Abstract
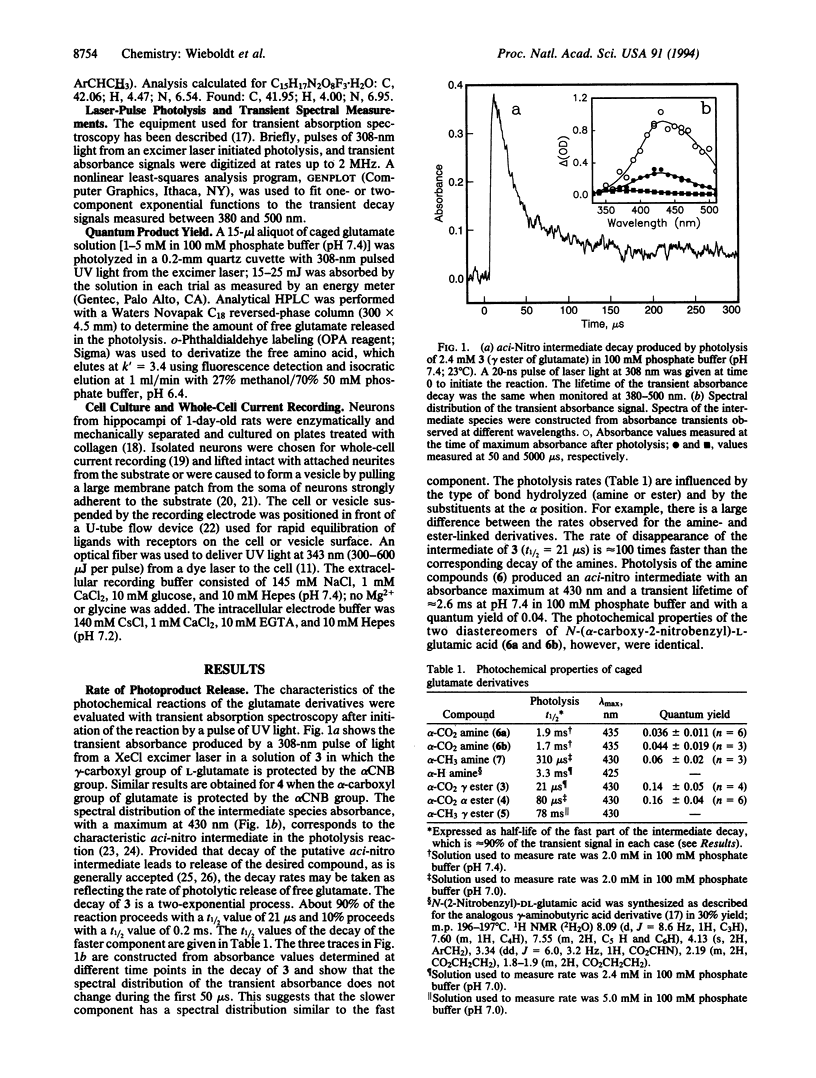
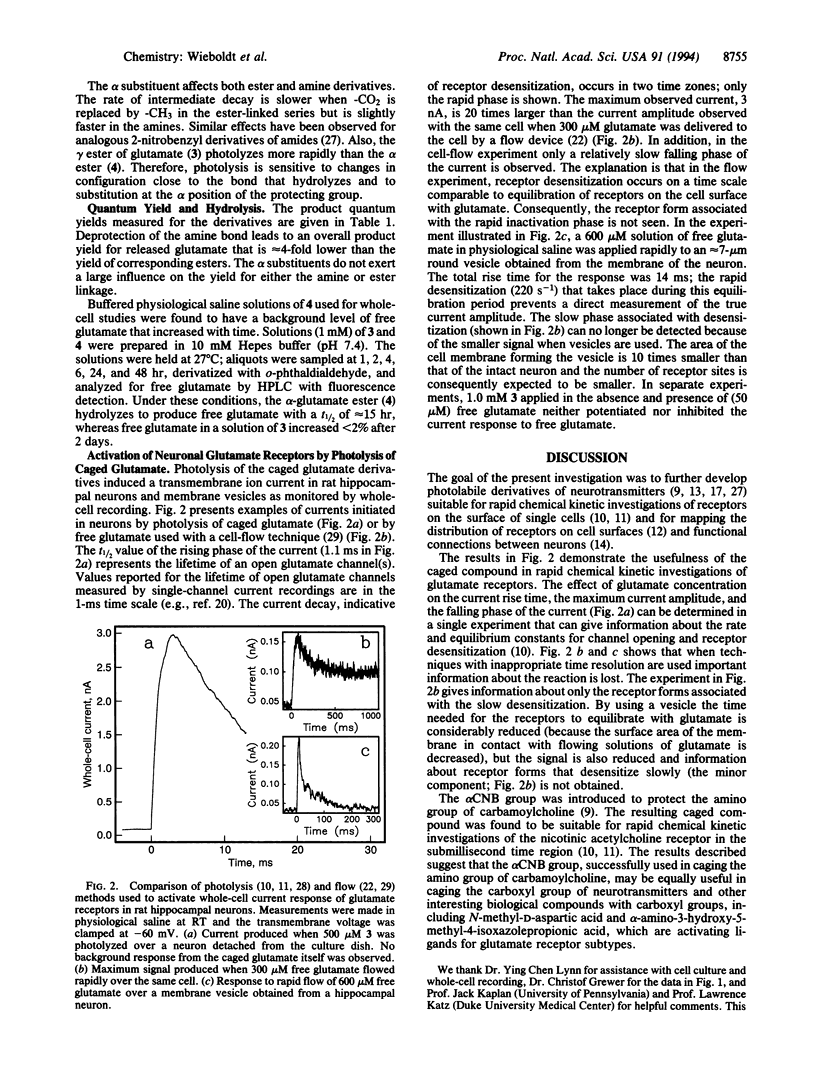
Newly synthesized photolabile derivatives of glutamate, caged glutamate, that release free glutamate on a microsecond time scale after a pulse of UV laser light are described. 2-Nitrobenzyl derivatives were attached to the amino or carboxyl groups of glutamate. Substitution with a -CO2- group at the benzylic carbon accelerates the photolysis reaction when compared to -H and -CH3 substituents. gamma-O-(alpha-Carboxy-2-nitrobenzyl)glutamate is stable at neutral pH. In 100 mM phosphate buffer at pH 7.0, the compound is photolyzed at 308 nm with a quantum product yield of 0.14. The half-life of the major component of the photolytic reaction, as judged by the transient absorbance change at 430 nm, is 21 microseconds (approximately 90%); the half-life of a minor component (approximately 10%) is 0.2 ms. The amino-linked derivatives have half-lives in the millisecond region and a 4-fold lower quantum yield. The potential of the newly synthesized compound for use in rapid chemical kinetic investigations of glutamate receptors is demonstrated. (i) The caged glutamate at 1 mM concentration does not desensitize glutamate receptors in rat hippocampal neurons. (ii) Caged glutamate (1 mM) does not inhibit activation of the receptors by 50 microM glutamate. (iii) Photolysis of the compound induces rapid onset of transmembrane currents in rat hippocampal neurons.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. R., Tsien R. Y. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- Callaway E. M., Katz L. C. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrie J. E., DeSantis A., Katayama Y., Khodakhah K., Messenger J. B., Ogden D. C., Trentham D. R. Postsynaptic activation at the squid giant synapse by photolytic release of L-glutamate from a 'caged' L-glutamate. J Physiol. 1993 Jun;465:1–8. doi: 10.1113/jphysiol.1993.sp019662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. D., Ferkany J. W., Enna S. J. Excitatory amino acid receptors: a gallery of new targets for pharmacological intervention. Life Sci. 1994;54(3):135–148. doi: 10.1016/0024-3205(94)00583-4. [DOI] [PubMed] [Google Scholar]
- Denk W. Two-photon scanning photochemical microscopy: mapping ligand-gated ion channel distributions. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6629–6633. doi: 10.1073/pnas.91.14.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Hess G. P. On the mechanism of a mammalian neuronal type nicotinic acetylcholine receptor investigated by a rapid chemical kinetic technique. Detection and characterization of a short-lived, previously unobserved, main receptor form in PC12 cells. Biochemistry. 1992 Jun 23;31(24):5477–5487. doi: 10.1021/bi00139a009. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn T., Matsubara N., Billington A. P., Udgaonkar J. B., Walker J. W., Carpenter B. K., Webb W. W., Marque J., Denk W., McCray J. A. Synthesis, photochemistry, and biological activity of a caged photolabile acetylcholine receptor ligand. Biochemistry. 1989 Jan 10;28(1):49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Niu L., Hess G. P. An acetylcholine receptor regulatory site in BC3H1 cells: characterized by laser-pulse photolysis in the microsecond-to-millisecond time region. Biochemistry. 1993 Apr 20;32(15):3831–3835. doi: 10.1021/bi00066a001. [DOI] [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udgaonkar J. B., Hess G. P. Chemical kinetic measurements of a mammalian acetylcholine receptor by a fast-reaction technique. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8758–8762. doi: 10.1073/pnas.84.24.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., McCray J. A., Hess G. P. Photolabile protecting groups for an acetylcholine receptor ligand. Synthesis and photochemistry of a new class of o-nitrobenzyl derivatives and their effects on receptor function. Biochemistry. 1986 Apr 8;25(7):1799–1805. doi: 10.1021/bi00355a052. [DOI] [PubMed] [Google Scholar]
- Walstrom K. M., Hess G. P. Mechanism for the channel-opening reaction of strychnine-sensitive glycine receptors on cultured embryonic mouse spinal cord cells. Biochemistry. 1994 Jun 21;33(24):7718–7730. doi: 10.1021/bi00190a027. [DOI] [PubMed] [Google Scholar]
- Wieboldt R., Ramesh D., Carpenter B. K., Hess G. P. Synthesis and photochemistry of photolabile derivatives of gamma-aminobutyric acid for chemical kinetic investigations of the gamma-aminobutyric acid receptor in the millisecond time region. Biochemistry. 1994 Feb 15;33(6):1526–1533. doi: 10.1021/bi00172a032. [DOI] [PubMed] [Google Scholar]



