Abstract
We set a model to replicate the vascular bone marrow niche by using endothelial colony forming cells (ECFCs), and we used it to explore the vascular niche function in patients with low-risk myelodysplastic syndromes (MDS). Overall, we investigated 56 patients and we observed higher levels of ECFCs in MDS than in healthy controls; moreover, MDS ECFCs were found variably hypermethylated for p15INK4b DAPK1, CDH1, or SOCS1. MDS ECFCs exhibited a marked adhesive capacity to normal mononuclear cells. When normal CD34 + cells were co-cultured with MDS ECFCs, they generated significant lower amounts of CD11b + and CD41 + cells than in co-culture with normal ECFCs. At gene expression profile, several genes involved in cell adhesion were upregulated in MDS ECFCs, while several members of the Wingless and int (Wnt) pathways were underexpressed. Furthermore, at miRNA expression profile, MDS ECFCs hypo-expressed various miRNAs involved in Wnt pathway regulation. The addition of Wnt3A reduced the expression of intercellular cell adhesion molecule-1 on MDS ECFCs and restored the defective expression of markers of differentiation. Overall, our data demonstrate that in low-risk MDS, ECFCs exhibit various primary abnormalities, including putative MDS signatures, and suggest the possible contribution of the vascular niche dysfunction to myelodysplasia.
Introduction
Homeostasis of hematopoietic system requires that hematopoietic stem cells (HSCs) can either rest dormant or proliferate and differentiate in response to peripheral stimuli [1]. The bone marrow (BM) environment guarantees for these definite activities by keeping HSCs embedded within specialized niches [2]. The BM niches are composed by several kinds of cells, including osteoblasts and mesenchymal and endothelial cells [3–5]. The osteoblasts govern the quiescence and maintain the pool of HSCs through specific pathways including Jagged-Notch, Tie2/angiopoietin, and transforming growth factor β/SMAD signaling [2]. During the past decade, several evidences have been gathered, showing that endothelial cells also play a critical role in hematopoiesis government [5–7]. In this study, we investigated the crosstalk between endothelial microenvironment and HSCs in myelodysplastic syndromes (MDS). These clonal disorders are characterized by an increased BM cellularity, due to excessive apoptosis, coupled with aberrant differentiation [8]. Although MDS constitute a very heterogeneous group of diseases, all of them share variable degrees of ineffective hematopoiesis and susceptibility to leukemic transformation [8]. Importantly, aberrant morphology of myelodysplastic cells mirrors specific functional defects, which significantly affects the disease course also in patients at low risk for leukemic evolution [9]. Indeed, patients with MDS are prone to serious infections even when the neutrophil count is apparently preserved and they can have serious bleeding episodes despite reasonable platelet counts [9]. In general, MDS patients have a transient and inadequate response to chemotherapy, probably as a result of the “dormant” status of MDS HSC within protective BM niches [10]. Therefore, it is conceivable that BM niches might contribute to the biologic demeanor of MDS. Because endothelial progenitors have been proven to contribute to the BM microvasculature, we used circulating endothelial colony forming cells (ECFCs) as a model to replicate the BM vascular niche [11,12]. We isolated ECFCs from a homogeneous group of patients diagnosed with low-risk MDS and evaluated the influence of MDS endothelium on the differentiation of normal hematopoietic cells.
Material and Methods
Patients
The study included 56 patients (25 males and 31 females, median age 69 years, range 46-90) affected by low-risk MDS according to the International Prognostic Scoring System [13]. At the time of study, patients were not receiving antiproliferative or demethylating drugs. Twenty-eight healthy blood donors (14 males and 14 females, median age 61.3 years, range 45 to 77) were used as controls. All blood samples were obtained after informed consent. The study was approved by the Institutional Ethical Committee.
Cell Cultures
ECFCs were obtained from peripheral blood of MDS patients or from healthy blood donors according to the method of Ingram et al., as previously described [14,15]. The endothelial nature of cells was confirmed by evaluating the expression of CD34, CD146, CD45, and vascular endothelial growth factor receptor by flow cytometry and reverse transcription–polymerase chain reaction (PCR) [15]. Confluent ECFCs at passages IV to VI were used for genotype profiling, hematopoietic cell co-cultures, and flow cytometry analysis. In selected experiments, Wnt3A or Wnt5A (R&D Systems, Space Import-Export srl, Milano, Italy) was added at 100 ng/ml. The influence of endothelial cells on normal hematopoietic differentiation was evaluated by co-culturing cord blood CD34 + cells over non-irradiated ECFC layers. CD34 + cells were isolated by immune-magnetic method with the purity always above 92%. Co-cultures were performed in 24 multiwell plates: 30 × 103 CD34 + cells per well were seeded on confluent ECFCS in Stem Span SFEM medium (Stem Cell Technology Inc., Vancouver, Canada). Different cocktails of cytokines were added to achieve the optimal commitment: 10 ng/ml each of stem cell factor (SCF), Granulocyte Macrophage - Colony Stimulating Factor, and Interleukin 3 for granulo-monocytic differentiation, 3 U/ml erythropoietin and 10 ng/ml SCF for erythroid differentiation, and 20 ng/ml Interleukin 6 and 10 ng/ml thrombopoietin for megakaryocytic differentiation (all purchased from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Every other day, 0.5 ml of supernatant was removed and replaced with fresh medium plus cytokines. CD34 + cultured without ECFCs served as controls.
Flow Cytometry
The differentiation toward different lineages was assessed by flow cytometry using a FACScanto flow cytometer (Becton-Dickinson, Franklin Lakes, NJ) equipped with a 488-nm excitation light source. The following monoclonal antibodies were used: phycoerytrin (PE)-mouse anti-human CD54, fluorescein isothiocyanate anti-human CD41a, PE anti-human CD11b, and PE anti-human CD71 (all purchased from Becton-Dickinson). Negative controls consisted of cells incubated with isotype-matched PE- or fluorescein isothiocyanate–conjugated irrelevant monoclonal antibodies. Results were expressed as percentage of positive cells. The adhesive capacity of ECFCs was assessed by evaluating the percentage of carboxyfluorescein succinimidyl ester (CFSE)–labeled mononuclear cells remaining adherent to the layer of ECFC after 2-hour incubation [15]. The expression of intercellular cell adhesion molecule-1 (ICAM-1) in ECFCs was assessed with the PE anti-human CD54 (Becton-Dickinson).
Real-Time Analysis, Methylation Assay, and Gene Expression Array
Real-time analysis, methylation assay, and gene expression array are detailed in the Supplementary Materials. All used primers and product lengths are shown in Tables S1 and S2.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA) and MedCalc version 10.2.0.0 (MedCalc Software, Mariakerke, Belgium). Statistical comparison of continuous variables was performed with the Mann-Whitney U test or paired t test, as appropriate. Comparison of categorical variables was performed using chi-square statistic and the Fisher exact test. P values less than .05 were considered as statistically significant.
Results
MDS Patients Have a Higher Output of ECFCs than Controls
ECFCs were achieved in 29 of 56 MDS patients and in 17 of 28 controls (P = .491), with no differences in age (P = .356) and sex (P = .651) distribution between subjects producing or not producing ECFCs. In addition, we did not observe any clinical and biologic differences in MDS patients with ECFC isolation frequency similar to normal individuals (≤ 0.9/107 cells) and in MDS patients with ECFC isolation frequency higher than normal individuals (> 0.9/107 cells). Among individuals with ECFCs, MDS patients had a significantly higher number of colonies than healthy controls (1.2 vs 0.2 ECFCs/107 cells, P < .0001, Figure 1). On the whole, the levels of ECFCs in our series of patients and the percentage of samples producing at least one ECFC are in good agreement with those reported by other authors using the same methodologies [16].
Figure 1.
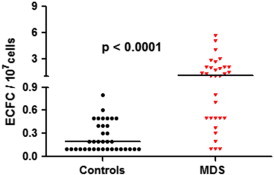
ECFCs in 29 patients with low IPSS risk MDS and in 17 healthy controls. MDS patients show significant higher levels of ECFCs (1.2 vs 0.2/107 cells; P < .0001).
ECFCs Isolated from MDS Patients Show a Hypermethylated Phenotype
Although recent studies evidenced that in myeloproliferative neoplasms cells from the endothelial compartment harbor the disease-specific signature, i.e., the JAK2V617F mutation [15,17], Della Porta et al. demonstrated that ECFCs isolated from MDS patients do not show the chromosomal aberrations found in the neoplastic clone [16]. Because aberrant DNA methylation of CpG-rich promoters of a variety of genes is very frequent in MDS [17], we investigated in ECFCs an eventual deregulation of the epigenetic machinery. We examined in ECFC samples obtained from 20 patients the methylation status of four genes that frequently undergo hypermethylation in MDS hematopoietic cells: p15INK4b (p15), DAPK1, CDH1, and SOCS1. We found that all patients showed at least one hypermethylated gene, with 13 patients having multiple hypermethylated genes (Table 1 and Figure 2). All investigated genes are functional to various pathways of normal endothelial cells, thus excluding that hypermethylation might result from physiological gene silencing. In addition, none of 14 investigated healthy controls was found hypermethylated.
Table 1.
Incidence of Aberrant DNA Methylation of CpG-Rich Promoters of p15INK4b, CDH1, DAPK1, and SOCS1 genes in ECFCs Obtained from 20 Patients with MDS and 14 Controls
| Controls (n = 14) |
MDS Patients (n = 20) |
P | |||
|---|---|---|---|---|---|
| Methylated | Unmethylated | Methylated | Unmethylated | ||
| p15INK4b | 0 | 14 | 8 | 12 | .01 |
| CDH1 | 0 | 14 | 7 | 13 | .026 |
| DAPK1 | 0 | 14 | 3 | 17 | ns |
| SOCS1 | 0 | 14 | 4 | 16 | ns |
| One of the above | 0/14 | 20/20 | < .0001 | ||
Figure 2.
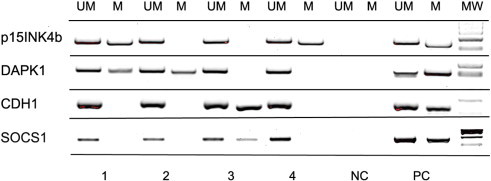
Methylation-specific PCR of p15INK4b (p15), DAPK1, CDH1, and SOCS1 genes in ECFCs isolated from MDS patients: representative results of ECFCs isolated from four patients. UM indicates unmethylated gene; M indicates methylated gene; NC indicates negative control; PC indicates positive control for unmethylated and methylated DNA; MW indicates the molecular weight markers. Other abbreviations are detailed in the text.
ECFCs from MDS Patients Show Increased Adhesion to Normal Hematopoietic Cells
It has been recently reported that mesenchymal stromal cells (MSCs) from MDS patients have a defective expression of adhesive molecules and chemokines [18,19], and we evaluated the proficiency of ECFCs obtained from MDS patients to adhere to normal mononuclear cells in comparison with ECFCs isolated from healthy subjects. Normal mononuclear cells tracked with CFSE were incubated over ECFC layers obtained from healthy individuals and MDS patients: After 2 hours, non-adherent cells were discharged and adherent cells were recovered and counted by flow cytometry. Results obtained are shown in Figure 3. On the whole, 40 ± 4% of tracked cells adhered to MDS ECFCs, in comparison with 13 ± 1% remaining adherent to normal ECFCs (P = .005). Altogether, these observations suggest that endothelial cells from MDS patients show an “adhesion-proficient” phenotype.
Figure 3.
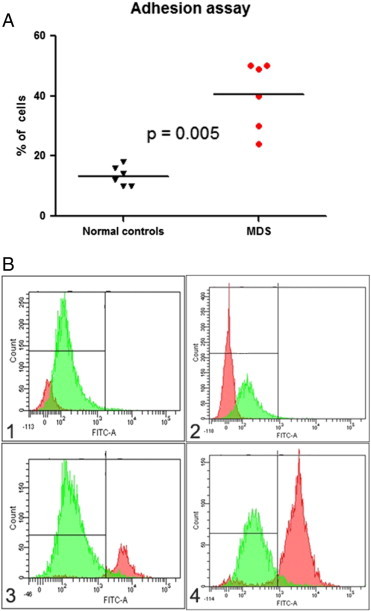
CFSE-labeled mononuclear cell adhesion to normal or MDS ECFCs. (A) Percentages of mononuclear cells adherent to ECFCs obtained from six healthy controls and from six MDS patients. (B) Representative experiment showing on the left the cytofluorimetric analysis of unstained peripheral blood mononuclear cells (negative control) and on the right the number of CFSE-labeled mononuclear cells bound by ECFC in a healthy control (upper panels) and in an MDS patient (lower panels). Abbreviations are detailed in the text.
The Contact with MDS Endothelial Cells Perturbs the Expansion and Differentiation of Normal CD34 + Cells
Preliminarily, we observed that underlying ECFCs supported the expansion and differentiation of cord blood CD34 + cells. In fact, when CD34 + cells were seeded over normal ECFCs in erythroid, granulo-monocytic, and megakaryocytic differentiation media, we observed at day 7 a mean increase of 1.6 ± 0.1, 1.4 ± 0.1, and 2.0 ± 0.1 in total nucleated cell (TNC) number, respectively, compared to cultures performed under the same conditions but without endothelial layers. This was the result of an increase in the number of both differentiated (i.e., CD71 +, CD11b +, and CD41 +) and undifferentiated (i.e., CD34 +) cells (Figure 4A). Accordingly, at the molecular level, we observed the harmonized up-regulation of genes related to the early phase of differentiation, including Friend of Gata (FOG), PU.1, and AML-1, and subsequently, the up-regulation of genes related to terminal differentiation, as Transferrin Receptor (TFR), Myeloperoxidase (MPO), and Glycoprotein Ib (GPIb) (Figure 4B). Indeed, we investigated whether MDS ECFCs had the same supportive properties exerted by normal ECFCs. We found that ECFCs isolated from MDS patients were scarcely able or even at all incapable to support the expansion and differentiation of hematopoietic cells (Figure 4A). Although this defect was apparently more evident in granulocyte and megakaryocytic cultures than in erythroid cultures, the pattern of expression of lineage-related genes was deeply perturbed, clearly suggesting an imbalance between proliferation and differentiation (Figure 4B). In particular, in granulo-monocytic and megakaryocytic cultures, lineage-related gene expression was abnormally low in both early (PU.1 and AML-1, respectively) and late (MPO and GPIb, respectively) phases of differentiation. In contrast, in cells pushed toward the erythroid lineage, FOG and TFR reached abnormally high expression values (Figure 4B).
Figure 4.
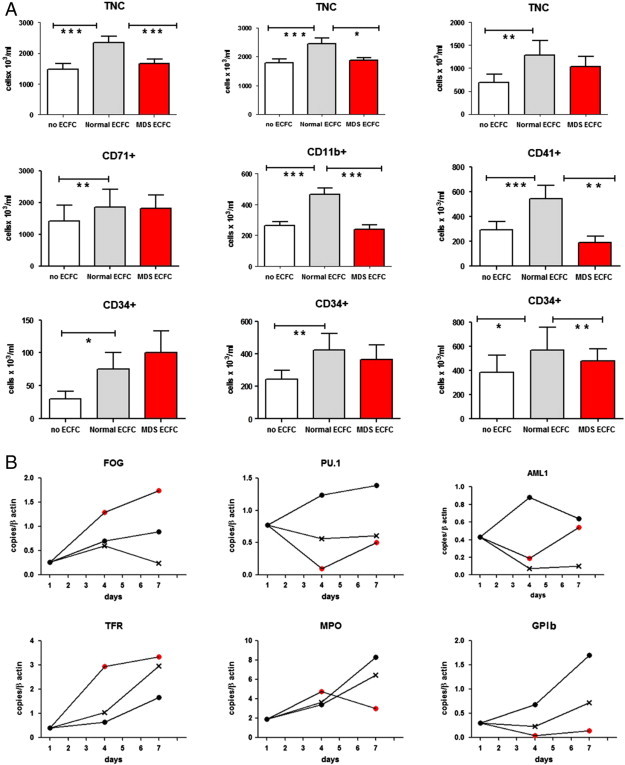
Co-cultures of normal cord blood CD34 + cells in the absence of endothelial layers or over ECFC obtained from healthy controls and from MDS patients. Results represent the mean of six experiments. (A) Day 7 concentrations of total nucleated cells, CD71 + or CD11b + or CD41 + cells, and CD34 + cells in cultures supporting erythroid, granulo-monocytic, and megakaryocytic differentiation, respectively. (B) Real-time PCR expression of early (FOG, PU.1, and AML-1) and late differentiation markers (TFR, MPO, and GPIb) in cells recovered at days 4 and 7 from the experiments illustrated in A. *P < .05; **P < .005; ***P < .001.  refers to cultures without ECFCs;
refers to cultures without ECFCs;  refers to cultures with normal ECFCs;
refers to cultures with normal ECFCs;  refers to cultures with MDS ECFCs.
refers to cultures with MDS ECFCs.
Normal and MDS ECFCs Have Different Gene Expression Profiles
To discover the alterations underlying the impaired capability of MDS ECFCs to sustain hematopoiesis in vitro, we evaluated through microarray analysis their gene expression profiles in comparison with normal ECFCs. We initially focused on genes involved in a wide range of endothelial cell pathways, including adhesion, angiogenesis, cell activation, cell survival, and apoptosis (the gene set is available in the Supplementary Materials). Overall, among six significant upregulated genes, four codified for cell adhesion molecules [ICAM-1, vascular cell adhesion molecule 1 (VCAM-1), l-selectin (SELL), and von Willebrand factor (vWF)]. Two additional genes found overexpressed were nitric oxide synthase and tumor necrosis factor ligand superfamily, member 10 (Table 2, upper section). In contrast, matrix metalloprotease-1 (MMP1), BCL2-like 1, and CASP2 and RIPK1 domain containing adaptor with death domain were significantly downregulated in MDS ECFCs compared to normal controls (Table 2, upper section). Some of these genes are implicated in the regulation of blood cell production. For example, nitric oxide delivery not only induces megakaryocyte apoptosis and platelet formation but also controls HSC production in embryonic and adult lives [20–22]. Likewise, MMP1 activates the hypoxia-inducible factor-1 pathway within niche cells, thereby inducing the transcription of hypoxia-inducible factor–responsive genes and terminal hematopoietic differentiation [23].
Table 2.
Genes Differentially Expressed in ECFCs Isolated from MDS Patients and Healthy Controls. The Upper Section Includes Genes Functional to Adhesion, Angiogenesis, Activation, Survival, and Apoptosis of Endothelial Cells; the Middle Section Includes Genes Belonging to the Wnt Pathway, and the Lower Section Includes miRNAs
| Gene | Fold Regulation | P |
|---|---|---|
| ICAM-1 | 14.06 | .012 |
| Constitutive nitric oxide synthase 3 | 13.17 | .015 |
| SELL | 7.26 | .041 |
| Tumor necrosis factor ligand superfamily, member 10 | 11.08 | .019 |
| VCAM-1 | 5.46 | .008 |
| vWF | 6.86 | .001 |
| BCL2-like 1 | − 8.51 | .006 |
| CASP2 and RIPK1 domain containing adaptor with death domain | − 4.92 | .032 |
| MMP1 | − 4.78 | .002 |
| FST | 5.38 | .043 |
| BMP4 | 4.85 | .001 |
| Amino-terminal enhancer of split | − 4.48 | .001 |
| Cyclin D1 | − 5.59 | .004 |
| C-terminal binding protein 1 | − 4.32 | .003 |
| Inhibitor of differentiation 2 | − 4.96 | .024 |
| Wnt3A | − 4.54 | .010 |
| Wnt5A | − 4.51 | .021 |
| Wnt5B | − 83.51 | .031 |
| miR-142-3p | − 6.99 | .003 |
| miR-424-5p | − 4.33 | .001 |
| miR-196b-5p | − 9.89 | .033 |
To explain our overall findings, we next examined additional pathways with putative effects on cell adhesion. Because the Wingless and int (Wnt) pathway is one of the main regulators of cell-to-cell interactions in hematopoietic tissues [24–26], we evaluated the expression profile of genes involved in the canonical and non-canonical Wnt pathways (gene sets, including ligands, receptors, and target of Wnt are available in the Supplementary Materials). We found that Wnt canonical signaling was significantly downregulated at multiple levels in MDS ECFCs (Table 2, middle section). Actually, MDS cells contained low RNA levels of several Wnt ligands (Wnt3A, Wnt5A, and Wnt5B), and several Wnt target genes were hence downregulated, including the transcriptional co-repressors amino-terminal enhancer of split and C-terminal binding protein 1, cyclin D1, and inhibitor of differentiation 2 [27]. On the contrary, the bone morphogenetic protein-4 (BMP4) and follistatin (FST), which are subjected to regulatory interactions with Wnt, were upregulated in MDS ECFCs in comparison with normal ECFCs (Table 2, middle section). Interestingly, both genes have specific activities in human hematopoiesis. In particular, BMP4 is a powerful inducer of erythroid differentiation in stress erythropoiesis and acts in concert with SCF and hypoxia to promote the proliferation and differentiation of erythroid progenitors in response to acute anemia [28]. The increased expression of BMP4 can partly explain why MDS ECFCs forced the expression of FOG and TFR during erythroid differentiation of normal progenitors. In contrast, FST regulates the hematopoietic cell adhesiveness to fibronectin, favoring the expansion of immature progenitors [29]. We hypothesized that the extensive hypo-expression of several Wnt genes could depend on the aberrant hypermethylation of their promoters. Nevertheless, our search for methylation of the Wnt5A gene promoter region was unsuccessful (data not shown). We then investigated the expression profile of a set of 84 miRNAs with predictable effects on the Wnt pathway. Actually, we demonstrated that, in comparison with normal ECFCs, MDS ECFCs contained significantly lower amounts of miR-142-3p, miR-424-5p, and miR-196b-5p (Table 2, lower section). Whereas all these miRNAs are variously involved in hematopoiesis [30,31] or angiogenesis [32], the low levels of miR-142-3p were particularly interesting, because it has been demonstrated to modulate the Wnt pathway to balance proliferation and differentiation of mesenchymal cells [33]. Interestingly, miR-142-3p was predicted to inhibit the expression of BMP4 at both “Ingenuity” and “microRNA.org-Target and Expression” software.
The Addition of Soluble Wnt3A Modifies the Adhesive Profile of MDS ECFCs
Overall, the analysis of gene expression profiles suggested that MDS endothelial progenitor cells show a defective expression of several Wnt pathway constituents. Previous studies demonstrated that canonical Wnt pathway signaling regulates hematopoietic homeostasis by shaping the niches supportive of hematopoietic stem/progenitor cells [25]. In particular, Wnt signaling modulates VCAM-1 expression by marrow stromal cells and controls the production of extracellular matrix components [34]. Therefore, our model suggests that the increased adhesiveness of MDS ECFCs may result from the ineffective Wnt signaling and that the aberrant attachment of hematopoietic cells to the endothelial environment could interfere with their normal differentiation programs. To test this hypothesis, we investigated whether the addition of 100 ng/ml of soluble Wnt3A and/or Wnt5A to ECFC cultures could influence the abnormally high RNA expression of ICAM-1, VCAM-1, vWF, and SELL. We observed that ICAM-1 RNA expression was the most significantly affected: either Wnt3A or Wnt5A was able to reduce ICAM-1 expression, but Wnt3A induced a more sustained and deep decrease (Figure 5A). These findings were further confirmed by flow cytometry. In particular, we found a significant reduction of ICAM-1 expression in MDS ECFC but not in normal ECFC after Wnt3A addition (Figure 5, B and C). In addition, we investigated the effect of Wnt3A addition on the capacity of MDS ECFCs to support hematopoietic differentiation. We found that the expression pattern of differentiation markers was positively affected by the Wnt3A addition, with a significant decrease in abnormally high TRF RNA values and a significant increase in both MPO and GPIb RNA expression (Figure 5D).
Figure 5.
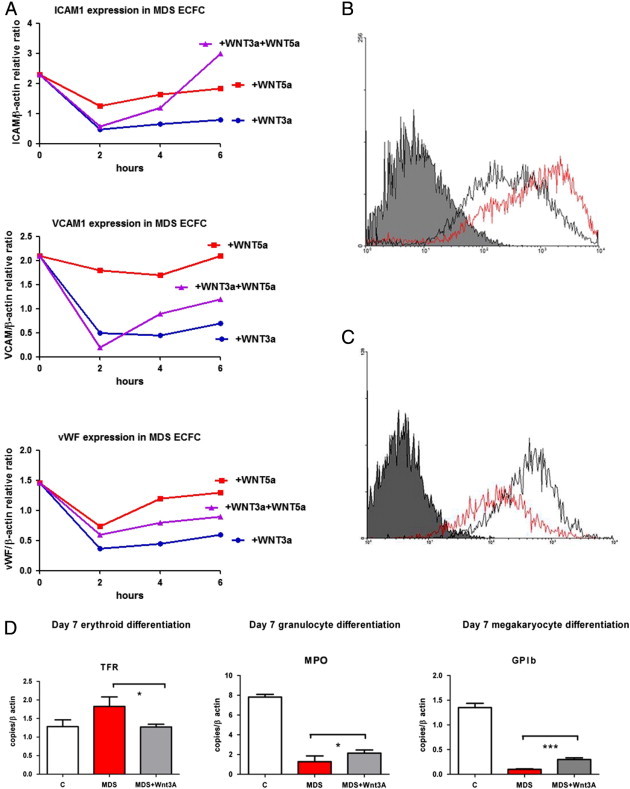
(A) Real-time PCR expression of VCAM-1, vWF, and ICAM-1 RNA in MDS ECFCs incubated with Wnt3A, Wnt5A, and Wnt3A plus Wnt5A (each at 100 ng/ml concentration). The data represent the mean values of three experiments (SDs are < 10% and have been omitted). (B and C) The flow cytometry expression of ICAM-1 in normal (gray line) and MDS (red line) ECFCs without Wnt3A (B) and after incubation with Wnt3A (C). We observed a significant reduction of ICAM-1 in MDS ECFCs. Data refer to a representative experiment; mean fluorescence intensity is reported in the x axis and number of events in the y axis. (D) Co-cultures of normal cord blood CD34 + cells with normal ECFCs (white columns), MDS ECFCs (red columns), or MDS ECFCs plus 100 ng/ml Wnt3A (gray columns). Real-time PCR expression of TRF, MPO, and GPIb RNA after day 7 of culture in erythroid, granulo-monocytic, and megakaryocytic differentiating media. Results represent the mean of five experiments. *P < .05; ***P < .001.
Discussion
This study demonstrates that endothelial progenitor cells isolated from patients with low-risk MDS have an increased adhesion capacity and fail to adequately sustain hematopoiesis, particularly in myeloid and megakaryocyte differentiation. Although the increased level of ECFCs in these patients has been previously reported [16], our findings suggest that the molecular defects underlying MDS endothelial progenitor dysfunctions are likely primary in their nature, since these cells differ from their normal counterparts in both genetic and epigenetic profiles. Since the transplant of allogeneic endothelial progenitor cells in myelosuppressed mice shortens the duration of aplasia and fosters blood cell recovery, it is conceivable that circulating ECFCs normally contribute to the BM microvasculature homeostasis [35]. Even though our observations have been gathered in an in vitro model where ECFCs represent the surrogate of the BM vascular niche, they suggest a possible “diseased vascular niche” in low-risk MDS patients.
The relevant role of endothelial cells in hematopoiesis was clearly demonstrated several decades ago by Knospe et al. [36]. At that time, the authors showed that in mice subjected to local BM curettage followed by radiation and HSC transplantation the earliest foci of hematopoiesis occurred just into the areas of sinusoidal vascular regeneration [36]. Subsequent studies highlighted that the engraftment and reconstitution of hematopoiesis are mediated by vascular endothelial growth factor receptor 2 through the regeneration of sinusoidal endothelial cells [37]. While these effects have been partly attributed to the secretion of soluble growth factors, additional evidences indicate that the direct contact between HSCs and endothelial cells is relevant to hematopoiesis regulation. For instance, the contact with HSCs triggers in endothelial cells the activation of Akt and the release of paracrine factors that balance HSC self-renewal and differentiation [38]. Similarly, in normal BM, the areas of SCF/c-kit–mediated hematopoiesis are in tight spatial co-localization with endothelial and perivascular cells [39]. In addition, E-selectin expressed by endothelial cells has been recently recognized as a new regulator of HSC proliferation and differentiation [40]. We show here that, in contrast to normal ECFCs, circulating endothelial cells obtained from low-risk MDS patients are scarcely efficient in supporting hematopoietic differentiation: This defect is associated to the increased adhesion to hematopoietic cells that is likely induced by the low expression of various members of the Wnt pathway. In particular, stromal Wnt3A has been indicated as a powerful negative regulator of several adhesion molecules, including ICAM-1 and VCAM-1 [34], and in our experiments, the addition of exogenous Wnt3A both reduced ICAM-1 expression and partially restored cell differentiation.
The microenvironment participation in determining clinical and biologic features of MDS has been extensively investigated. Tennant et al. first showed that the adherent layers obtained from BM of MDS patients were significantly defective in supporting hematopoiesis in long-term Dexter-type cultures [41]. Tauro et al. observed heterogeneous abnormalities of MDS stromal cells including altered matrix molecule expression and changes in superoxide production, possibly contributing to the abnormal survival and development of hematopoietic cells [42]. Moreover, BM stromal cells of MDS patients exhibit abnormal production of several cytokines contributing to the impaired immune function observed in these patients [43]. More recently, Ferrer at al. showed that primary MSCs obtained from MDS patients express low levels of adhesion and cell surface molecules and, when co-cultured with CD34 + cells from healthy donors, produce lower numbers of cobblestone area–forming cells and fewer colony-forming units [18]. Similarly, Geyh et al. found that MSCs from patients with all MDS subtypes exhibit impaired growth and osteogenic differentiation, associated with specific methylation patterns and altered expression of several molecules involved in the interaction with HSCs, including osteopontin, Jagged1, Kit-ligand, and angiopoietin as well as several chemokines [19]. Finally, Pavlaki et al. observed that MSCs obtained from low- to intermediate-risk patients displayed downregulated canonical Wnt pathway due to up-regulation of inhibitors [44]. When pharmacologically activated, Wnt signaling led to an increased cell proliferation and restored their osteogenic differentiation ability [44]. The findings obtained in our model suggest for the first time that specific primitive perturbations could occur also at the level of the MDS vascular niche. Primitive abnormalities of endothelial cells or endothelial cell progenitors have been previously demonstrated in chronic myeloproliferative neoplasms. In these conditions, the “diseased vascular niche” facilitates clonal stem cell proliferation, favors extramedullary hematopoiesis through mobilization and homing of neoplastic HSCs in new niches in the spleen, and contributes to vascular complications [15,45,46]. In contrast, in MDS patients, endothelial niche dysfunctions could sustain BM hypercellularity embedded in dense microvasculature scaffolds. In addition, the pathologic involvement of the circulating endothelial compartment might be implicated in the association between MDS with systemic vasculitis [47] or in thrombotic proficiency of MDS patients subjected to specific therapies despite concomitant thrombocytopenia [48].
The canonical and non-canonical Wnt pathways consist of a complex network of interrelated and reciprocally connected signals [24–26]. Previous studies carried out in MDS hematopoietic cells reported hypermethylation of several Wnt antagonists, contributing to the activation of the Wnt pathway and the expression of β-catenin [49,50]. In these patients, a correlation between risk of leukemic transformation and nuclear β-catenin expression or Wnt antagonist hypermethylation was noticed, confirming the linkage between Wnt pathway activation and leukemogenesis [49,50]. Our observations, in contrast, suggest that the defective function of the Wnt pathway in vascular niches could mainly affect the physiological differentiation processes of hematopoietic cells, resulting in the myelodysplasia observed also in patients at low risk for leukemic transformation. Accordingly, harvest of immature progenitors (i.e., CD34 + cells) in cultures supported by MDS endothelial cells was not so different from that obtained in the presence of normal endothelial cells. Furthermore, we found that supplementation of exogenousWnt3A to cultures partly reverted the impaired hematopoietic differentiation of hematopoietic cells.
Conclusion
Overall, our observations add a new tile to the complex mosaic of the MDS pathogenesis and offer an innovative perspective on these diseases, pointing to the primary dysfunctions of the vascular niche as important drivers for myelodysplasia.
Footnotes
This work was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC IG-11799). Author’s contributions: L.T., M.M., E.R.N., S.C., M.G.I., and A.C. collected and analyzed the data; L.T. and L.M.L. designed the research and wrote the paper; M.M., E.R.N., S.C., M.G.I., and A.C. performed the experiments; M.M. provided statistical analysis; E.F. and M.T.V provided patient samples and clinical information. Disclosure of potential conflicts of interest: The authors report no potential conflicts of interest.
This article refers to supplementary materials, which are designated by Tables S1 and S2 and are available online at www.neoplasia.com.
Appendix A. Supplementary Materials
Table S1. Primers and Probes Used in Reverse Transcription–PCR Analysis of Normal and MDS ECFCs and of Normal Hematopoietic Cells
Table S2. Primers Used in Promoter Methylation Analysis of ECFCs
References
- 1.Wilson A., Laurenti E., Oser G., van der Wath R.C., Blanco-Bose W., Jaworski M., Offner S., Dunant C.F., Eshkind L., Bockamp E. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 2.Wilson A., Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 3.Yin T., Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Y., Nilsson S.K. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. 2012;19:250–255. doi: 10.1097/MOH.0b013e328353c714. [DOI] [PubMed] [Google Scholar]
- 5.Scadden D.T. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Niu C., Ye L., Huang H., He X., Tong W.G., Ross J., Haug J., Johnson T., Feng J.Q. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 7.Doan P.L., Chute J.P. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26:54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 8.Komrokji R.S., Zhang L., Bennett J.M. Myelodysplastic syndromes classification and risk stratification. Hematol Oncol Clin North Am. 2010;24:443–457. doi: 10.1016/j.hoc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg P.L. The multifaceted nature of myelodysplastic syndromes: clinical, molecular, and biological prognostic features. J Natl Compr Canc Netw. 2013;11:877–884. doi: 10.6004/jnccn.2013.0105. [DOI] [PubMed] [Google Scholar]
- 10.Corey S.J., Minden M.D., Barber D.L., Kantarjian H., Wang J.C., Schimmer A.D. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 11.Slayton W.B., Li X.M., Butler J., Guthrie S.M., Jorgensen M.L., Wingard J.R., Scott E.W. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–2955. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- 12.Yoder M.C., Mead L.E., Prater D., Krier T.R., Mroueh K.N., Li F., Krasich R., Temm C.J., Prchal J.T., Ingram D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg P., Cox C., LeBeau M.M., Fenaux P., Morel P., Sanz G., Sanz M., Vallespi T., Hamblin T., Oscier D. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 14.Ingram D.A., Mead L.E., Tanaka H., Meade V., Fenoglio A., Mortell K., Pollok K., Ferkowicz M.J., Gilley D., Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 15.Teofili L., Martini M., Iachininoto M.G., Capodimonti S., Nuzzolo E.R., Torti L., Cenci T., Larocca L.M., Leone G. Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood. 2011;117:2700–2707. doi: 10.1182/blood-2010-07-297598. [DOI] [PubMed] [Google Scholar]
- 16.Della Porta M.G., Malcovati L., Rigolin G.M., Rosti V., Bonetti E., Travaglino E., Boveri E., Gallì A., Boggi S., Ciccone M. Immunophenotypic, cytogenetic and functional characterization of circulating endothelial cells in myelodysplastic syndromes. Leukemia. 2008;22:530–537. doi: 10.1038/sj.leu.2405069. [DOI] [PubMed] [Google Scholar]
- 17.Issa J.P. The myelodysplastic syndrome as a prototypical epigenetic disease. Blood. 2013;121:3811–3817. doi: 10.1182/blood-2013-02-451757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer R.A., Wobus M., List C., Wehner R., Schönefeldt C., Brocard B., Mohr B., Rauner M., Schmitz M., Stiehler M. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013;98:1677–1685. doi: 10.3324/haematol.2013.083972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geyh S., Oz S., Cadeddu R.P., Fröbel J., Brückner B., Kündgen A., Fenk R., Bruns I., Zilkens C., Hermsen D. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013;27:1841–1851. doi: 10.1038/leu.2013.193. [DOI] [PubMed] [Google Scholar]
- 20.Battinelli E., Willoughby S.R., Foxall T., Valeri C.R., Loscalzo J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc Natl Acad Sci U S A. 2001;98:14458–14463. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.North T.E., Goessling W., Peeters M., Li P., Ceol C., Lord A.M., Weber G.J., Harris J., Cutting C.C., Huang P. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasnov P., Michurina T., Packer M.A., Stasiv Y., Nakaya N., Moore K.A., Drazan K.E., Enikolopov G. Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Mol Med. 2008;14:141–149. doi: 10.2119/2007-00011.Krasnov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida C., Kusubata K., Tashiro Y., Gritli I., Sato A., Ohki-Koizumi M., Morita Y., Nagano M., Sakamoto T., Koshikawa N. MT1-MMP plays a critical role in hematopoiesis by regulating HIF-mediated chemokine/cytokine gene transcription within niche cells. Blood. 2012;119:5405–5416. doi: 10.1182/blood-2011-11-390849. [DOI] [PubMed] [Google Scholar]
- 24.Ichii M., Frank M.B., Iozzo R.V., Kincade P.W. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119:1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamane T., Kunisada T., Tsukamoto H., Yamazaki H., Niwa H., Takada S., Hayashi S.I. Wnt signaling regulates hemopoiesis through stromal cells. J Immunol. 2001;167:765–772. doi: 10.4049/jimmunol.167.2.765. [DOI] [PubMed] [Google Scholar]
- 26.Staal F.J., Luis T.C. Wnt signaling in hematopoiesis: crucial factors for self-renewal, proliferation, and cell fate decisions. J Cell Biochem. 2010;109:844–849. doi: 10.1002/jcb.22467. [DOI] [PubMed] [Google Scholar]
- 27.Mosimann C., Hausmann G., Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 28.Wu D.C., Paulson R.F. Hypoxia regulates BMP4 expression in the murine spleen during the recovery from acute anemia. PLoS One. 2010;5:e11303. doi: 10.1371/journal.pone.0011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maguer-Satta V., Forissier S., Bartholin L., Martel S., Jeanpierre S., Bachelard E., Rimokh R. A novel role for fibronectin type I domain in the regulation of human hematopoietic cell adhesiveness through binding to follistatin domains of FLRG and follistatin. Exp Cell Res. 2006;312:434–442. doi: 10.1016/j.yexcr.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Fan H.B., Liu Y.J., Wang L., Du T.T., Dong M., Gao L., Meng Z.Z., Jin Y., Chen Y., Deng M. miR-142-3p acts as an essential modulator of neutrophil development in zebrafish. Blood. 2014;124:1320–1330. doi: 10.1182/blood-2013-12-545012. [DOI] [PubMed] [Google Scholar]
- 31.Faraoni I., Laterza S., Ardiri D., Ciardi C., Fazi F., Lo-Coco F. MiR-424 and miR-155 deregulated expression in cytogenetically normal acute myeloid leukaemia: correlation with NPM1 and FLT3 mutation status. J Hematol Oncol. 2012;5:26. doi: 10.1186/1756-8722-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.How C., Hui A.B., Alajez N.M., Shi W., Boutros P.C., Clarke B.A., Yan R., Pintilie M., Fyles A., Hedley D.W. MicroRNA-196b regulates the homeobox B7-vascular endothelial growth factor axis in cervical cancer. PLoS One. 2013;8:e67846. doi: 10.1371/journal.pone.0067846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carraro G., Shrestha A., Rostkovius J., Contreras A., Chao C.M., El Agha E., Mackenzie B., Dilai S., Guidolin D., Taketo M.M. miR-142-3p balances proliferation and differentiation of mesenchymal cells during lung development. Development. 2014;141:1272–1281. doi: 10.1242/dev.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra S., Kincade P.W. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp Hematol. 2009;37:19–30. doi: 10.1016/j.exphem.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salter A.B., Meadows S.K., Muramoto G.G., Himburg H., Doan P., Daher P., Russell L., Chen B., Chao N.J., Chute J.P. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knospe W.H., Gregory S.A., Husseini S.G., Fried W., Trobaugh F.E., Jr. Origin and recovery of colony-forming units in locally curetted bone marrow of mice. Blood. 1972;39:331–340. [PubMed] [Google Scholar]
- 37.Hooper A.T., Butler J.M., Nolan D.J., Kranz A., Iida K., Kobayashi M., Kopp H.G., Shido K., Petit I., Yanger K. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi H., Butler J.M., O'Donnell R., Kobayashi M., Ding B.S., Bonner B., Chiu V.K., Nolan D.J., Shido K., Benjamin L. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding L., Saunders T.L., Enikolopov G., Morrison S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler I.G., Barbier V., Nowlan B., Jacobsen R.N., Forristal C.E., Patton J.T., Magnani J.L., Lévesque J.P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 41.Tennant G.B., Walsh V., Truran L.N., Edwards P., Mills K.I., Burnett A.K. Abnormalities of adherent layers grown from bone marrow of patients with myelodysplasia. Br J Haematol. 2000;111:853–862. [PubMed] [Google Scholar]
- 42.Tauro S., Hepburn M.D., Bowen D.T., Pippard M.J. Assessment of stromal function, and its potential contribution to deregulation of hematopoiesis in the myelodysplastic syndromes. Haematologica. 2001;86:1038–1045. [PubMed] [Google Scholar]
- 43.Kornblau S.M., McCue D., Singh N., Chen W., Estrov Z., Coombes K.R. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood. 2010;116:4251–4261. doi: 10.1182/blood-2010-01-262071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlaki K., Pontikoglou C.G., Demetriadou A., Batsali A.K., Damianaki A., Simantirakis E., Kontakis M., Galanopoulos A., Kotsianidis I., Kastrinaki M.C. Impaired proliferative potential of bone marrow mesenchymal stromal cells in patients with myelodysplastic syndromes is associated with abnormal WNT signaling pathway. Stem Cells Dev. 2014;23:1568–1581. doi: 10.1089/scd.2013.0283. [DOI] [PubMed] [Google Scholar]
- 45.Rosti V., Villani L., Riboni R., Poletto V., Bonetti E., Tozzi L., Bergamaschi G., Catarsi P., Dallera E., Novara F. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood. 2013;121:360–368. doi: 10.1182/blood-2012-01-404889. [DOI] [PubMed] [Google Scholar]
- 46.Sozer S., Fiel M.I., Schiano T., Xu M., Mascarenhas J., Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113:5246–5249. doi: 10.1182/blood-2008-11-191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oostvogels R., Petersen E.J., Chauffaille M.L., Abrahams A.C. Systemic vasculitis in myelodysplastic syndromes. Neth J Med. 2012;70:63–68. [PubMed] [Google Scholar]
- 48.Yang X., Brandenburg N.A., Freeman J., Freeman J., Salomon M.L., Zeldis J.B., Knight R.D., Bwire R. Venous thromboembolism in myelodysplastic syndrome patients receiving lenalidomide: results from postmarketing surveillance and data mining techniques. Clin Drug Investig. 2009;29:161–171. doi: 10.2165/00044011-200929030-00003. [DOI] [PubMed] [Google Scholar]
- 49.Xu J., Suzuki M., Niwa Y., Hiraga J., Nagasaka T., Ito M., Nakamura S., Tomita A., Abe A., Kiyoi H. Clinical significance of nuclear non-phosphorylated beta-catenin in acute myeloid leukaemia and myelodysplastic syndrome. Br J Haematol. 2008;140:394–401. doi: 10.1111/j.1365-2141.2007.06914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H., Fan R., Wang X.Q., Wu D.P., Lin G.W., Xu Y., Li W.Y. Methylation of Wnt antagonist genes: a useful prognostic marker for myelodysplastic syndrome. Ann Hematol. 2013;92:199–209. doi: 10.1007/s00277-012-1595-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers and Probes Used in Reverse Transcription–PCR Analysis of Normal and MDS ECFCs and of Normal Hematopoietic Cells
Table S2. Primers Used in Promoter Methylation Analysis of ECFCs


