Abstract
Pathogenic bacteria in the genus Xanthomonas cause diseases on over 350 plant species, including cannabis (Cannabis sativa L.). Because of regulatory limitations, the biology of the Xanthomonas-cannabis pathosystem remains largely unexplored. To gain insight into the evolution of Xanthomonas strains pathogenic to cannabis, we sequenced the genomes of two geographically distinct Xanthomonas strains, NCPPB 3753 and NCPPB 2877, which were previously isolated from symptomatic plant tissue in Japan and Romania. Comparative multilocus sequence analysis of housekeeping genes revealed that they belong to Group 2, which comprises most of the described species of Xanthomonas. Interestingly, both strains lack the Hrp Type III secretion system and do not contain any of the known Type III effectors. Yet their genomes notably encode two key Hrp pathogenicity regulators HrpG and HrpX, and hrpG and hrpX are in the same genetic organization as in the other Group 2 xanthomonads. Promoter prediction of HrpX-regulated genes suggests the induction of an aminopeptidase, a lipase and two polygalacturonases upon plant colonization, similar to other plant-pathogenic xanthomonads. Genome analysis of the distantly related Xanthomonas maliensis strain 97M, which was isolated from a rice leaf in Mali, similarly demonstrated the presence of HrpG, HrpX, and a HrpX-regulated polygalacturonase, and the absence of the Hrp Type III secretion system and known Type III effectors. Given the observation that some Xanthomonas strains across distinct taxa do not contain hrpG and hrpX, we speculate a stepwise evolution of pathogenicity, which involves (i) acquisition of key regulatory genes and cell wall-degrading enzymes, followed by (ii) acquisition of the Hrp Type III secretion system, which is ultimately accompanied by (iii) successive acquisition of Type III effectors.
Keywords: comparative genomics, Xanthomonas, hemp, cell-wall degrading enzymes, type II secretion system, type III secretion system, hrp genes, PIP box
Introduction
Plant pathogenic bacteria in the genus Xanthomonas collectively cause major losses worldwide on over 350 plant species, including crops such as banana, tomato, pepper, sugar cane, and many cereals. Over 20 Xanthomonas species are divided into two main phylogenetic groups based on 16S rDNA and gyrB sequence analysis (Hauben et al., 1997; Parkinson et al., 2007) and subdivided into pathovars loosely corresponding to host specificity. Group 1, also known as the early branching group, comprises highly diverse Xanthomonas species including important sugarcane and cereal pathogens (e.g., Xanthomonas sacchari, Xanthomonas albilineans and Xanthomonas translucens, Hauben et al., 1997; Parkinson et al., 2007). Group 2, the largest and best-described group, includes species such as Xanthomonas oryzae, Xanthomonas citri, Xanthomonas vasicola, Xanthomonas euvesicatoria, Xanthomonas axonopodis and Xanthomonas campestris (Hauben et al., 1997; Parkinson et al., 2007). This diverse genus of bacteria infects and associates with many plant hosts, but individual strains typically possess very restricted host ranges limited to a single genus.
Xanthomonas spp. employ a suite of virulence factors to colonize plant tissue, including adhesins, cell wall-degrading enzymes, extracellular polysaccharide and protein secretion systems (Büttner and Bonas, 2010). The Hrp (hypersensitive response and pathogenicity) Type III secretion system (T3SS) is a major virulence trait found in most pathogenic Xanthomonas spp. and serves as a molecular syringe to deliver effector proteins into host cells to suppress defenses and modulate plant physiology to promote pathogen growth (White et al., 2009). Plants also evolved resistance proteins that recognize pathogen avirulence effectors and inhibit infection often via a hypersensitivity response (HR), a form of programmed cell death (Bent and Mackey, 2007). A majority of sequenced pathogenic Xanthomonas strains have limited host ranges likely due to the plant recognition of Type III (T3)-secreted avirulence effectors (White et al., 2009). In Xanthomonas spp., HrpX, an AraC-type regulator, is the transcriptional activator of the genes encoding the T3SS and many of its associated effectors (Koebnik et al., 2006; Tang et al., 2006). HrpG, an OmpR-family and major pathogenicity regulator, positively regulates expression of hrpX (Tang et al., 2006). Mutant strains lacking either hrpX and hrpG are unable to activate expression of the T3SS and thus are non pathogenic (Wengelnik et al., 1996; Tang et al., 2006; Mole et al., 2007). The importance of the T3SS and many T3-secreted effectors during infection is heavily studied, but the evolutionary history of the acquisition of genes encoding the T3SS, associated T3-secreted effectors and regulators, HrpX and HrpG, remains unclear.
Hemp or cannabis (Cannabis sativa L.) is a major, global cash crop with many applications such as seed for human consumption, oil, fiber for clothing or ropes, pulp for paper, plastic and composite material (www.hemp.com). Since 2010 worldwide hemp production has increased, and recent surges of hemp production in the United States, China, Australia, Canada, and many other countries have made hemp a multi-million dollar industry (www.hemp.com, www.faostat.fao.org). A draft genome is now available for C. sativa cv. Purple Kush (van Bakel et al., 2011), potentially providing a base for molecular and evolutionary understanding of this plant species. Hemp plant production is limited by bacteria, fungi, nematodes, and viruses (McPartland et al., 2000), but because of regulatory constraints, little is known about hemp diseases such as bacterial leaf spot of cannabis caused by Xanthomonas species.
Symptoms associated with Xanthomonas bacterial leaf spot include water-soaking lesions followed by necrosis accompanied by a yellow halo (Severin, 1978; Netsu et al., 2014). The host range of these Xanthomonas strains appears to be quite large unlike most xanthomonads (Severin, 1978; Netsu et al., 2014). Under laboratory conditions, these bacteria caused symptoms on a wide range of plants including cannabis, tomato, mulberry, geranium and Ficus erecta (Severin, 1978; Netsu et al., 2014). These strains further trigger an HR on tobacco, but do not elicit any response after inoculation on common bean (Severin, 1978; Netsu et al., 2014). The factors that contribute to pathogenicity and host range of cannabis-infecting Xanthomonas are unknown.
To gain insight into the evolution and pathogenicity of bacterial pathogens of cannabis, we sequenced two geographically distinct Xanthomonas strains, NCPPB 3753 and 2877, which were previously isolated from symptomatic hemp leaf tissue from Japan and Romania, respectively (Severin, 1978; Netsu et al., 2014). We tested their ability to infect barley, a previously unreported, compatible monocot host. We determined with comparative whole genome analysis based on average nucleotide identity (ANI) and multilocus sequence analysis (MLSA) the relationship of these cannabis strains to each other and other xanthomonads. We provide evidence that NCPPB 3753 and NCPPB 2877 form a unique species in the genus Xanthomonas herein called Xanthomonas cannabis. We further describe likely virulence traits encoded by their genomes. Most notably these strains lack a Hrp T3SS but possess the major hrp virulence regulators HrpX and HrpG. Based on our comparative genomic analysis in X. cannabis, we provide a putative model for acquisition of the T3SS, T3-secreted effectors and the hrp regulators in Xanthomonas spp.
Results/methods/discussion
Phenotypic evaluation
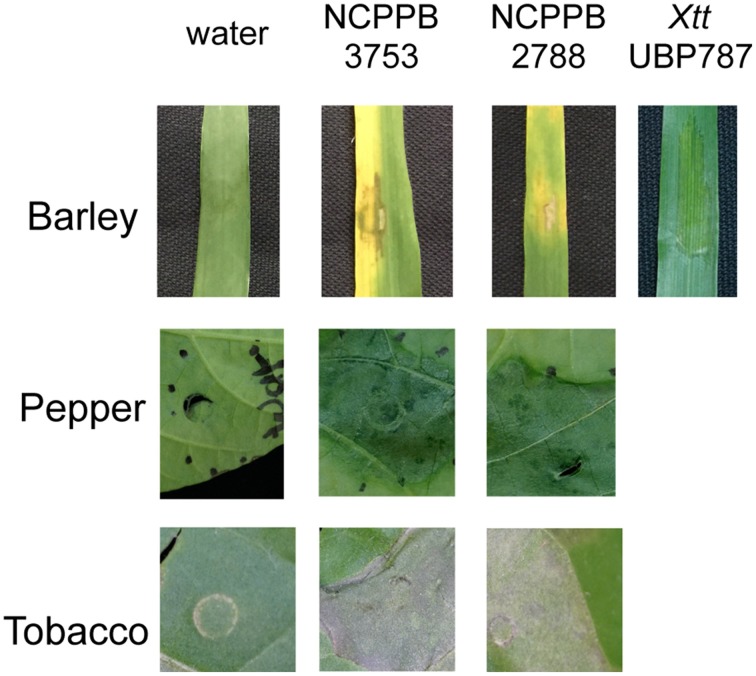
Two representative strains of X. cannabis (also known as Xanthomonas campestris pv. cannabis), isolated from symptomatic hemp leaves (C. sativa L.), were chosen for genome sequencing. Type strain NCPPB 2877 was isolated by I. Sandru at the Lovrin station in the Timiş judeţ (Romania) in 1974 (Severin, 1978), and strain NCPPB 3753 was isolated by Y. Takiwawa in the Kanuma region of Tochigi Prefecture (Japan) in 1982 (isolate SUPP546; Netsu et al., 2014). X. cannabis strains were previously reported to cause disease on many dicot host plants. To determine if X. cannabis could infect a monocot host, we inoculated barley (Hordeum vulgare L. cv. Morex) leaves with the cannabis strains by infiltration. Overnight cultures grown using PSA (Tsuchiya et al., 1982) or NB medium (Sigma-Aldrich, USA) were pelleted and resuspended in water. Plant leaves were infiltrated by a needleless syringe with a water-bacterial suspension or water as a control. Leaves developed necrosis around the zone of infiltration followed by leaf yellowing (Figure 1). These symptoms closely resembled the leaf spot symptoms on C. sativa observed by previous characterizations (Severin, 1978; Netsu et al., 2014). Similar symptoms were observed with lower inoculum (OD600 = 0.05 and 0.1). Tomato is a compatible host for X. cannabis (Severin, 1978), and therefore we decided to test X. cannabis virulence of pepper, another solanaceous plant. Pepper leaves were infiltrated with strains NCPPB 2877 and NCPPB 3753 as with barley. Pepper plants displayed water-soaked lesions 48 h post inoculation (Figure 1). Both cannabis strains elicited an HR when inoculated on tobacco (Figure 1), but the nature of this HR remains to be determined.
Figure 1.
Phenotypic analysis of X. cannabis on different plants. Symptom development and HR were evaluated on barley, pepper, and tobacco. Leaves were inoculated by needleless infiltration with water-bacterial suspensions of X. cannabis NCPPB 3753, NCPPB 2877 (OD600 = 0.5) or water as a control. Images were taken 48 h post inoculation for barley and tobacco, or 4 days post inoculation for pepper. The barley pathogen, X. translucens pv. translucens strain UPB787, served as a positive control for symptoms on barley. Plants were grown in a growth chamber at 22°C, 50% humidity and 16 h of light.
Genome sequencing and annotation
The genomes of strains NCPPB 2877 and NCPPB 3753 were sequenced using the Illumina Hi-Seq2500 platform (Fasteris SA, Switzerland). The shotgun sequencing yielded 2,921,175 100-bp paired-end reads (730 Mb) for strain NCPPB 2877 and 2,464,521 paired-end reads (616 Mb) for strain NCPPB 3753, with insert sizes ranging from of 250 bp to 1.5 kb. Draft genome sequences were assembled using the Edena algorithm v3.131028 (Hernandez et al., 2014), yielding 257 contigs ≥200 bp (N50 = 38,306 bp) with 69 × coverage for strain NCPPB 2877 and 260 contigs (N50 = 35,229 bp) with 73 × coverage for strain NCPPB 3753. For comparison, draft genome sequences were also assembled using the Velvet algorithm v1.1.04 (Zerbino and Birney, 2008), yielding 564 contigs ≥200 bp (N50 = 15,608 bp) for strain NCPPB 2877 and 469 contigs (N50 = 20,963 bp) for strain NCPPB 3753. Because of their better quality, Edena-derived contigs were annotated with GeneMarkS + release 2.9 (revision 452131) (Borodovsky and Lomsadze, 2014), as implemented in the NCBI Prokaryotic Genome Annotation Pipeline (http://www.ncbi.nlm.nih.gov/genome/annotation_prok/), which predicted a total of 4095 genes within 4,756,730 bp for strain NCPPB 2877 and 4160 genes within 4,837,471 bp for strain NCPPB 3753. These whole genome shotgun projects have been deposited at DDBJ/EMBL/GenBank under the accession no. JSZE00000000 (NCPPB 2877) and JSZF00000000 (NCPPB 3753). The versions described in this paper are the first versions, JSZE01000000 and JSZF01000000.
Comparison of the two genome sequences
ANI provides a robust method to determine bacterial species definition based on whole genome sequence comparison and is considered the new standard for species definition (Konstantinidis and Tiedje, 2005; Figueras et al., 2014). To determine if NCPPB 2877 and NCPPB 3753 are the same species, the ANI was calculated for both genome sequences using JSpecies (Richter and Rosselló-Móra, 2009). BLAST-based comparison revealed 99.2% ANI for the 92.5% sequences that could be aligned, and MUMmer-based comparison revealed 99.1% ANI for the 96.9% sequences that could be aligned, thus confirming that both strains belong to the same species.
Using our web-based pipeline for prediction of satellites (http://www.biopred.net/VNTR/), we then evaluated whether or not both strains belong to a clonal complex. For satellite prediction, the following parameters were chosen (Zhao et al., 2012): algorithm, TRF; region length, 30–1000 bp; unit length, 5–12 bp; and at least 6 tandem repeats with a similarity of at least 80% among the repeats. In total, 45 microsatellites were predicted, 35 of which were found to be present in both genome sequences. For 34 of them, repeat numbers could be derived; while one locus was not informative because it was located at the end of two contigs and thus not completely assembled in NCPPB 3753. To provide further evidence that the calculated repeat numbers were meaningful, the corresponding loci were also analyzed in the Velvet-based genome assemblies. Strikingly, there was not a single discrepancy between the Edena- and Velvet-based data, except for the fact that some satellite loci were not completely assembled by Velvet while they were complete in the Edena assembly. For the complete loci, 28 loci (82%) were different between the two strains with respect to repeat numbers. For the six loci with identical repeat numbers DNA sequence analysis revealed that five of them were identical due to homoplasy, i.e., these loci evolved by convergent evolution to the same number of repeats. Thus, both strains differ by almost all (97%) of their completely assembled microsatellite loci, a finding that indicates that both strains do not belong to a clonal complex.
Taxonomic position of the two cannabis pathogens
Comparison of 16S rDNA sequences is a method of choice to elucidate the taxonomic positions of bacterial strains, and was previously used to analyze and delineate 20 species of Xanthomonas (Hauben et al., 1997). It was found that the genus Xanthomonas exhibited a relatively high level of 16S rDNA sequence identity, with on average 14 single-nucleotide polymorphisms (SNPs) between two different Xanthomonas species (Hauben et al., 1997). The 16S rDNA sequences of both cannabis pathogens were found to be identical. When we compared the 16S rDNA sequence of the cannabis pathogens with those of the 20 Xanthomonas type strains, the cannabis pathogen grouped with Group 2 strains, which contains the majority of characterized Xanthomonas species. Interestingly, GenBank comparison revealed that another recently sequenced strain that was isolated from symptomatic bean plants in Rwanda, Nyagatare, contains the same 16S rDNA sequence (Aritua et al., 2015).
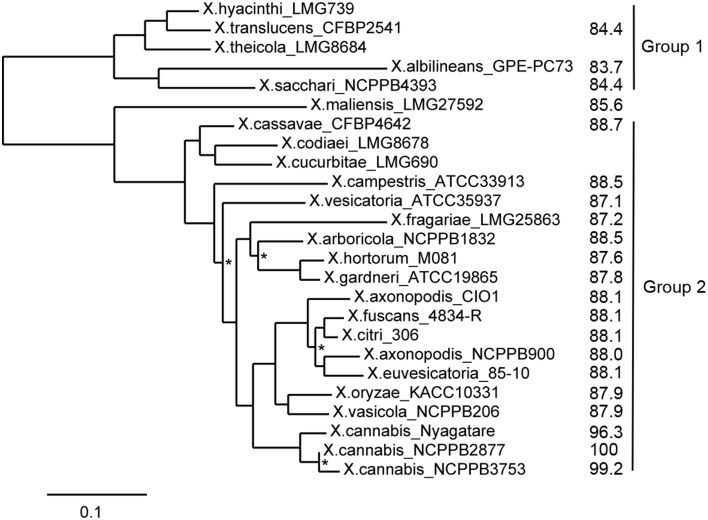
Previously X. cannabis strains were also called X. campestris pv. cannabis based on the similarity of the 16S rDNA sequence to X. campestris, but it has been suggested that the name should be changed to X. cannabis (Netsu et al., 2014). Since the resolution of the 16S rDNA sequence is very low within Group 2 strains (Hauben et al., 1997) and often only distinguishes a species by one or two SNPs, we performed whole-genome comparisons including one representative strain per species for which genome sequences were available (Figure 2). The pairwise ANI of the two cannabis strains against any of the representative strains was below 90%, regardless of which algorithm (BLAST or MUMmer) was used, indicating that these two strains belong to an unique and distinct Xanthomonas species (Figure 2). We suggest that X. cannabis is the appropriate name for this bacterial species based on our ANI analysis and as previously suggested by Netsu et al. (2014).
Figure 2.
ANI and MLSA-based phylogenetic tree of xanthomonads. Phylogenetic analysis was performed on the Phylogeny.fr platform (Dereeper et al., 2008). Sequences were aligned with MUSCLE (v3.7) configured for highest accuracy (MUSCLE with default settings). After alignment, ambiguous regions (i.e., containing gaps and/or poorly aligned) were removed with Gblocks (v0.91b) using default parameters. The phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v3.0). The HKY85 substitution model was selected assuming an estimated proportion of invariant sites and 4 gamma-distributed rate categories to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data. Reliability for internal branch was assessed using the aLRT test (SH-Like). Graphical representation and edition of the phylogenetic tree were performed with TreeDyn (v198.3). All nodes were supported by bootstrap values above 0.9, except for those marked with an asterisk. Calculated ANI values in comparison to NCPPB 2877 and the two major phylogenetic groups are indicated on the right side.
Guided by the observation that their 16S rDNA sequences were identical to that of the Nyagatare strain, we compared the genomes of X. cannabis NCPPB 3753 and NCPPB 2877 and X. sp. Nyagatare. JSpecies calculations revealed that the two cannabis strains were 96.3–96.4% identical to the Nyagatare strain, when calculated over the 88.6–91.0% of the genome sequence that could be aligned by the more robust MUMmer algorithm (Richter and Rosselló-Móra, 2009). These values are slightly above the ≈95–96% transition zone, above which strains can be considered to belong to the same taxonomically circumscribed prokaryotic species (Konstantinidis and Tiedje, 2005). Therefore the Nyagatare strain most likely belongs to the X. cannabis species. It would be interesting to perform functional studies to determine the similarities and differences between these closely related X. cannabis strains because the Nyagatare strain is a reported bean pathogen and X. cannabis strains elicit no response when inoculated on bean (Netsu et al., 2014).
Partial sequencing of the gyrB and other housekeeping genes for MLSA grouped all Xanthomonas species into four major MLSA subgroups (Parkinson et al., 2007; Young et al., 2008). We used MLSA of seven housekeeping genes (atpD, dnaK, efp, glnA, gyrB, lepA, and rpoD) and ANI calculations to understand the relationship of the cannabis and Nyagatare strains compared to other xanthomonads. The X. cannabis NCPPB 2877 and NCPPB 3753 and Nyagatare strains appear to form a distinct grouping according to MLSA (Figure 2). Based on the comparisons of ANI and of seven concatenated internal portions of the genes, we overall conclude that the cannabis and Nyagatare strains belong to a single species, X. cannabis because: (1) they are above the 95–96% ANI threshold for species definition and (2) they appear to belong to a new MLSA clade (Figure 2). This new clade corresponds to the novel species-level clade (slc) 1, as suggested by Parkinson and co-workers, which also contains the pathovars esculenti and zinniae (Parkinson et al., 2009). Following the pathovar designation, we suggest to name the two cannabis strains X. cannabis pv. cannabis, and the bean-pathogenic Nyagatare strain X. cannabis pv. phaseoli.
Comparison of pathogenicity-related gene clusters
Several gene clusters are considered to be important for pathogenicity of xanthomonads and their possible contribution to host- and tissue-specificity has been studied previously (Lu et al., 2008). We therefore analyzed whether and to which extent these gene clusters are conserved in the cannabis strains.
The rpf (regulation of pathogenicity factors) gene cluster plays a role in the intercellular signal-response system that links synthesis and perception of the diffusible signal factor (DSF) cis-11-methyl-2-dodecenoic acid to the synthesis of extracellular enzymes, extracellular polysaccharide, and biofilm dispersal (Dow, 2008). The genetic organization of the cannabis rpf gene cluster resembles that of X. euvesicatoria strain 85–10, yet it contains an additional gene, rpfI, downstream of gene XCV1913, which is not present in X. euvesicatoria strain 85–10. Moreover, the proteins RpfF, responsible for DSF synthesis, and the two-component regulatory system RpfC/RpfG, which is involved in DSF perception and signal transduction (Dow, 2008), are highly conserved in the cannabis pathogens.
The gum gene clusters encode proteins that are involved in the exopolysaccharide (EPS) biosynthesis (Becker et al., 1998). The core gum gene cluster, consisting of gumB to gumM, all transcribed in the same direction, is entirely conserved in the cannabis pathogen. As in other xanthomonads, the first gene, gumB, is located downstream of a proline-specific tRNA gene. The gumA gene which is located upstream of the tRNA gene and downstream pheS and pheT is most likely not a bona fide gum gene. The accessory gumN gene, which for instance is disrupted in X. euvesicatoria strain 85–10 and X. oryzae pv. oryzicola strain BLS256, appears to be intact in the cannabis pathogen. gumO and gumP, annotated as a 3-oxoacyl-(acyl carrier protein) synthase and a metal-dependent hydrolase, respectively, are located directly downstream of gumN. The contribution of these two genes to EPS biosynthesis is questionable since it is not present in several xanthomonads infecting monocotyledons, such as X. sacchari, X. translucens and X. oryzae, and it is also not present in the recently sequenced Xanthomonas maliensis strain 97M, which was isolated from rice leaves in Mali. On the other side, this unique conservation in bacteria colonizing eudicots could suggest a role in host specificity at the level of a division.
Lipopolysaccharide (LPS) is another bacterial polysaccharide, which is firmly attached to the outer membrane, and an aberrant structure of the LPS O-chain has been linked to virulence defects (Mhedbi-Hajri et al., 2011). The gene cluster responsible for LPS biosynthesis is always present between the highly conserved etfA and metB genes of Xanthomonas. A remarkably high degree of variation both in number and in identity of LPS genes has been found in different xanthomonads, even within a single species or pathovar (Patil et al., 2007). In the two cannabis pathogens, 14 genes are predicted to participate in the LPS biosynthesis. The gene content and genetic organization is largely identical to a few other Xanthomonas strains belonging to different species: Xanthomonas gardneri strain ATCC 19865, Xanthomonas vesicatoria strain ATCC 35937, and strains of X. campestris (B100, CN14, CN15, CN16, JX, Xca5, and 756C); except that the X. campestris strains have a short, additionally predicted gene, wxcH, just downstream of the etfA gene, which is not present (or frame-shifted) in the other species. Interestingly, the relatively closely related Nyagatare strain shares only the four etfA-proximal genes, wxcK to wxcN, and the two metB-proximal genes, wzm and wzt, with the cannabis strains, while between them three large genes without orthologs in other xanthomonads are predicted. As observed before on a smaller set of Xanthomonas genome sequences (Patil et al., 2007; Lu et al., 2008), the cannabis pathogens underscore the fact that the LPS gene cluster varies highly between and within species, suggesting multiple horizontal gene transfers and re-assortments. Yet, it is unclear what evolutionary pressure drives this variation—is it escape from recognition by plant immune receptors, and/or is it escape from recognition by bacteriophages?
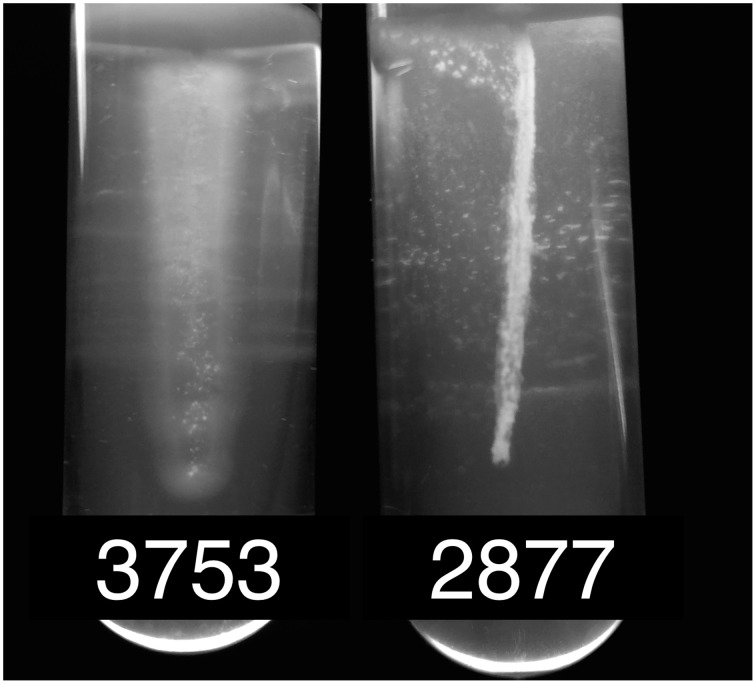
Motility is an important feature of bacteria that is governed by flagella-based swimming and/or pilus-based twitching or gliding (Rossez et al., 2015). The flagellum is a complex machinery, the biosynthesis of which depends on dozens of genes, collectively called fle, flg, flh, and fli genes, which are organized in large gene clusters. The cannabis strain NCPPB 2877 contains the full gene complement of flagellar biosynthesis. In contrast, strain NCPPB 3753 appears to have lost two large regions in its two genes clusters. One deletion encompasses the genes flgG to flgJ and the 5′ portion of flgK. Downstream of flgF, remnants of an IS element were present in the genome assembly. The second, even larger deletion goes from the 3′ portion of fliK until the end of flhA, totaling to more than 11 genes. Absence of flagella under standard culture conditions was confirmed for strain NCPPB 3753 by a swimming assay in semisolid medium (Figure 3). Briefly overnight cultures grown in liquid NB medium were fixed to an OD600 = 1.0 and stabbed into semi-solid NYGA medium (0.3% agar). Motility was defined as observed turbid growth outside the stab zone of inoculation in the semi-solid medium. Tubes inoculated with NCPPB 3753 were turbid after 4 days of growth (Figure 3), which demonstrates that this strain is motile. Growth of NCPPB 2877 was confined to the stab zone of inoculation (Figure 3), which supports our hypothesis that NCPPB 2877 is non-motile because it lacks a flagellum.
Figure 3.
Motility of X. cannabis strains. Bacterial motility was determined by stab inoculation in motility medium of either strain NCPPB 3753 or NCPPB 2877. Motility was evaluated qualitatively by turbid growth (motile) compared to localized, fixed growth (non-motile) around the zone of inoculation. Bacteria were grown in semi-solid NYGA agar medium (0.3%) as previously described (Sun et al., 2006).
Loss of flagella in xanthomonads is not without precedent (Darrasse et al., 2013). When Darrasse and co-workers tested 300 Xanthomonas strains representing different species and pathovars, five percent of the tested strains turned out to carry a deletion in the flagellar cluster and were non-motile. Co-isolation of flagellate and non-flagellate variants from an outbreak suggested that flagellar motility is not essential for fitness within the plant and that mixed populations could be a strategy to avoid detection by the plant defense system (Darrasse et al., 2013). Indeed, many plants have evolved receptor-like kinases (e.g., FLS2) that detect peptide epitopes of the FliC subunit of the flagellum (e.g., flg22 and flgII-28) (Sun et al., 2006), and it has been reported that otherwise isogenic flagellin-deficient Xanthomonas bacteria have a colonization advantage over flagellated bacteria in citrus leaves (Shi et al., 2015). Interestingly, when we analyzed the FliC flagellin sequence of the two cannabis strains, we detected a flg22 variant that deviates from the consensus sequence by as much as nine amino acid residues and is identical to the flg22 sequences from other xanthomonads, such as X. oryzae and X. vasicola (Supplemental Figure S1). In contrast, the related Nyagatare strain has a flg22 sequence that only deviates from the consensus by four residues and is similar to the elicitation-active flagellins that are recognized by FLS2 in Arabidopsis (Sun et al., 2006). These findings suggest that the cannabis strains use at least two out of several strategies to escape from recognition by the plant immune surveillance system, either by loss of the flagellum or by allelic variation of flagellin epitope(s) (Rossez et al., 2015).
The cannabis pathogen contains two different Type II protein secretion systems, the Xcs and the Xps system, as found in several other xanthomonads (Lu et al., 2008). The xcs gene cluster, consisting of 12 genes, xcsC to xcsN, is located downstream of a TonB-dependent receptor gene and upstream of a GntR-family regulator gene, which is also the case for X. euvesicatoria strain 85–10 and strains of X. campestris. The related Nyagatare strain contains another four genes between xcsN and the GntR-family regulator gene, a feature that is shared with the X. citri pv. citri strain 306 and the Xanthomonas arboricola strain 3004. Interestingly, the xcs gene cluster is also present in the X. maliensis strain 97M, but absent in the Group 1 strains X. albilineans GPE PC-73, X. sacchari NCPPB 4393, and X. translucens pv. cerealis CFBP 2541. The second Type II secretion system, encoded by the xps gene cluster, consists of 11 genes, xpsE to xpsN, followed by xpsD, and is found between the adhesion gene xadA and the pnuC gene. This position in the bacterial chromosome is largely conserved among Xanthomonas strains belonging to different Groups, such as the X. sacchari strain NCPPB 4393, the X. translucens pv. cerealis strain CFBP 2541, the X. maliensis strain 97M, and strains of Group 2.
Many Gram-negative plant-pathogenic bacteria have evolved another protein secretion system, the T3SS, which plays a pivotal role in the pathogen-host interaction (Büttner, 2012). The Hrp system serves as a molecular syringe that injects T3 effector proteins into the cytosol of the host's cells at the benefit of the bacterium. Yet, many injected effectors can also betray the pathogen to the host plant, triggering a strong defense response, which typically is accompanied by an effector-triggered HR (White et al., 2009). Notably the early-branching Group 1 sugarcane pathogen X. albilineans does not have an Hrp T3SS, a finding that could be attributed to the reduced genome size of this pathogen (Pieretti et al., 2009). Since then, new genome sequences became available, including other Group 1 strains belonging to the species X. sacchari (Studholme et al., 2011). Some of these strains do not appear to have undergone extensive genome reduction, yet, they do not encode a T3SS. When we analyzed the gene content of the cannabis strains we realized that they do not have any of the hrc, hrp, or hpa genes encoding the T3SS. We were also not able to detect any traces of a T3-effector gene when using the set of described genes as queries (http://www.xanthomonas.org/t3e.html). In contrast, the Nyagatare strain has a full gene complement for the Hrp T3SS and also possesses a couple of effector proteins (Aritua et al., 2015). This is an interesting finding for several reasons. First, how do the cannabis pathogens suppress plant defense, a function that became more and more linked to the activity of T3-effectors (e.g., Canonne et al., 2011; Schulze et al., 2012; Sinha et al., 2013; Li et al., 2015; Stork et al., 2015)? Second, which molecular entities are triggering the HR in Nicotiana tabacum if not type III effectors? Interestingly, a type II-secreted pectate lyase, XagP, from X. axonopodis pv. glycines was previously found to be associated with HR induction on tobacco and pepper, but not on cucumber, sesame and tomato, thus giving a prime example that cell wall-degrading enzymes could trigger an HR (Kaewnum et al., 2006). We therefore speculate that similar or other secreted enzymes that disturb the integrity of the plant cell wall could be responsible for HR triggered by the cannabis pathogen independent of a T3SS. Last but not least, these Group 2 genome sequences may serve as a valuable resource to predict new T3 effectors. As a relative of other xanthomonads it will provide a valuable negative training or filtering set for prediction algorithms.
Analysis of the HrpX regulon
Previous work has shown that the Hrp T3SS and many of its secreted effectors are controlled by a regulatory cascade, consisting of HpaR2/HpaS, HrpG, and HrpX (Büttner and Bonas, 2010; Li et al., 2014). However, HrpG and HrpX regulate pathogenesis beyond the T3SS alone (Guo et al., 2011). We therefore wondered if these components are present in Xanthomonas strains that do not possess the Hrp T3SS, and if so, what genes would be controlled by these components. BLAST searches revealed that all four regulatory genes are conserved in the cannabis pathogens (Table 1). hpaR2 and hpaS are also conserved in the X. maliensis strain and in strains from Group 1, such as X. albilineans, X. sacchari and X. translucens. Their location is always between the glmS and glmU gene, with some extra genes for an efflux pump between glmS and hpaR2. In contrast, hrpG and hrpX were not found in strains of X. albilineans or X. sacchari, but they are present in X. translucens. Interestingly, however, hrpG and hrpX from X. translucens cluster together with the rest of the T3SS hrp gene cluster while all other xanthomonads have hrpG and hrpX at a distinct genomic location between radA and hsp70 (Wichmann et al., 2013). We could not determine their genomic organization for X. maliensis due to the highly fragmented genome assembly.
Table 1.
Presence of type III secretion systems, type III effectors, hrp/hpa regulatory genes, and homologs of predicted HrpX regulon members of X. cannabis pv. cannabis in representative strains of Xanthomonas.
| Group | Species | Strain | GenBank accession no. | Hrp T3SS | T3Es | HrpG HrpX | HpaS HpaR2 | PehA | PehD | Amino-peptidase | LPL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | X. albilineans | GPE PC73 | FP565176 | no | no | no | YES | YES | YES | no | YES |
| 1 | X. sacchari | NCPPB 4393 | AGDB01000001 | no | no | no | YES | YES | no | YES | YES |
| 1 | X. translucens pv. cerealis | CFBP 2541 | JWHD01000001 | YES | YES | YES | noa | YES | YES | Ψ | YES |
| X. maliensis | 97M | AQPR01000001 | no | no | YES | YES | no | PIP | PIP[5] | PIP[1] | |
| 2 | X. campestris pv. campestris | ATCC 33913 | AE008922 | YES | YES | YES | YES | PIP | PIP[1] | PIP[1] | PIP |
| 2 | X. vesicatoria | ATCC 35937 | AEQV01000004 | YES | YES | YES | YES | PIP | PIP | PIP[1] | PIP |
| 2 | X. vasicola pv. vasculorum | NCPPB 206 | AKBM01000001 | YES | YES | YES | YES | PIP | no | PIP[1] | PIP |
| 2 | X. oryzae pv. oryzae | KACC110331 | AE013598 | YES | YES | YES | nob | Ψ | YES | no | PIP |
| 2 | X. fuscans subsp. fuscans | 4834-R | FO681494 | YES | YES | YES | YES | PIP | PIP | PIP[1] | PIP |
| 2 | X. citri pv. citri | 306 | AE008923 | YES | YES | YES | YES | PIP | PIP | PIP[1] | PIP |
| 2 | X. axonopodis pv. manihotis | CIO1 | AKCZ01000001 | YES | YES | YES | YES | PIP | no | PIP[1] | PIP-Ψ |
| 2 | X. axonopodis pv. vasculorum | NCPPB 900 | JPHD01000001 | YES | YES | YES | noc | PIP | PIP | PIP[1] | PIP |
| 2 | X. euvesicatoria | 85-10 | AM039952 | YES | YES | YES | YES | PIP | PIP | PIP[1] | PIP |
| 2 | X. cassavae | CFBP 4642 | ATMC01000001 | YES | YES | YES | YES | PIP | PIP | PIP[2] | PIP[3] |
| 2 | X. arboricola pv. celebensis | NCPPB 1832 | JPHC01000001 | YES | YES | YES | YES | PIP | no | PIP[1] | PIP[1] |
| 2 | X. hortorum pv. carotae | M081 | AEEU01000001 | YES | YES | YES | YES | PIP | PIP | PIP[1] | PIP |
| 2 | X. gardneri | ATCC 19865 | AEQX01000001 | YES | YES | YES | YES | PIP | PIP | PIP[1] | PIP |
| 2 | X. fragariae | LMG 25863 | AJRZ01000001 | YES | YES | YES | YES | PIP | PIP | Ψ | Ψ |
| 2 | X. cannabis pv. phaseoli | Nyagatare | JRQI01000001 | YES | YES | YES | YES | PIP | PIP | PIP | PIP |
| 2 | X. cannabis pv. cannabis | NCPPB 2877 | JSZE01000001 | no | no | YES | YES | PIP | PIP | PIP | PIP |
| 2 | X. cannabis pv. cannabis | NCPPB 3753 | JSZF01000001 | no | no | YES | YES | PIP | PIP | PIP | PIP |
“YES” indicates presence of a homolog, “no” indicates absence of the protein(s).
“Ψ” indicates pseudogenes, i.e., the gene is split into two or more fragments.
“PIP” indices the presence of the gene along with a canonical PIP box and a properly spaced −10 promoter motif. Numbers in square brackets indicate the number of single-nucleotide variants with respect to the canonical PIP box and −10 promoter motif.
hpaR2 is eroded while an N-terminally truncated form of hpaS is present in X. translucens pv. cerealis strain CFBP 2541. Remnants are found in two other X. translucens strains (ART-Xtg27 and DSM 18974). Interestingly, the hpaR2/hpaS locus is intact in X. translucens strain DAR61454. This is an example of ongoing gene erosion in one species of Xanthomonas.
The hpaR2/hpaS locus got destroyed by an IS element in X. oryzae pv. oryzae strain KACC 10331. Yet, hpaR2/hpaS is present in X. oryzae pv. oryzicola strain BLS256. Remnants are found in the African X. oryzae pv. oryzae strain NAI8 and in X. oryzae strains from the United States. This finding illustrates ongoing gene erosion in another species of Xanthomonas.
hpaR2 is absent while an N-terminally truncated form of hpaS is present in X. axonopodis pv. vasculorum strain NCPPB 900.
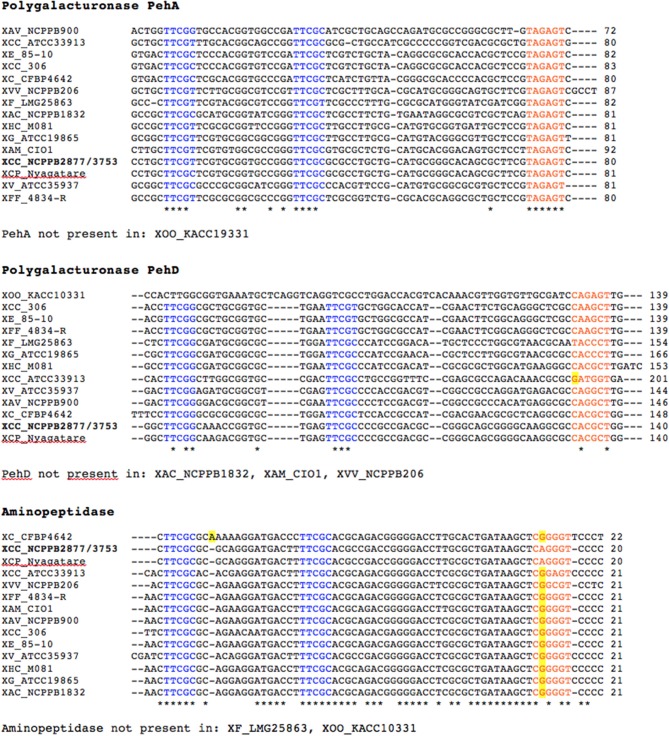
Within the Hpa-Hrp regulatory cascade, HrpX is the most downstream component that directly induces the synthesis of pathogenicity factors, such as the Hrp T3SS, T3 effectors and cell wall-degrading enzymes, by binding to a conserved cis element, called PIP (plant-induced promoter) box, within the promoter regions of the corresponding genes (Koebnik et al., 2006). For gene activation, both the PIP box as well as a properly spaced −10 promoter motif are required (Furutani et al., 2006). We therefore analyzed the genome sequences of the cannabis pathogens for the presence of the promoter motif TTCGB-N15-TTCGB-N30−32-TYNNNT (B represents C, G, or T; Y represents C or T) (Koebnik et al., 2006). Using this conservative query, four genes were strongly predicted to belong to the HrpX regulon, two polygalacturonases (PehA, synonymous with PghAxc, and PehD, synonymous with PghBxc and PglA; Hsiao et al., 2008; Wang et al., 2008), one putative aminopeptidase and one putative lysophospholipase (LPL). Indeed, the two polygalacturonases have been previously shown to be regulated by HrpX in X. campestris (Wang et al., 2008). For X. citri, microarray transcriptome studies have shown previously that three genes with a canonical PIP box and −10 promoter motif (PehA, PehD, LPL; Table 1) are positively regulated by HrpX (Guo et al., 2011). Finally, our own RNAseq data show that the LPL is under control of HrpX in an African X. oryzae strain (unpublished data). We highly suspected that the four predicted genes with a canonical PIP box and properly spaced −10 promoter motif were indeed under control of HrpX in the cannabis strains.
Genome mining revealed that these four genes are present in most xanthomonads from Groups 1 and 2 (Table 1). Yet, in some lineages, one or the other gene apparently got lost. For instance, X. sacchari, X. maliensis, X. arboricola, X. axonopodis pv. manihotis, X. oryzae, and X. vasicola lack one or the other polygalacturonase, and the aminopeptidase is absent from X. albilineans and X. oryzae. In a few cases, these genes appear to have suffered from pseudogenization (Table 1) but this needs confirmation by targeted DNA sequencing due to the risk of sequence errors in some of the draft genome sequences.
Since these four genes appear to be under control of HrpX in the cannabis pathogens, we looked for evidence that the same regulation occurs in the other xanthomonads. We therefore compared the upstream regions of these four genes in a representative set of Xanthomonas strains. Strikingly, we found PIP boxes and properly spaced −10 promoter motifs for most of the genes in most of the Xanthomonas strains (Figure 4, Supplemental Figure S2), except for the Group 1 strains X. albilineans, X. sacchari, and X. translucens. Multiple sequence alignments of the promoter regions of the three genes show that the PIP boxes are conserved in sequence, context and position (PIP boxes ~140–150 bp before start codon of pehA, ~90 bp before start codon of the aminopeptidase gene, and ~210–250 bp before start codon of pehD) indicate that the PIP boxes of the polygalacturonase and aminopeptidase genes evolved early after separation of Group 2 from Group 1. In contrast, the PIP boxes of the lysophospholipase gene do not align with each other and reveal four subgroupings, which are compatible with a MLSA-based phylogeny of Group 2 strains (Supplemental Figure S2). This finding could suggest that the PIP box evolved several times independently at early times after separation of the four MLSA subgroups, or that the surrounding sequences evolved too extensively to allow the PIP boxes to be aligned using standard parameters.
Figure 4.
Promoter sequences of predicted HrpX regulon members and their homologs in strains of Xanthomonas. Promoter regions encompassing 350 bp upstream of the translational start codon of a representative set of Xanthomonas strains were aligned by MUSCLE. PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of each sequence block. Deviations from the PIP consensus sequence are highlighted in yellow. The following Xanthomonas strains were analyzed: XAC (X. arboricola pv. celebensis) NCPPB 1832, XAM (X. axonopodis pv. manihotis) CIO1, XAV (X. axonopodis pv. vasculorum) NCPPB 900, XCC (X. campestris pv. campestris) ATCC 33913, XCC (X. cannabis pv. cannabis) NCPPB 2877 and NCPPB 3753, XCP (X. cannabis pv. phaseoli) Nyagatare, XC (X. cassavae) CFBP 4642, XCC (X. citri pv. citri) 306, XE (X. euvesicatoria) 85-10, XF (X. fragariae) LMG 25863, XFF (X. fuscans subsp. fuscans) 4834-R, XG (X. gardneri) ATCC 19865, XHC (X. hortorum pv. carotae) M081, XOO (X. oryzae pv. oryzae) KACC 19331, XVV (X. vasicola pv. vasculorum) NCPPB 206, and XV (X. vesicatoria) ATCC 35937. *Denotes conserved nucleotide.
To test if HrpX and HrpG could activate expression of the putative targets, we quantified gene expression of pehA, one of the four genes with a PIP box, in various mutant backgrounds of X. cannabis NCPPB 3753. HrpG* from X. euvesicatoria 85–10 is a HrpG variant that mimics the active form of HrpG (Wengelnik et al., 1999). Mutant strains with hrpG* induce expression of hrpX and further genes targeted by HrpX, which includes primarily promoters with PIP boxes (Wengelnik et al., 1999). We therefore transformed X. cannabis cells with either pBBR1MCS-5 (empty vector), pBBR1MCS-5::hrpX or pBBR1MCS-5::hrpG*, as previously described (Koebnik et al., 2006). hrpG* from X. euvesicatoria was cloned into pBBR1MCS-5, as described for hrpX (Wengelnik et al., 1999; Koebnik et al., 2006). Positive transformants were confirmed by PCR (data not shown). Quantitative PCR (qPCR) was used to determine the relative expression of putative HrpX-target genes in X. cannabis NCPPB 3753 pBBR1MCS-5::hrpG* and NCPPB 3653 pBBR1MCS-5::hrpX compared to NCPPB 3753 pBBR1MCS-5 (empty vector) as a control. atpD was used as a normalization control. pehA, pehD, and the gene encoding the putative LPL were dramatically induced in NCPPB 3753 ectopically expressing hrpG* or hrpX, compared to the empty vector control (Figure 5). NCPPB 3753 pBBR1MCS-5::hrpG* specifically upregulated (fold-change [SE]) pehA (54.8 ± 17.3), pehD (425.8 ± 16.6) and LPL (377.1 ± 36.5) compared to the empty vector control. A similar trend was observed with expression of the three genes (26.2 ± 4.1, 49.4 ± 11.3, 50.7 ± 11.9, respectively) in NCPPB 3753 pBBR1MCS-5::hrpX compared to the empty vector control. We did not detect differential expression of the putative aminopeptidase in our hrp regulatory variant backgrounds under the conditions mentioned above (data not shown). We only tested one set of primers for this gene under one condition, and further experiments need to be performed to determine if this negative result suggests that this gene is not a target of HrpX. We overall conclude that the promoter of pehA, pehD, and LPL are bona fide targets of HrpX, validating our bioinformatic analysis. This supports the hypothesis that HrpG and HrpX regulate pathogenesis beyond T3SS alone (Tang et al., 2006). Further investigation of the role of HrpX and HrpX targets remains to be performed, but X. cannabis is a potentially interesting model to understand the role of HrpX beyond the T3SS.
Figure 5.
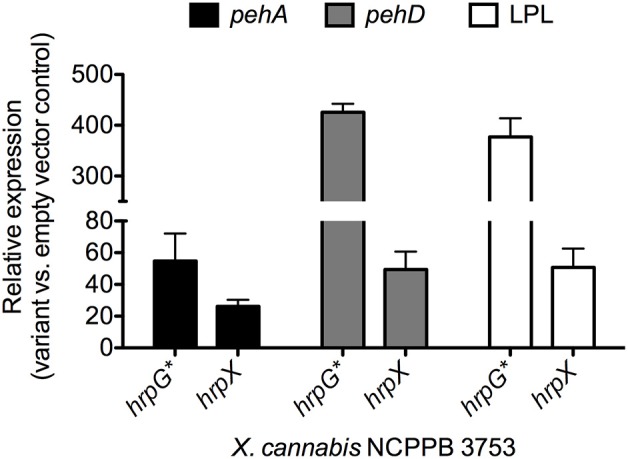
Gene expression analysis of genes with HrpX-inducible PIP boxes. Bacteria were grown overnight in liquid NB (Sigma Aldrich, USA) supplemented with gentamycin (20 μg l−1) and transferred to fresh 10 mL NB media with gentamycin for a final OD600 = 0.5. Bacteria were incubated for 3 h, shaking at 28°C. Transcriptional profiles and RNA was preserved with 5% phenol in ethanol as previously described (Jahn et al., 2008; Jacobs et al., 2012). Bacterial RNA was extracted with Trizol (Invitrogen, USA), cleaned up with Zymogen RNA concentrator (Zymo Research, USA) and treated with Turbo DNase (Invitrogen, USA) following manufacturer's protocols. RNA (1 μg per sample) was reverse transcribed with Superscript III (Invitrogen, USA) following the manufacturer's recommendation. qPCR with SYBR MESA BLUE Master Mix (Eurogentec, Belgium) was performed following the manufacturer's protocol on a Roche LightCycler 480 Real-Time PCR instrument (Roche Diagnostics Corporation, USA) with reaction parameters of 10-min polymerase activation at 95°C, then 40 cycles, with an individual cycle consisting of 15 s at 95°C and 1 min at 60°C. Relative gene expression analysis of pehA, pehD and the gene encoding the putative lysophospholipase (LPL) were calculated by the delta-delta-Ct method (Livak and Schmittgen, 2001) for NCPPB 3753 variants ectopically expressing hrpG* or hrpX compared to NCPPB 3753 pBBR1MCS-5. Expression of atpD was used as a normalization internal control gene. Primers are listed in Supplemental Table S1.
One of the polygalacturonase genes, pehA, is not only under control of HrpX but was found to be regulated by Clp and RpfF in X. campestris (Hsiao et al., 2008). Since Clp and RpfF are conserved over all clades it is tempting to speculate that this layer of regulation has evolved before separation of the clades. However, comparison of the pehA promoter regions in a representative set of Xanthomonas strains revealed that the predicted Clp-binding site is not conserved (Supplemental Figure S3). Notably, multiple sequence alignment instead revealed a conserved sequence motif the position of which is shifted by 8 bp with respect to the predicted Clp-binding site (Supplemental Figure S3). In fact, this conserved box is as similar to the canonical Clp box as is the previously predicted Clp-binding site (Dong and Ebright, 1992), and its position is compatible with the mapping of the Clp-binding site by lacZ reporter fusions (Hsiao et al., 2008). Probably, the Clp-binding site was slightly mispredicted at that time due to the absence of sufficient sequence data from diverse Xanthomonas strains, which help to uncover regulatory DNA elements.
Conclusions
Stimulated by two new genome sequences from cannabis-pathogenic xanthomonads, we explored the world of pathogenicity determinants in the genus Xanthomonas. A plethora of research data as well as our own analyses let us speculate about a stepwise evolution of pathogenicity in Xanthomonas. We developed a model (Figure 6) of evolution and acquisition of Xanthomonas pathogenicity factors based on a model proposed by Lu et al. (2008). Given the observation that some Xanthomonas strains across distinct taxa do not contain hrpG and hrpX, we speculate a stepwise evolution of pathogenicity (Figure 6), which involves (i) acquisition of key regulatory genes and cell wall-degrading enzymes, followed by (ii) acquisition of the Hrp type III secretion system, which is ultimately accompanied by (iii) successive acquisition of type III effectors. In parallel, as soon as hrpG and hrpX were acquired, a subset of genes, which can contribute to pathogenicity, evolved PIP boxes in their promoter regions thus ensuring their efficient expression during plant colonization.
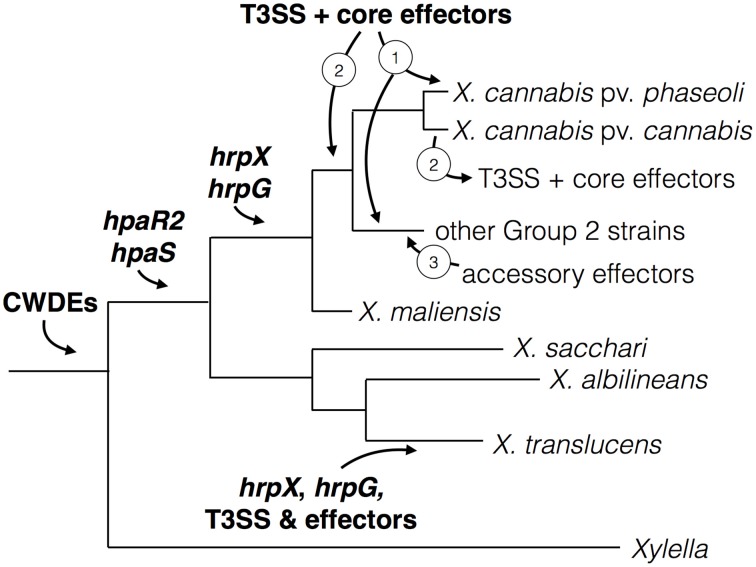
Figure 6.
Model for virulence gene and regulator acquisition in X. cannabis. The acquisition of various virulence traits was likely sequential in Xanthomonas spp., which represented by a schematic phylogenetic tree. Cell-wall modification by cell-wall degrading enzymes (CWDEs) is an ancient trait found in both Xylella and Xanthomonas, which suggests their acquisition predates the separation of these two pathogenic genera. Genes encoding the regulators HpaR2 and HpaS were subsequently acquired before the separation of the Xanthomonas Group 2 and early branching Group 1. Then the regulatory genes hrpX and hrpG were gained by Group 2 strains and further promoters (e.g., PIP boxes) evolved to adapt to these virulence regulators. This is further supported by the absence of PIP boxes in front of non-hrp genes in Group 1 strains (X. albilineans and X. sacchari). Because the organization of hrpX and hrpG and the T3SS is more similar to R. solanacearum, different than Group 2 xanthomonads and lacking in most Group 1 species (X. sacchari and X. albilineans), we suspect an independent acquisition of this system in X. translucens. hrpG and hrpX are present in a similar location in all sequenced Group 2 species. We hypothesize that the T3SS and core effectors were either (1) acquired independently by individual pathovars or (2) acquired an earlier point and lost in some pathovars. After the acquisiton of the T3SS, we posit that accessory effectors were acquired (3) by horizontal gene transfer to alter host range and/or promote susceptibility by suppressing plant immunity.
Basic pathogenicity factors, such as the Xps type II secretion system and its secreted cell wall-degrading enzymes, as well as the rpf gene cluster, were probably already present in the ancestor of Xanthomonas and Xylella. Perhaps, these components were already expressed in response to environmental conditions, as we can still observe for the genes that are controlled by RpfF and/or Clp. When the xanthomonads had separated into distinct genetic clades (Group 1 vs. Group 2), hrpG and hrpX were acquired, and perhaps evolved to be cross-regulated by the HpaR2/HpaS two-component regulatory system. It is conceivable that the X. translucens lineage acquired hrpX and hrpG together with the Hrp type III secretion system since the two regulatory genes are physically linked to the hrp gene cluster (Wichmann et al., 2013), reminiscent of the situation in Ralstonia solanacearum. Notably, the other early-branching xanthomonads, such as X. albilineans and X. sacchari, did not acquire hrpX nor hrpG. In contrast, all other xanthomonads have the oppositely transcribed hrpG and hrpX genes in the same synteny group between radA and hsp70, suggesting a unique acquisition event.
Interestingly, there are xanthomonads that possess hrpG and hrpX but not the Hrp Type III secretion system nor any type III effectors, such as the cannabis pathogen, the new X. maliensis clade (Triplett et al., 2015), and a recently sequenced strain of X. arboricola (Ignatov et al., 2015). This could be taken as evidence that the Hrp system was either (1) acquired later or (2) got lost in some lineages. We favor the hypothesis that the T3SS and core effectors were gained in the case of the Nyagatare strain and not lost from X. cannabis NCPPB 2788 and NCPPB 3753. There seems to be no indication of any remnant features of the T3SS or effectors in the two X. cannabis genomes. Moreover, the Nyagatare strain possesses the T3SS and only core effectors, which appear to be located on a pathogenicity island flanked by insertion/transposable elements. More systematic genome sequencing of underexamined genetic lineages will shed light on this evolutionary puzzle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work benefited from a grant from the National Science Foundation to JJ (DBI - 1306196), a grant from the Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture (093604) to CP, and a grant from the Agence Nationale de la Recherche (ANR-2010-BLAN-1723) to RK. We are grateful to Lionel Moulin and Lucie Poulin, IRD Montpellier, for advice on ANI analyses and for technical assistance, respectively.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00431/abstract
Flg22 epitope variants found in various strains of Xanthomonas. On top, the prototype elicitor-active flg22 peptide from Pseudomonas aeruginosa is shown (Felix et al., 1999). Below, homologous sequences from different species and pathovars of Xanthomonas are aligned. Residues that deviate from the prototype sequence are in red. The peptide that corresponds to eliciting flagellin variants of X. campestris pv. campestris is highlighted in green, while the peptide that corresponds to non-eliciting flagellin variants highlighted in yellow (Sun et al., 2006).
Comparison of promoter sequences of the Xanthomonas lysophospholipase gene. Promoter regions encompassing 350 bp upstream of the translational start codon of a representative set of Xanthomonas strains were aligned by MUSCLE. PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of the lower sequence block. Deviations from the PIP consensus sequence are highlighted in yellow. For the set of analyzed strains, compare with Figure 4.
Comparison of promoter sequences of the Xanthomonas pehA gene. Promoter regions encompassing 350 bp upstream of the translational start codon of a representative set of Xanthomonas strains were aligned by MUSCLE. Consensus CAP/CLP binding boxes, according to Dong and Ebright (1992), are indicated above the multiple sequence alignment, first aligned with the Clp-binding site as determined by Hsiao et al. (2008), for X. campestris pv. campestris (highlighted in yellow, six conserved residues are shown in green), and then aligned with the Clp-binding site as proposed by us (conserved residues are shown in red). The PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of the lower sequence block. For better comparison with Figure 4, PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of the lower sequence block. Deviations from the PIP consensus sequence are highlighted in yellow. For the set of analyzed strains, compare with Figure 4.
References
- Aritua V., Musoni A., Kato F., Abang M. M., Buruchara R., Sapp M., et al. (2015). The draft genome sequence of Xanthomonas species strain Nyagatare, isolated from diseased bean in Rwanda. FEMS Microbiol. Lett. 362. 10.1093/femsle/fnu055 [DOI] [PubMed] [Google Scholar]
- Becker A., Katzen F., Pühler A., Ielpi L. (1998). Xanthan gum biosynthesis and application: a biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 50, 145–152. [DOI] [PubMed] [Google Scholar]
- Bent A. F., Mackey D. (2007). Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 45, 399–436. 10.1146/annurev.phyto.45.062806.094427 [DOI] [PubMed] [Google Scholar]
- Borodovsky M., Lomsadze A. (2014). Gene identification in prokaryotic genomes, phages, metagenomes, and EST sequences with GeneMarkS suite. Curr. Protoc. Microbiol. 32, 7. 10.1002/9780471729259.mc01e07s32 [DOI] [PubMed] [Google Scholar]
- Büttner D. (2012). Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol. Mol. Biol. Rev. 76, 262–310. 10.1128/MMBR.05017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D., Bonas U. (2010). Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 34, 107–133. 10.1111/j.1574-6976.2009.00192.x [DOI] [PubMed] [Google Scholar]
- Canonne J., Marino D., Jauneau A., Pouzet C., Brière C., Roby D., et al. (2011). The Xanthomonas type III effector XopD targets the Arabidopsis transcription factor MYB30 to suppress plant defense. Plant Cell 23, 3498–3511. 10.1105/tpc.111.088815 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Darrasse A., Carrère S., Barbe V., Boureau T., Arrieta-Ortiz M. L., Bonneau S., et al. (2013). Genome sequence of Xanthomonas fuscans subsp. fuscans strain 4834-R reveals that flagellar motility is not a general feature of xanthomonads. BMC Genomics 14:761 10.1186/1471-2164-14-761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., et al. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469. 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Ebright R. H. (1992). DNA binding specificity and sequence of Xanthomonas campestris catabolite gene activator protein-like protein. J. Bacteriol. 174, 5457–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow M. (2008). Diversification of the function of cell-to-cell signaling in regulation of virulence within plant pathogenic xanthomonads. Sci. Signal. 1:pe23. 10.1126/stke.121pe23 [DOI] [PubMed] [Google Scholar]
- Felix G., Duran J. D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276. 10.1046/j.1365-313X.1999.00265.x [DOI] [PubMed] [Google Scholar]
- Figueras M. J., Beaz-Hidalgo R., Hossain M. J., Liles M. R. (2014). Taxonomic affiliation of new genomes should be verified using average nucleotide identity and multilocus phylogenetic analysis. Genome Announc. 2, e00927–e00914. 10.1128/genomeA.00927-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani A., Nakayama T., Ochiai H., Kaku H., Kubo Y., Tsuge S. (2006). Identification of novel HrpXo regulons preceded by two cis-acting elements, a plant-inducible promoter box and a −10 box-like sequence, from the genome database of Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 259, 133–141. 10.1111/j.1574-6968.2006.00265.x [DOI] [PubMed] [Google Scholar]
- Guo Y., Figueiredo F., Jones J., Wang N. (2011). HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. 24, 649–661. 10.1094/MPMI-09-10-0209 [DOI] [PubMed] [Google Scholar]
- Hauben L., Vauterin L., Swings J., Moore E. R. (1997). Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. Int. J. Syst. Bacteriol. 47, 328–335. 10.1099/00207713-47-2-328 [DOI] [PubMed] [Google Scholar]
- Hernandez D., Tewhey R., Veyrieras J. B., Farinelli L., Østerås M., François P., et al. (2014). De novo finished 2.8 Mbp Staphylococcus aureus genome assembly from 100 bp short and long range paired-end reads. Bioinformatics 30, 40–49. 10.1093/bioinformatics/btt590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y. M., Zheng M. H., Hu R. M., Yang T. C., Tseng Y. H. (2008). Regulation of the pehA gene encoding the major polygalacturonase of Xanthomonas campestris by Clp and RpfF. Microbiology 154, 705–713. 10.1099/mic.0.2007/012930-0 [DOI] [PubMed] [Google Scholar]
- Ignatov A. N., Kyrova E. I., Vinogradova S. V., Kamionskaya A. M., Schaad N. W., Luster D. G. (2015). Draft genome sequence of Xanthomonas arboricola strain 3004, a causal agent of bacterial disease on barley. Genome Announc. 3, e01572–e01514. 10.1128/genomeA.01572-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. M., Babujee L., Meng F., Milling A., Allen C. (2012). The in planta transcriptome of Ralstonia solanacearum: conserved physiological and virulence strategies during bacterial wilt of tomato. MBio 3, e00114–e00112. 10.1128/mBio.00114-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn C., Charkowski A., Willis D. K. (2008). Evaluation of isolation methods and RNA integrity for bacterial RNA quantitation. J. Microbiol. Methods 75, 318–324. 10.1016/j.mimet.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Kaewnum S., Prathuangwong S., Burr T. J. (2006). A pectate lyase homolog, xagP, in Xanthomonas axonopodis pv. glycines is associated with hypersensitive response induction on tobacco. Phytopathology 96, 1230–1236. 10.1094/PHYTO-96-1230 [DOI] [PubMed] [Google Scholar]
- Koebnik R., Krüger A., Thieme F., Urban A., Bonas U. (2006). Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J. Bacteriol. 188, 7652–7660. 10.1128/JB.00795-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis K. T., Tiedje J. M. (2005). Genomic insights that advance the species definition for prokaryotes. Proc. Natl. Acad. Sci. U.S.A. 102, 2567–2572. 10.1073/pnas.0409727102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. F., Lu G. T., Li L., Su H. Z., Feng G. F., Chen Y., et al. (2014). Identification of a putative cognate sensor kinase for the two-component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris. Environ. Microbiol. 16, 2053–2071. 10.1111/1462-2920.12207 [DOI] [PubMed] [Google Scholar]
- Li S., Wang Y., Wang S., Fang A., Wang J., Liu L., et al. (2015). The type III effector AvrBs2 in Xanthomonas oryzae pv. oryzicola suppresses rice immunity and promotes disease development. Mol. Plant Microbe Interact. [Epub ahead of print]. 10.1094/MPMI-10-14-0314-R [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu H., Patil P., Van Sluys M. A., White F. F., Ryan R. P., Dow J. M., et al. (2008). Acquisition and evolution of plant pathogenesis-associated gene clusters and candidate determinants of tissue-specificity in Xanthomonas. PLoS ONE 3:e3828. 10.1371/journal.pone.0003828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J. M., Clarke R. C., Watson D. P. (2000). Hemp Diseases and Pests: Management and Biological Control: An Advanced Treatise. Oxfordshire: CABI Publishing. [Google Scholar]
- Mhedbi-Hajri N., Jacques M. A., Koebnik R. (2011). Adhesion mechanisms of plant-pathogenic Xanthomonadaceae. Adv. Exp. Med. Biol. 715, 71–89. 10.1007/978-94-007-0940-9_5 [DOI] [PubMed] [Google Scholar]
- Mole B. M., Baltrus D. A., Dangl J. L., Grant S. R. (2007). Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol. 15, 363–371. 10.1016/j.tim.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Netsu O., Kijima T., Takikawa Y. (2014). Bacterial leaf spot of hemp caused by Xanthomonas campestris pv. cannabis in Japan. J. Gen. Plant Pathol. 80, 164–168. 10.1007/s10327-013-0497-8 [DOI] [Google Scholar]
- Parkinson N., Aritua V., Heeney J., Cowie C., Bew J., Stead D. (2007). Phylogenetic analysis of Xanthomonas species by comparison of partial gyrase B gene sequences. Int. J. Syst. Evol. Microbiol. 57, 2881–2887. 10.1099/ijs.0.65220-0 [DOI] [PubMed] [Google Scholar]
- Parkinson N., Cowie C., Heeney J., Stead D. (2009). Phylogenetic structure of Xanthomonas determined by comparison of gyrB sequences. Int. J. Syst. Evol. Microbiol. 59, 264–274. 10.1099/ijs.0.65825-0 [DOI] [PubMed] [Google Scholar]
- Patil P. B., Bogdanove A. J., Sonti R. V. (2007). The role of horizontal transfer in the evolution of a highly variable lipopolysaccharide biosynthesis locus in xanthomonads that infect rice, citrus and crucifers. BMC Evol. Biol. 7:243 10.1186/1471-2148-7-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti I., Royer M., Barbe V., Carrere S., Koebnik R., Cociancich S., et al. (2009). The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genomics 10:616. 10.1186/1471-2164-10-616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M., Rosselló-Móra R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U.S.A. 106, 19126–19131. 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossez Y., Wolfson E. B., Holmes A., Gally D. L., Holden N. J. (2015). Bacterial flagella: twist and stick, or dodge across the kingdoms. PLoS Pathog. 11:e1004483. 10.1371/journal.ppat.1004483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze S., Kay S., Büttner D., Egler M., Eschen-Lippold L., Hause G., et al. (2012). Analysis of new type III effectors from Xanthomonas uncovers XopB and XopS as suppressors of plant immunity. New Phytol. 195, 894–911. 10.1111/j.1469-8137.2012.04210.x [DOI] [PubMed] [Google Scholar]
- Severin V. (1978). Ein neues Bakterium an Hanf – Xanthomonas campestris pathovar cannabis. Arch. Phytopathol. Pflanzenschutz 14, 7–15. [Google Scholar]
- Shi Q., Febres V. J., Jones J. B., Moore G. A. (2015). Responsiveness of different citrus genotypes to the Xanthomonas citri ssp. citri-derived pathogen-associated molecular pattern (PAMP) flg22 correlates with resistance to citrus canker. Mol. Plant Pathol. 16, 507–520. 10.1111/mpp.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D., Gupta M. K., Patel H. K., Ranjan A., Sonti R. V. (2013). Cell wall degrading enzyme induced rice innate immune responses are suppressed by the type 3 secretion system effectors XopN, XopQ, XopX and XopZ of Xanthomonas oryzae pv. oryzae. PLoS ONE 8:e75867 10.1371/journal.pone.0075867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork W., Kim J. G., Mudgett M. B. (2015). Functional analysis of plant defense suppression and activation by the Xanthomonas core type III effector XopX. Mol. Plant Microbe Interact. 28, 180–194. 10.1094/MPMI-09-14-0263-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme D. J., Wasukira A., Paszkiewicz K., Aritua V., Thwaites R., Smith J., et al. (2011). Draft genome sequences of Xanthomonas sacchari and two banana-associated xanthomonads reveal insights into the Xanthomonas group 1 clade. Genes 2, 1050–1065. 10.3390/genes2041050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Dunning F. M., Pfund C., Weingarten R., Bent A. F. (2006). Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18, 764–779. 10.1105/tpc.105.037648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Xiao Y., Zhou J. M. (2006). Regulation of the type III secretion system in phytopathogenic bacteria. Mol. Plant Microbe Interact. 19, 1159–1166. 10.1094/MPMI-19-1159 [DOI] [PubMed] [Google Scholar]
- Triplett L. R., Verdier V., Campillo T., Van Malderghem C., Cleenwerck I., Maes M., et al. (2015). Characterization of a novel clade of Xanthomonas isolated from rice leaves in Mali and proposal of Xanthomonas maliensis sp. nov. Antonie Van Leeuwenhoek 107, 869–881. 10.1007/s10482-015-0379-5 [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Mew T. W., Wakimoto S. (1982). Bacteriological and pathological characteristics of wild types and induced mutants of Xanthomonas campestris pv. oryzae. Phytopathology 72, 43–46. [Google Scholar]
- van Bakel H., Stout J. M., Cote A. G., Tallon C. M., Sharpe A. G., Hughes T. R., et al. (2011). The draft genome and transcriptome of Cannabis sativa. Genome Biol. 12:R102. 10.1186/gb-2011-12-10-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Rong W., He C. (2008). Two Xanthomonas extracellular polygalacturonases, PghAxc and PghBxc, are regulated by type III secretion regulators HrpX and HrpG and are required for virulence. Mol. Plant Microbe Interact. 21, 555–563. 10.1094/MPMI-21-5-0555 [DOI] [PubMed] [Google Scholar]
- Wengelnik K., Rossier O., Bonas U. (1999). Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J. Bacteriol. 181, 6828–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengelnik K., Van den Ackerveken G., Bonas U. (1996). HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant Microbe Interact. 9, 704–712. 10.1094/MPMI-9-0704 [DOI] [PubMed] [Google Scholar]
- White F. F., Potnis N., Jones J. B., Koebnik R. (2009). The type III effectors of Xanthomonas. Mol. Plant Pathol. 10, 749–766. 10.1111/j.1364-3703.2009.00590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann F., Vorhölter F., Hersemann L., Widmer F., Blom J., Niehaus K., et al. (2013). The noncanonical type III secretion system of Xanthomonas translucens pv. graminis is essential for forage grass infection. Mol. Plant Pathol. 14, 576–588. 10.1111/mpp.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M., Park D. C., Shearman H. M., Fargier E. (2008). A multilocus sequence analysis of the genus Xanthomonas. Syst. Appl. Microbiol. 31, 366–377. 10.1016/j.syapm.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Zerbino D. R., Birney E. (2008). Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821–829. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Poulin L., Rodriguez-R L. M., Forero-Serna N., Liu S. H., Wonni I., et al. (2012). Development of a variable number of tandem repeats typing scheme for the bacterial rice pathogen Xanthomonas oryzae pv. oryzicola. Phytopathology 102, 948–956. 10.1094/PHYTO-04-12-0078-R [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flg22 epitope variants found in various strains of Xanthomonas. On top, the prototype elicitor-active flg22 peptide from Pseudomonas aeruginosa is shown (Felix et al., 1999). Below, homologous sequences from different species and pathovars of Xanthomonas are aligned. Residues that deviate from the prototype sequence are in red. The peptide that corresponds to eliciting flagellin variants of X. campestris pv. campestris is highlighted in green, while the peptide that corresponds to non-eliciting flagellin variants highlighted in yellow (Sun et al., 2006).
Comparison of promoter sequences of the Xanthomonas lysophospholipase gene. Promoter regions encompassing 350 bp upstream of the translational start codon of a representative set of Xanthomonas strains were aligned by MUSCLE. PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of the lower sequence block. Deviations from the PIP consensus sequence are highlighted in yellow. For the set of analyzed strains, compare with Figure 4.
Comparison of promoter sequences of the Xanthomonas pehA gene. Promoter regions encompassing 350 bp upstream of the translational start codon of a representative set of Xanthomonas strains were aligned by MUSCLE. Consensus CAP/CLP binding boxes, according to Dong and Ebright (1992), are indicated above the multiple sequence alignment, first aligned with the Clp-binding site as determined by Hsiao et al. (2008), for X. campestris pv. campestris (highlighted in yellow, six conserved residues are shown in green), and then aligned with the Clp-binding site as proposed by us (conserved residues are shown in red). The PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of the lower sequence block. For better comparison with Figure 4, PIP half boxes are shown in blue and the −10 promoter motif is shown in orange. Distance to the translational start codon is indicated on the right side of the lower sequence block. Deviations from the PIP consensus sequence are highlighted in yellow. For the set of analyzed strains, compare with Figure 4.