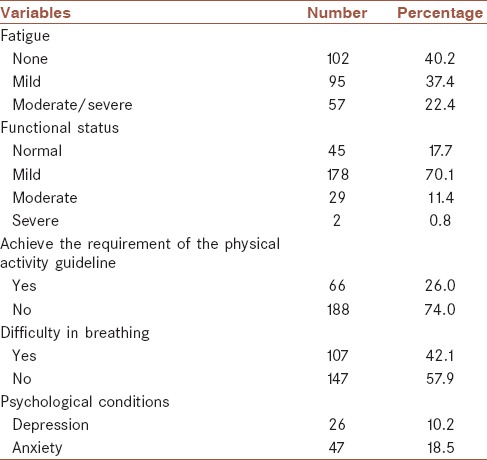
Relationship between severity of fatigue (score of brief fatigue inventory) and other variables
As shown in Table 2, Spearman rank correlation analysis showed that moderate to severe fatigue was negatively associated with preoperative FEV1/FVC and physical activity, and positively correlated with functional status, concurrent lung diseases, breathing difficulty, depression, and anxiety. Multivariate logistic regression analysis demonstrated that functional status, concurrent lung diseases, depression, and anxiety were independent risk factors for fatigue, whereas physical activity was a protective factor [Table 3].
Table 2.
Relationship between severity of fatigue (score of BFI) and other variables
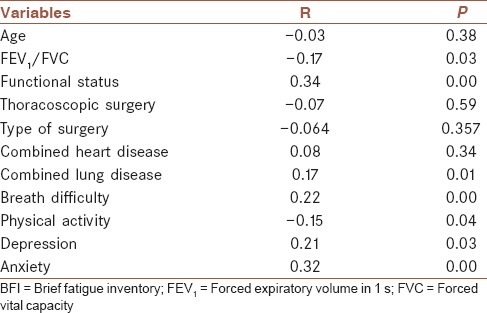
Table 3.
Logistic regression analysis on moderate to severe fatigue
DISCUSSION
In this study, the BFI scale with good reliability and validity was applied to a large sample population to assess the incidence of fatigue in postoperative patients with early-stage NSCLC. Results showed that more than half of the patients had fatigue symptoms, indicating that the incidence of fatigue was high in patients with lung cancer and persisted several years into the postoperative period without cancer recurrence. Previous study performed by Huang showed that the prevalence of fatigue was 57% in stage IA or IB NSCLC, which was in accordance with ours (59.8%).[9] They also presented that 41% of participants had mild fatigue and 16.8% had moderate or severe fatigue (BFI ≥4),[9] and in current study, 37.4% had mild fatigue and 22.4% had moderate to severe fatigue (BFI ≥4), These results together with ours confirmed that fatigue is highly prevalent among early-stage NSCLC survivors.
We have also proved the influence of fatigue on an individual's functional status, especially the relationship between moderate to severe fatigue and dysfunction. The study showed that 21.1% of patients with moderate to severe fatigue showed obvious dysfunction, such as inability to work or care for themselves, these results were also in line with Huang's study, which showed that among the individuals reporting moderate or severe fatigue, 23.7% had significant functional impairment compared to 2.8% with mild or no fatigue.[9] One of the characteristics of this study was that it not only analyzed the presence of fatigue, but also emphasized the severity of fatigue and its influence on functional status.
This study also analyzed the association of moderate to severe fatigue with other factors and identified risk factors that included psychological status (depression and anxiety) and combined lung diseases (including COPD and asthma) because lung diseases like COPD could limit physical activity and increase fatigue.[18] At the same time, an altered psychological state like anxiety, could aggravate dyspnea, leading to more severe anxiety and difficulty breathing, thus forming a vicious cycle.[19] The current study results provided information regarding the prevalence of anxiety and depression in postoperative patients with early-stage NSCLC, 18.5% had anxiety and 10.2% had depression, indicating that lung cancer and its related treatment may have had greater negative effects on the psychological well-being of the participants. One previous report found that in postoperative breast carcinoma survivors, 16.7% had anxiety and 21.1% had been at risk for depression even 1-year after tumor resection,[20] The results and ours confirmed the close links between fatigue and psychological status and highlighted the significance of carefully assessing the psychological status in NSCLC survivors who report problems with fatigue.
Physical activity was shown to be a protective factor; exercise, and relaxation training could help alleviate anxiety, depression, and other symptoms.[21] In univariate analysis, fatigue was also related to difficulty breathing, suggesting that symptoms such as fatigue, difficulty breathing, depression, and anxiety might be a symptom cluster, always occurring together, which is consistent with the results of previous studies.[22] These findings provide useful information for clinicians treating early-stage NSCLC patients with fatigue. The relatively high prevalence of fatigue suggests that routine screening of all lung cancer patients after surgery for clinically significant posttreatment fatigue is needed. If moderate or severe fatigue is present, patients should also be assessed for depressive and anxiety symptoms; once identified, a comprehensive approach, including psychotherapy (such as cognitive behavioral therapy), antidepressants with dual anxiolytic effects, and/or exercise and relaxation training should be adopted.
The strengths of this study include a relatively large number of participants and a good response rate. Furthermore, this was the first domestic fatigue assessment of early-stage lung cancer patients carried out 1-5 years after surgery. This study identified several indicators of moderate to severe fatigue that could be modified by human intervention suggesting that fatigue in cancer survivors can be mitigated through timely diagnosis and treatment. However, there were several limitations to our study. First, 17 patients did not participate in the survey, 12 due to passive refusal; therefore, the incidence of fatigue may be underestimated. Second, we did not assess objective fatigue-associated biological indicators such as hemoglobin, thyroid function, and testosterone, which are potential causes of fatigue. Third, this was a cross-sectional study, so it was not possible to establish causality between the observed risk factors.
CONCLUSION
The findings of the current study demonstrate that more than half of the patients with early-stage NSCLS experienced fatigue several years after surgery, and that moderate to severe fatigue clearly affected the patients’ daily functional status. Fatigue was closely associated with anxiety, depression, and lung diseases (combined and including COPD and asthma), whereas physical activity was helpful in relieving the symptoms of fatigue. As fatigue and these other symptoms formed a symptom cluster, comprehensive treatment methods might achieve more effective therapeutic results.
AUTHOR'S CONTRIBUTION
XH contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. WZ and YZ contributed in the analysis and interpretation of data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Paoletti L, Pastis NJ, Denlinger CE, Silvestri GA. A decade of advances in treatment of early-stage lung cancer. Clin Chest Med. 2011;32:827–38. doi: 10.1016/j.ccm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Hartvig P, Aulin J, Hugerth M, Wallenberg S, Wagenius G. Fatigue in cancer patients treated with cytotoxic drugs. J Oncol Pharm Pract. 2006;12:155–64. doi: 10.1177/1078155206070774. [DOI] [PubMed] [Google Scholar]
- 5.Sarna L, Cooley ME, Brown JK, Chernecky C, Elashoff D, Kotlerman J. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care. 2008;17:455–67. [PMC free article] [PubMed] [Google Scholar]
- 6.Brunelli A, Socci L, Refai M, Salati M, Xiumé F, Sabbatini A. Quality of life before and after major lung resection for lung cancer: A prospective follow-up analysis. Ann Thorac Surg. 2007;84:410–6. doi: 10.1016/j.athoracsur.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Win T, Sharples L, Wells FC, Ritchie AJ, Munday H, Laroche CM. Effect of lung cancer surgery on quality of life. Thorax. 2005;60:234–8. doi: 10.1136/thx.2004.031872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarna L, Padilla G, Holmes C, Tashkin D, Brecht ML, Evangelista L. Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol. 2002;20:2920–9. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 9.Hung R, Krebs P, Coups EJ, Feinstein MB, Park BJ, Burkhalter J, et al. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J Pain Symptom Manage. 2011;41:426–35. doi: 10.1016/j.jpainsymman.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozturk A, Sarihan S, Ercan I, Karadag M. Evaluating quality of life and pulmonary function of long-term survivors of non-small cell lung cancer treated with radical or postoperative radiotherapy. Am J Clin Oncol. 2009;32:65–72. doi: 10.1097/COC.0b013e31817e6ec2. [DOI] [PubMed] [Google Scholar]
- 11.Wang XS, Hao XS, Wang Y, Guo H, Jiang YQ, Mendoza TR, et al. Validation study of the Chinese version of the Brief Fatigue Inventory (BFI-C) J Pain Symptom Manage. 2004;27:322–32. doi: 10.1016/j.jpainsymman.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Mystakidou K, Tsilika E, Parpa E, Mendoza TR, Pistevou-Gombaki K, Vlahos L, et al. Psychometric properties of the Brief Fatigue Inventory in Greek patients with advanced cancer. J Pain Symptom Manage. 2008;36:367–73. doi: 10.1016/j.jpainsymman.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Evers PD, Logan JE, Sills V, Chin AI. Karnofsky performance status predicts overall survival, cancer-specific survival, and progression-free survival following radical cystectomy for urothelial carcinoma. World J Urol. 2014;32:385–91. doi: 10.1007/s00345-013-1110-7. [DOI] [PubMed] [Google Scholar]
- 14.Macfarlane D, Chan A, Cerin E. Examining the validity and reliability of the Chinese version of the International Physical Activity Questionnaire, long form (IPAQ-LC) Public Health Nutr. 2011;14:443–50. doi: 10.1017/S1368980010002806. [DOI] [PubMed] [Google Scholar]
- 15.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 16.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–8. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 17.Leung CM, Wing YK, Kwong PK, Lo A, Shum K. Validation of the Chinese-Cantonese version of the hospital anxiety and depression scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr Scand. 1999;100:456–61. doi: 10.1111/j.1600-0447.1999.tb10897.x. [DOI] [PubMed] [Google Scholar]
- 18.Baghai-Ravary R, Quint JK, Goldring JJ, Hurst JR, Donaldson GC, Wedzicha JA. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med. 2009;103:216–23. doi: 10.1016/j.rmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Kunik ME, Azzam PN, Souchek J, Cully JA, Wray NP, Krishnan LL, et al. A practical screening tool for anxiety and depression in patients with chronic breathing disorders. Psychosomatics. 2007;48:16–21. doi: 10.1176/appi.psy.48.1.16. [DOI] [PubMed] [Google Scholar]
- 20.Tan XF, Xia F. Long-term fatigue state in postoperative patients with breast cancer. Chin J Cancer Res. 2014;26:12–6. doi: 10.3978/j.issn.1000-9604.2014.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: Efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: Conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]


