Abstract
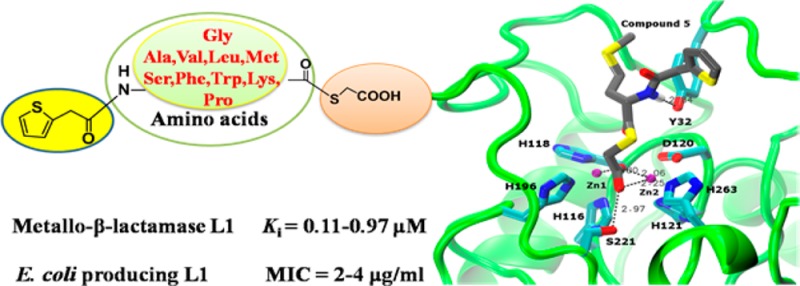
In light of the biomedical significance of metallo-β-lactamases (MβLs), ten new mercaptoacetic acid thioester amino acid derivatives were synthesized and characterized. Biological activity assays indicated that all these synthesized compounds are very potent inhibitors of L1, exhibiting an IC50 value range of 0.018–2.9 μM and a Ki value range of 0.11–0.95 μM using cefazolin as substrate. Partial thioesters also showed effective inhibitory activities against NDM-1 and ImiS with an IC50 value range of 12–96 and 3.6–65 μM, respectively. Also, all these thioesters increased susceptibility of E. coli cells expressing L1 to cefazolin, indicated by a 2–4-fold reduction in MIC of the antibiotic. Docking studies revealed potential binding modes of the two most potent L1 inhibitors to the active site in which the carboxylate group interacts with both Zn(II) ions and Ser221. This work introduces a highly promising scaffold for the development of metallo-β-lactamase L1 inhibitors.
Keywords: Antibiotic resistance, metallo-β-lactamase, subclass B3, L1, inhibitor, mercaptoacetic acid thioester
β-Lactam antibiotics, which include penicillins, cephalosporins, and carbapenems, remain the most important and frequently used antimicrobial agents, constituting more than 50% of the antibiotics prescribed worldwide.1 However, the overuse of antibiotics in the clinical setting as well as animal production has resulted in resistance2 conferred, among other resistance mechanisms, by the production of β-lactamases. More than a thousand β-lactamases have been isolated, and these enzymes have been categorized into classes A to D, depending on their amino acid sequence homologies.1 Enzymes from classes A, C, and D comprise the serine β-lactamases. They use a catalytic mechanism that is characterized by the nucleophilic attack of an active-site serine on the β-lactam carbonyl, ultimately causing cleavage of the β-lactam ring. Class B enzymes, or metallo-β-lactamases (MβLs), require either one or two Zn(II) ions per enzyme molecule for full catalytic activity, and these enzymes inactivate all clinically important β-lactams except aztreonam. According to amino acid sequence homologies MβLs are divided into subclasses B1–B3, based on amino acid sequence homologies.3 MβLs from subclasses B1 and B3 can inactivate nearly all β-lactam antibiotics. Contrarily, B2 enzymes possess a narrow substrate preference for carbapenems. There are no known clinical inhibitors of the MβLs to date.1
The emergence of antibiotic resistance is a global public health problem. Given the enormous clinical importance of MβLs, there have been many attempts to identify novel MβL inhibitors.4,5 Toney et al. reported biphenyl tetrazoles and succinic acids to be effective inhibitors of MβL IMP-1.6 Mercaptocarboxylate,7 3-subsutituted phthalic acid derivatives,8 and N-arylsulfonyl hydrazones9 also displayed inhibitory activities on IMP-1. A 2-picolinic acid based inhibitor was found to inhibit the B2 subclass CphA.10 Also some broad-spectrum inhibitors of the MβLs have been reported, including mercaptophosphonate compounds, thiomandelic acid, and thiols11
MβLs inhibitors containing both sulfur and carboxylate capable of metal ligation are the most potent inhibitors.12−14 Payne et al. have reported several mercaptoacetic acid thioesters as irreversible inhibitors of β-lactamase BcII. Mercaptoacetic acid is delivered to the metal-coordinating cysteine as a result of the thioester conversion by the enzyme.15 However, isothermal titration calorimetry (ITC) indicated that inhibition was not accomplished through chelation of the Zn(II) ions because the presence of a phenyl substituent retarded the cleavage of the thioester bond.16 MALDI-TOF spectrometry showed that two molecules of mercaptoacetate covalently bind to L1, a subclass B3 enzyme responsible for penicillin resistance,17 when the enzyme is incubated with benzylacetyl-d-alanylthioacetic acid. The activities of the modified enzyme and native L1 are the same.18 The structure–activity relationships (SAR) of the thioester inhibitors of the MβL IMP-1 showed that introducing more hydrophobic moieties on the C-terminus greatly enhanced their activity.19 In order to further probe the SAR of thioester inhibitors against L1 and design an effective scaffold for the development of metallo-β-lactamase L1 inhibitors, in this work, we synthesized a series of 2-thiopheneacetyl mercaptoacetic acid thioesters with various N-terminal substituents and studied their biological activities.
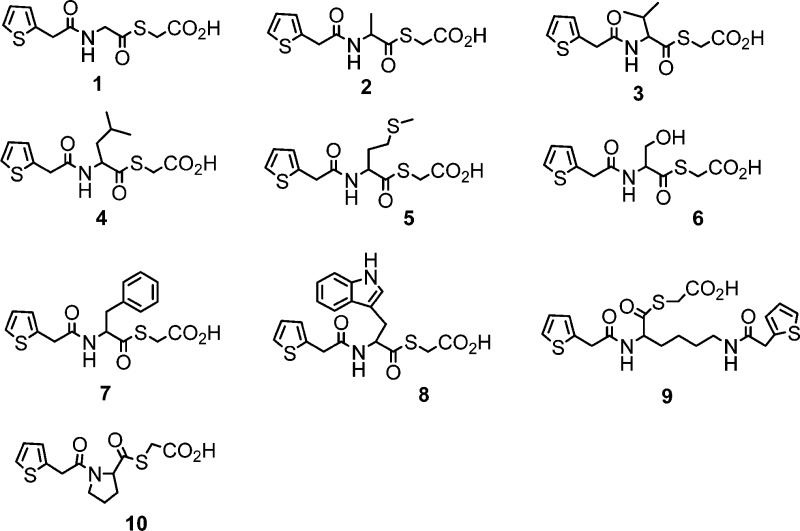
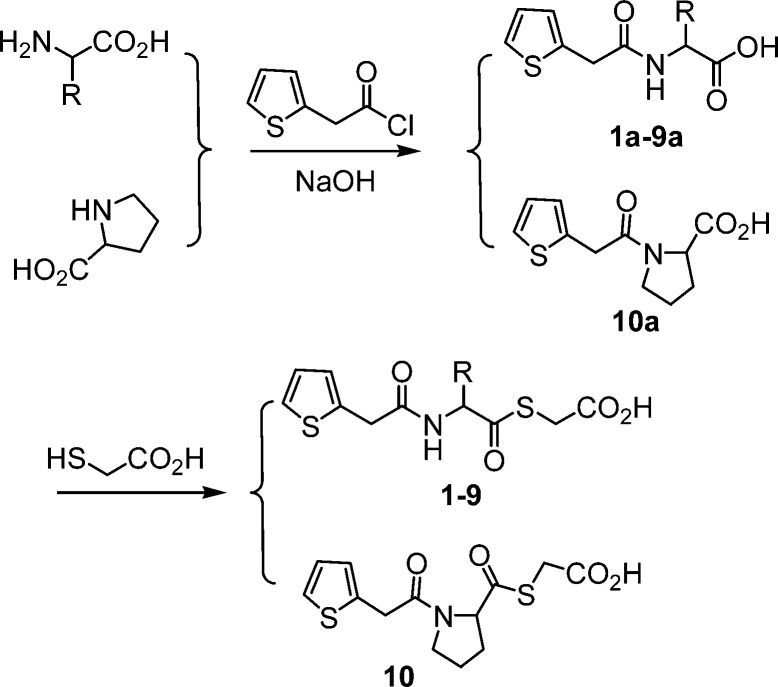
Ten 2-thiopheneacetyl mercaptoacetic acid thioesters (Figure 1) were synthesized by a previously reported method.18,20 The synthetic route is shown in Scheme 1. 2-thiopheneacetyl chloride reacted with amino acids under basic conditions to give the corresponding amides 1a–10a. In the presence of triethylamine, the amides further reacted with mercaptoacetic acid to offer the target products 2-thiopheneacetyl mercaptoacetic acid thioesters 1–10. These synthesized mercaptoacetic acid thioesters were characterized by 1H and 13C NMR and confirmed by MS (see Supporting Information). These compounds basically represent N- and C-terminally modified derivatives of the natural amino acids glycine (1), alanine (2), valine (3), leucine (4), methionine (5), serine (6), phenylalanine (7), tryptophan (8), lysine (9), and proline (10). In 9, two amino groups and carboxyl group of lysine are modified.
Figure 1.
Structures of the synthesized mercaptoacetic acid thioesters.
Scheme 1. Synthetic Route of Mercaptoacetic Acid Thioesters.
To test whether these mercaptoacetic acid thioesters were specific inhibitors of L1 or potentially broad-spectrum inhibitors of all MβLs, MβLs from the three subclasses (CcrA from B1a, NDM-1 from B1b, ImiS from B2, and L1 from B3) were overexpressed in Escherichia coli and purified as reported previously.4,21−23 Steady-state kinetic studies and inhibition experiments of the mercaptoacetic acid thioesters as inhibitors against MβLs were conducted on an Agilent UV8453 spectrometer. Hydrolysis of substrate (cefazolin for CcrA, NDM-1, and L1; imipenem for ImiS) was monitored at 262 and 300 nm, respectively. The initial reaction rates were determined in the absence or presence of inhibitors in triplicate, and the average values were recorded.
The inhibitor concentrations causing 50% decrease of enzyme activity (IC50) were determined and are presented in Table 1. It is clear to be observed that all mercaptoacetic acid thioesters tested effectively inhibit L1 with an IC50 value range of 0.018–2.9 μM, and 5 showed the lowest IC50 value of 18 nM, revealing that mercaptoacetic acid thioesters specifically target L1. The thioesters 1–7 inhibit ImiS with an IC50 value range of 4–65 μM, while 8–10 had no inhibitory activity against ImiS. Also, apart from thioesters 3 and 5 inhibiting CcrA with IC50 values of 39 and 123 μM, respectively, the other compounds had no activity against this enzyme. It is worth noting that the mercaptoacetic acid thioesters 1–4 and 7 significantly inhibit NDM-1 with an IC50 value range of 12–96 μM, as pathogens expressing NDM-1 have been called superbugs.24
Table 1. Inhibitory Activity of Mercaptoacetic Acid Thioesters against MβLsa.
| IC50 (μM) |
||||
|---|---|---|---|---|
| inhibitor | NDM-1a | CcrAa | ImiSb | L1a |
| 1 | 28.7 | 61.5 | 0.84 | |
| 2 | 95.7 | 46.3 | 0.91 | |
| 3 | 12.4 | 38.5 | 18.9 | 0.45 |
| 4 | 28.5 | 3.6 | 0.069 | |
| 5 | 123 | 13.3 | 0.018 | |
| 6 | 60.1 | 1.49 | ||
| 7 | 36.3 | 65.4 | 2.90 | |
| 8 | 0.29 | |||
| 9 | 1.08 | |||
| 10 | 0.45 | |||
Blank spaces represent no inhibition. The substrate used was cefazolin (a) and imipenem (b).
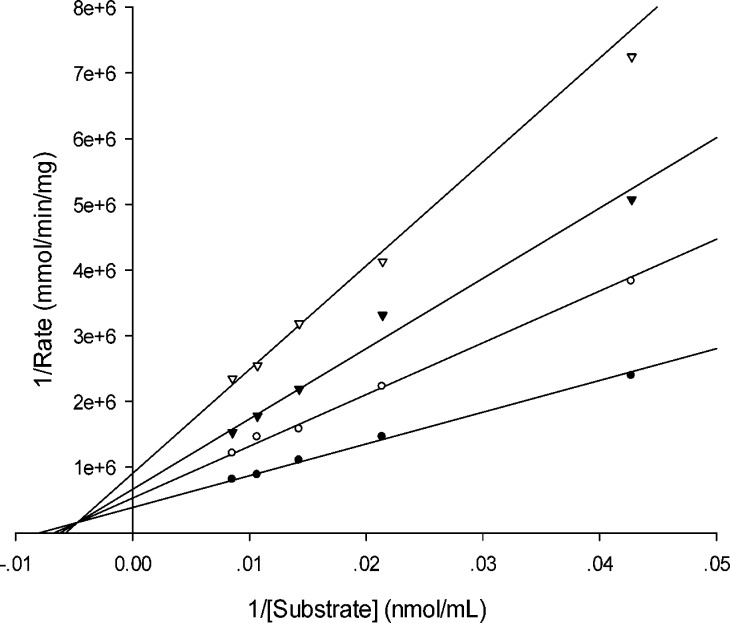
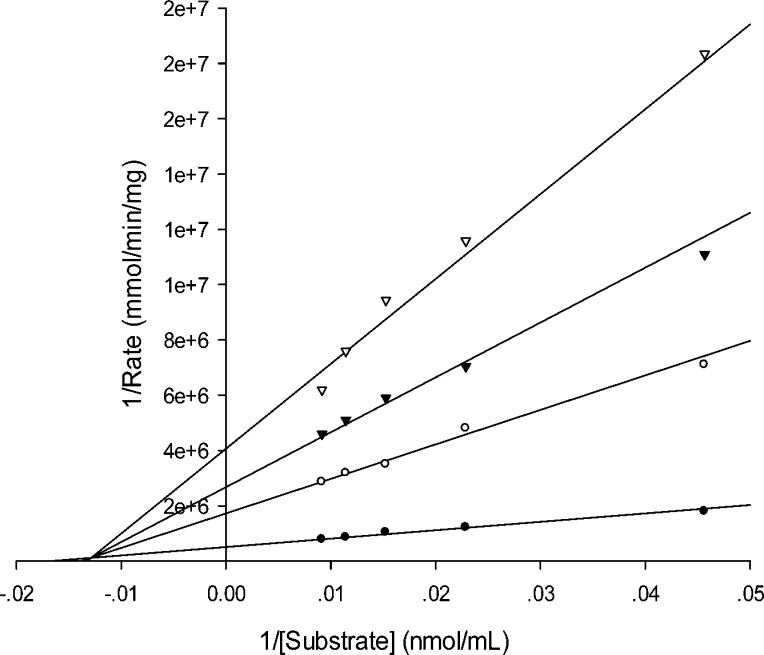
Given the specificity of these mercaptoacetic acid thioesters targeting L1, in order to further identify the inhibition model of the inhibitors, we determined inhibition constants Ki of the thioesters against L1. The data listed in Table 2 show that these compounds are very potent inhibitors with a Ki value range of 0.11–0.95 μM. The Lineweaver–Burk plots with the thioester inhibitors indicated that 1–10 are mixed inhibitors of L1. Exemplary, inhibitors 5 and 8 are presented to show their inhibition modes against L1 in Figure 2 and 3.
Table 2. Inhibitory Activity of Mercaptoacetic Acid Thioesters against Metallo-β-lactamase L1a.
| inhibitor | Ki (μM) | inhibitor | Ki (μM) |
|---|---|---|---|
| 1 | 0.28 ± 0.07 | 6 | 0.17 ± 0.02 |
| 2 | 0.41 ± 0.09 | 7 | 0.95 ± 0.09 |
| 3 | 0.28 ± 0.02 | 8 | 0.17 ± 0.03 |
| 4 | 0.21 ± 0.02 | 9 | 0.19 ± 0.02 |
| 5 | 0.11 ± 0.03 | 10 | 0.87 ± 0.09 |
Ki values were determined using cefazolin as substrate and L1 as the enzyme in 50 mM Tris, at 25 °C. The inhibitor concentrations were ranged from 0–2.5 μM.
Figure 2.
Lineweaver–Burk plot of L1-catalyzed hydrolysis in the absence and presence of compound 5. The substrate used was cefazolin. Inhibitor 5 concentrations were 0 μM (•), 0.625 μM (○), 1.25 μM (▼), and 2.50 μM (▽).
Figure 3.
Lineweaver–Burk plot of L1-catalyzed hydrolysis in the absence and presence of compound 8. The substrate used was cefazolin. Inhibitor 8 concentrations were 0 μM (•), 0.625 μM (○), 1.25 μM (▼), and 2.50 μM (▽).
The minimum inhibitory concentrations (MICs) of antibiotics in the absence or presence of compounds 1–10 were determined as previously reported method.25 Four strains of E. coli BL21(DE3) containing plasmids pMSZ02-CcrA, pET26b-NDM-1, pET26b-ImiS, and pET26b-L1, as well as an E. coli control without plasmid, were used to assess these inhibitors (Table 3). MIC data indicate that all mercaptoacetic acid thioester inhibitors resulted in 2–4-fold MIC reduction of cefazolin for E. coli BL21(DE3) containing the L1-encoding plasmid (E. coli-L1), while the MICs for the other strains were not affected significantly (no more than one dilution factor).
Table 3. Antibacterial Activities (MIC, μg/mL) of the Mercaptoacetic Acid Thioester Inhibitors at a Final Concentration of 16 μg/mLa.
| tested strains | inhibitor black | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. colia | 64 | 64 | >64 | >64 | 64 | >64 | 64 | 64 | 64 | 64 | 64 |
| E. colib | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| E.coli-NDM-1a | 8 | 4 | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 4 | 8 |
| E.coli-CcrAa | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| E. coli-ImiSb | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| E. coli-L1a | 8 | 4 | 2 | 4 | 2 | 2 | 4 | 4 | 2 | 4 | 2 |
The antibiotics used were cefazolin (a) and imipenem (b).
In order to elucidate possible binding modes of the most potent L1 inhibitors, 5 and 8, these compounds were docked into the active site of the L1 crystal structure26 using the same procedure as reported previously.27 The top ranked conformations shown in Figure 4A,B exhibit common features. One of the carboxylate oxygens bridges the two Zn(II) ions, while the other oxygen coordinates Zn2 and hydrogen bonds with Ser221. Thus, one oxygen assumes the role of the bridging water/hydroxide, while the other one assumes the role of the C4 carboxylate of hydrolyzed moxalactam in the crystal structure26 (Figure 4C). The hydrophobic portions of the molecules (the 2-thiopheneacetyl amido group and the amino acid side chains) are located where the C7 substituent of moxalactam is located in the crystal structure. The amide nitrogens form hydrogen bonds with the Tyr32 side chain, which is formed between the carbonyl of the C7 substituent and Tyr32 in the crystal structure. The fact that compound 6 (serine derivative) also exhibits a low Ki value (0.17 μM), while the other compounds have higher values, implies that a hydrophilic component (polar sulfur in 5, hydroxyl group in 6, and indole nitrogen in 8) is favorable, possibly due to interaction with bulk solvent. Indeed, in the crystal structure the carboxylate of the moxalactam C7 substituent is in this location pointing toward the solvent. While also endowed with a hydrophilic amide group, compound 9 may simply be too big to be accommodated as efficiently in the L1 active site. Because of the combination of these specific interactions with L1, these compounds can be interpreted as analogues of a β-lactam hydrolysis product. We hope to validate this model in the near future with a crystal structure.
Figure 4.
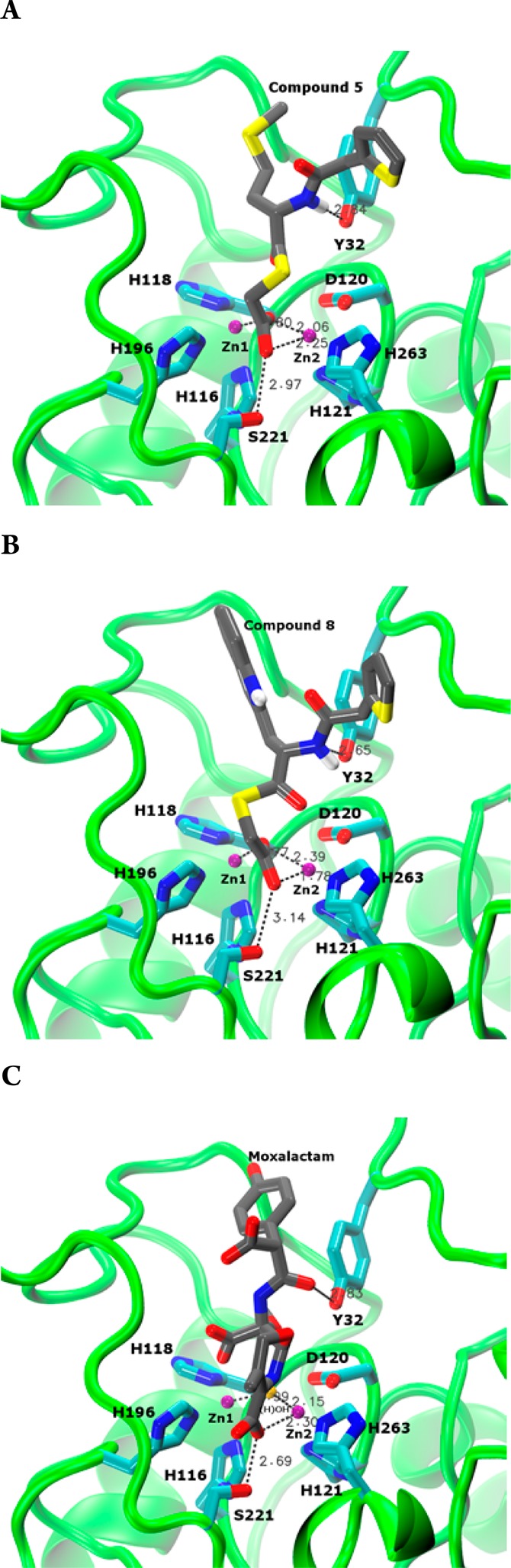
Lowest-energy docking conformations of compounds 5 (A) and 8 (B) docked into the active site of L1 (backbone shown as green cartoon) and hydrolyzed moxalactam cocrystallized with L126 (C) rendered as sticks (H, white; C, gray; N, blue; O, red; S, yellow). Zn(II) ions are shown as magenta spheres. The side chains of Zn(II) ligands as well as Tyr32 and Ser221 are rendered as sticks (C, cyan; N, blue; O, red). The water/hydroxide molecule (H)OH in panel C is shown as a yellow sphere. Characteristic short distances between the compounds and Zn(II) ions, (H)OH, Tyr32, and Ser221 are indicated by dashed lines.
In summary, ten novel mercaptoacetic acid thioesters based on natural amino acids were synthesized and characterized. Biological activity assays indicated that these compounds are very potent mixed inhibitors of MβL L1 and inhibit gowth of E. coli cells expressing L1 in the presence of cefazolin. Docking studies suggest that the carboxylate group interacts with the two Zn(II) ions and Ser221 in a similar fashion to a moxalactam hydrolysis product cocrystallized with L1. This work provides a highly promising scaffold for the development of metallo-β-lactamase L1 inhibitors.
Supporting Information Available
Detailed synthesis procedure, NMR and ESI mass data for target compounds; methods for enzyme overexpression and purification; inhibition assay; determination of MIC and Ki; and docking study. These materials are available free of charge via the Internet at The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00098.
Author Contributions
∥ These authors contributed equally to this work.
Author Contributions
The manuscript was written through contributions of all authors.
This work was supported by grants 21272186 and 81361138018 (to K.W.Y.) from the National Natural Science Foundation of China and key grant 2014KW23-03 (to K.W.Y.) for international cooperation of Shaanxi province.
The authors declare no competing financial interest.
Supplementary Material
References
- Drawz S. M.; Bonomo R. A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. F.; Meroueh S. O.; Mobashery S. Bacterial resistance to β-lactam antibiotics: Compelling opportunism, compelling opportunity. Chem. Rev. 2005, 105, 395–424. [DOI] [PubMed] [Google Scholar]
- Bebrone C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 2007, 74, 1686–701. [DOI] [PubMed] [Google Scholar]
- Yang H.; Aitha M.; Hetrick A. M.; Richmond T. K.; Tierney D. L.; Crowder M. W. Mechanistic and Spectroscopic Studies of Metallo-β-lactamase NDM-1. Biochemistry 2012, 51, 3839–47. [DOI] [PubMed] [Google Scholar]
- Bush K. The ABCD’s of β-lactamase nomenclature. J. Infect. Chemother. 2013, 19, 549–59. [DOI] [PubMed] [Google Scholar]
- Toney J. H.; Hammond G. G.; Fitzgerald P. M.; Sharma N.; Balkovec J. M.; Rouen G. P.; Olson S. H.; Hammond M. L.; Greenlee M. L.; Gao Y. D. Succinic acids as potent inhibitors of plasmid-borne IMP-1 metallo-β-lactamase. J. Biol. Chem. 2001, 276, 31913–8. [DOI] [PubMed] [Google Scholar]
- Concha N. O.; Janson C. A.; Rowling P.; Pearson S.; Cheever C. A.; Clarke B. P.; Lewis C.; Galleni M.; Frere J. M.; Payne D. J.; Bateson J. H.; Abdel-Meguid S. S. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: Binding determinants of a potent broad-spectrum inhibitor. Biochemistry 2000, 39, 4288–98. [DOI] [PubMed] [Google Scholar]
- Hiraiwa Y.; Morinaka A.; Fukushima T.; Kudo T. Metallo-β-lactamase inhibitory activity of phthalic acid derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5162–5. [DOI] [PubMed] [Google Scholar]
- Siemann S.; Evanoff D. P.; Marrone L.; Clarke A. J.; Viswanatha T.; Dmitrienko G. I. N-Arylsulfonyl hydrazones as inhibitors of IMP-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 2002, 46, 2450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfall L. E.; Garau G.; Lienard B. M.; Dideberg O.; Schofield C. J.; Frere J. M.; Galleni M. Competitive inhibitors of the CphA metallo-β-lactamase from Aeromonas hydrophila Antimicrob. Agents Chemother. 2007, 51, 2136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard B. M.; Garau G.; Horsfall L.; Karsisiotis A. I.; Damblon C.; Lassaux P.; Papamicael C.; Roberts G. C.; Galleni M.; Dideberg O.; Frere J. M.; Schofield C. J. Structural basis for the broad-spectrum inhibition of metallo-β-lactamases by thiols. Org. Biomol. Chem. 2008, 6, 2282–94. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez I.; Hopkins J.; Papamicael C.; Franceschini N.; Amicosante G.; Rossolini G. M.; Galleni M.; Frère J. M.; Dideberg O. J. Structure of Chryseobacterium meningosepticum zinc β-lactamase in complex with the inhibitor, D-captopril. Biol. Chem. 2003, 278, 23868–73. [DOI] [PubMed] [Google Scholar]
- Wachino J.; Yamaguchi Y.; Mori S.; Kurosaki H.; Arakawa Y.; Shibayama K. Structural insights into the subclass B3 metallo-β-lactamase SMB-1 and the mode of inhibition by the common metallo-β-lactamase inhibitor mercaptoacetate. Antimicrob. Agents Chemother. 2013, 57, 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridoon; Islam N. U. An update on the status of potent inhibitors of metallo-β-lactamases. Sci. Pharm. 2013, 81, 309–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. J.; Bateson J. H.; Gasson B. C.; Proctor D.; Khushi T.; Farmer T. H.; Tolson D. A.; Bell D.; Skett P. W.; Marshall A. C.; Reid R.; Ghosez L.; Combret Y.; Marchand-Brynaert J. Inhibition of metallo-β-lactamases by a series of mercaptoacetic acid thiol ester derivatives. Antimicrob. Agents Chemother. 1997, 41, 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. J.; Bateson J. H.; Gasson B. C.; Khushi T.; Proctor D.; Pearson S. C.; Reid R. Inhibition of metallo-β-lactamases by a series of thiol ester derivatives of mercaptophenylacetic acid. FEMS Microbiol. Lett. 1997, 157, 171–5. [DOI] [PubMed] [Google Scholar]
- Yang K. W.; Crowder M. W. Inhibition studies on the metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Arch. Biochem. Biophys. 1999, 368, 1–6. [DOI] [PubMed] [Google Scholar]
- Feng L.; Yang K. W.; Zhou L. S.; Xiao J. M.; Yang X.; Zhai L.; Zhang Y. J.; Crowder M. W. N-Heterocyclic dicarboxylic acids: broad-spectrum inhibitors of metallo-β-lactamases with co-antibacterial effect against antibiotic-resistant bacteria. Bioorg. Med. Chem. Lett. 2012, 22, 5185–9. [DOI] [PubMed] [Google Scholar]
- Greenlee M. L.; Laub J. B.; Balkovec J. M.; Hammond M. L.; Hammond G. G.; Hammond M. L.; Pompliano D. L.; Epstein-Toney J. H. Synthesis and SAR of thioester and thiol inhibitors of IMP-1 metallo-β-lactamase. Bioorg. Med. Chem. Lett. 1999, 9, 2549–54. [DOI] [PubMed] [Google Scholar]
- Banerjee P. S.; Zuniga E. S.; Ojima I.; Carrico I. S. Targeted and armed oncolytic adenovirus via chemoselective modification. Bioorg. Med. Chem. Lett. 2011, 21, 4985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Benkovic S. J. Purification, characterization, and kinetic studies of a soluble Bacteroides fragilis metallo-β-lactamase that provides multiple antibiotic resistance. J. Biol. Chem. 1998, 273, 22402–8. [DOI] [PubMed] [Google Scholar]
- Crawford P. A.; Sharma N.; Chandrasekar S.; Sigdel T.; Walsh T. R.; Spencer J.; Crowder M. W. Over-expression, purification, and characterization of metallo-β-lactamase ImiS from Aeromonas Veronii bv. sobria. Protein Expr. Purif. 2004, 36, 272–9. [DOI] [PubMed] [Google Scholar]
- Crowder M. W.; Walsh T. R.; Banovic L.; Pettit M.; Spencer J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1998, 42, 921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; McLeod S.; MacCormack K.; Sriram S.; Gao N.; L. Breeze A. L.; Hu J. Real-time monitoring of New Delhi metallo-β-lactamase activity in living bacterial cells by 1H NMR spectroscopy. Angew. Chem., Int. Ed. 2014, 53, 2130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. M.; Feng L.; Zhou L. S.; Gao H. Z.; Zhang Y. L.; Yang K. W. Novel fluorescent cephalosporins: synthesis, antimicrobial activity and photodynamic inactivation of antibiotic resistant bacteria. Eur. J. Med. Chem. 2013, 59, 150–9. [DOI] [PubMed] [Google Scholar]
- Spencer J.; Read J.; Sessions R. B.; Howell S.; Blackburn G. M.; Gamblin S. J. Antibiotic recognition by binuclear metallo-β-lactamases revealed by X-ray crystallography. J. Am. Chem. Soc. 2005, 127, 14439–44. [DOI] [PubMed] [Google Scholar]
- Zhang Y. L.; Yang K. W.; Zhou Y. J.; LaCuran A. E.; Oelschlaeger P.; Crowder M. W. Diaryl-substituted azolylthioacetamides: inhibitor discovery of New Delhi metallo-β-lactamase-1 (NDM-1). ChemMedChem 2014, 9, 2445–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.