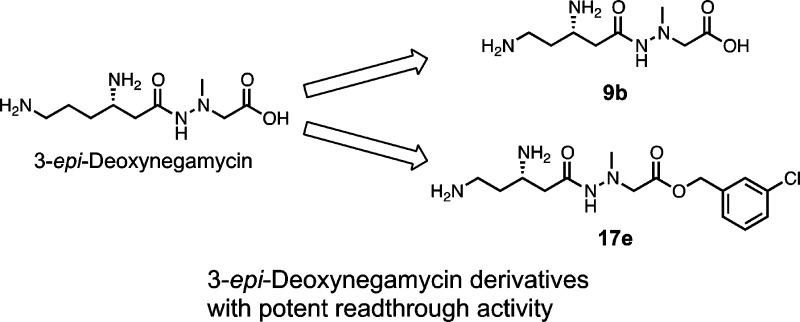
Abstract
(+)-Negamycin (1), a natural dipeptidic antibiotic bearing a hydrazide structure, exhibits a readthrough activity toward the nonsense mutation of the dystrophin gene and restores dystrophin expression in muscles of Duchenne muscular dystrophy model mdx mice. Herein to develop more potent readthrough compounds, we performed a structure–activity relationship (SAR) study of 3-epi-deoxynegamycin (2), which is also another natural readthrough compound with little antimicrobial activity, focusing on the main carbon chain length. We found that one carbon atom shorter derivative 9b shows a higher readthrough activity than 1 and 2. Further derivatization at the carboxylic acid part of 9b demonstrates that its meta-chlorobenzyl ester derivative 17e, which has a higher ClogP value, exhibits a more potent readthrough activity than 9b. Interestingly, in the cell-free protein expression system, the readthrough activity of 17e drastically decreases compared to that in the cell-based assay. These results suggest that benzyl ester-type derivatives enhance the hydrophobicity and function as prodrugs to produce active compound 9b in living cell systems.
Keywords: (+)-Negamycin, 3-epi-deoxynegamycin, readthrough drug, Duchenne muscular dystrophy, prodrug
There are an estimated 1800 types of hereditary diseases caused by nonsense mutations. One such disease, Duchenne muscular dystrophy (DMD),1 is a serious progressive X-linked recessive genetic disorder that occurs in 1 out of every 3500 newborn boys.2 About 20% of DMD cases are caused by a nonsense mutation of the dystrophin gene with a premature termination codon (PTC). Once PTC is induced into the gene, protein translation is terminated, producing a nonfunctional truncated dystrophin protein. In addition, such PTC-containing mRNA is easily degraded by a nonsense-mediated mRNA decay (NMD) pathway, which is a ubiquitous mRNA surveillance system that protects an organism from the deleterious effects of truncated proteins in eukaryotes.3 Although dystrophin has a very important biological role in which it fastens the basement membrane firmly to the cytoskeleton by attaching to the laminin of the extracellular matrix through binding to sarcoglycan and dystroglycan,4 a functional dystrophin protein almost completely disappears in DMD patients. A deficiency in the dystrophin function results in a wide range of movement disorders. Currently, there is no effective treatment for DMD except for a mild steroid treatment.5,6
Recently, the “readthrough” strategy has attracted attention as a new therapeutic methodology to treat nonsense mutation-mediated genetic diseases.7 Readthrough compounds can interact with ribosomes to promote a translational “skip” of PTCs, resulting in the production of a full-length functional protein. Additionally, readthrough compounds should exhibit a positive effect against NMD.8,9 To date, several forms of aminoglycoside antibiotics such as gentamicin and G418 have been reported to show readthrough activities,7,10−12 but their nephrotoxicity13 and ototoxicity14 are serious side effects for long-term DMD treatment.
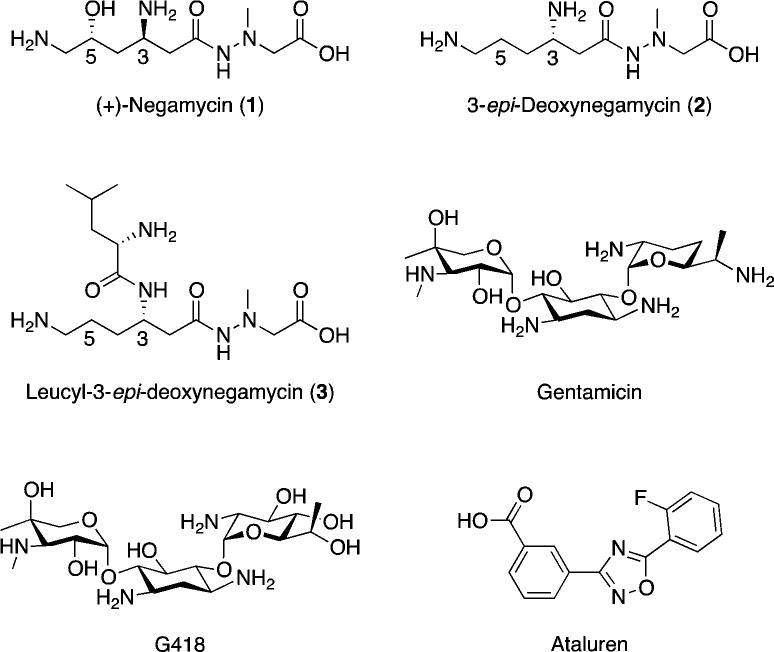
As a nonaminoglycoside readthrough compound, Ataluren, which was developed from a chemical library, promotes dystrophin production in primary muscle cells from humans and mdx mice.15 In contrast, (+)-negamycin (1) (Figure 1), an antibiotic with a dipeptide-like hydrazide structure, isolated in 1970 from culture filtrates of three strains related to Streptomyces purpeofuscus,16 exhibits efficacious antimicrobial activity against Gram-negative microorganisms. In 2003, Arakawa et al. reported that 1 induces the expression of the dystrophin protein in skeletal and cardiac muscles of mdx mice but shows a lower toxicity than gentamicin.17
Figure 1.
Structures of the readthrough compounds.
Our previous study revealed that other native negamycin analogues18 [i.e., 3-epi-deoxynegamycin (2) and leucyl-3-epi-deoxynegamycin (3)] show a higher readthrough activity than 1 without an antimicrobial activity. Due to the structural difference between 1 and 2, namely, the lack of the 5-OH group and the opposite stereochemistry of the amino group at the 3-position in β-amino acid residue of the left side of 2, native analogue 2 probably does not induce an antimicrobial activity in the prokaryotic ribosomal system, but it is tolerated by the eukaryotic ribosomal system to elicit a readthrough activity. Consequently, specific drug efficacies of analogues 2 and 3 to the readthrough activity have been realized. These analogues appear to be preferable lead compounds to develop more potent readthrough compounds without antimicrobial activity.19
In the present study, we design and synthesize a series of derivatives for analogue 2 and perform a structure–activity relationship (SAR) study with an emphasis on the effects of (1) the side chain length of the β-amino acid residue, (2) the position of the 2-amino group on the left side, and (3) the carboxyl group esterification on the right side on the readthrough activity. One carbon atom shorter derivative 9b exhibits a higher readthrough activity than 2 and its benzyl ester-type derivatives further increase the potency by functioning as a relatively hydrophobic prodrug.
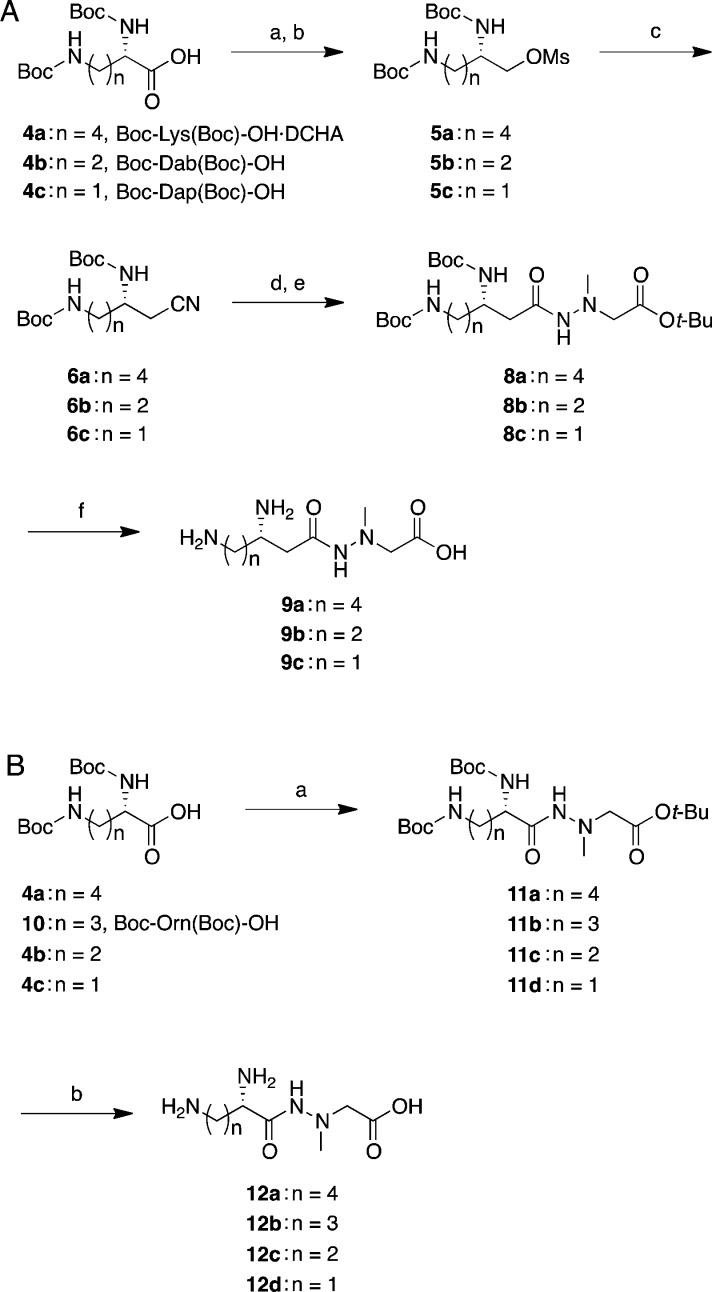
Tables 1 and 2 depict the series of new derivatives of analogue 2. The synthesis of derivatives 9a–c with modified left sides was carried out based on the previously reported synthetic procedure for 2.19 Briefly, as depicted in Scheme 1, Boc-protected amino acid derivatives 4a–c were reduced to the corresponding alcohols using NaBH4 in tetrahydrofuran (THF)/water via a mixed anhydride method. Then they were protected with a mesyl group (Ms) by treating the crude mixture with MsCl in the presence of Et3N in CH2Cl2 to obtain methanesulfonates 5a–c in 58–66% yields. Methanesulfonates 5a–c were subsequently treated with KCN in the presence of a phase transfer catalyst 18-crown-6 in N,N-dimethylformaldehyde (DMF) or acetonitrile to obtain nitriles 6a–c. Then nitriles 6a–c were hydrolyzed under the basic conditions by the addition of KOH in ethanol/water (2:1) and subsequent coupling with t-butyl 2-(1-methylhydrazinyl)acetate·p-toluenesulfonic acid (PTSA) salt 7(20) using the EDC-HOBt (EDC, 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide; HOBt, 1-hydroxybenzonitrile) method.21 Resulting hydrazides 8a–c were treated with 4 M HCl/dioxane to deprotect two N-Boc groups and the t-butyl ester. Purification by reversed-phase (RP) HPLC afforded 9a–c.
Table 1. Readthrough Activity of Derivatives 9a–c and 12a–d.
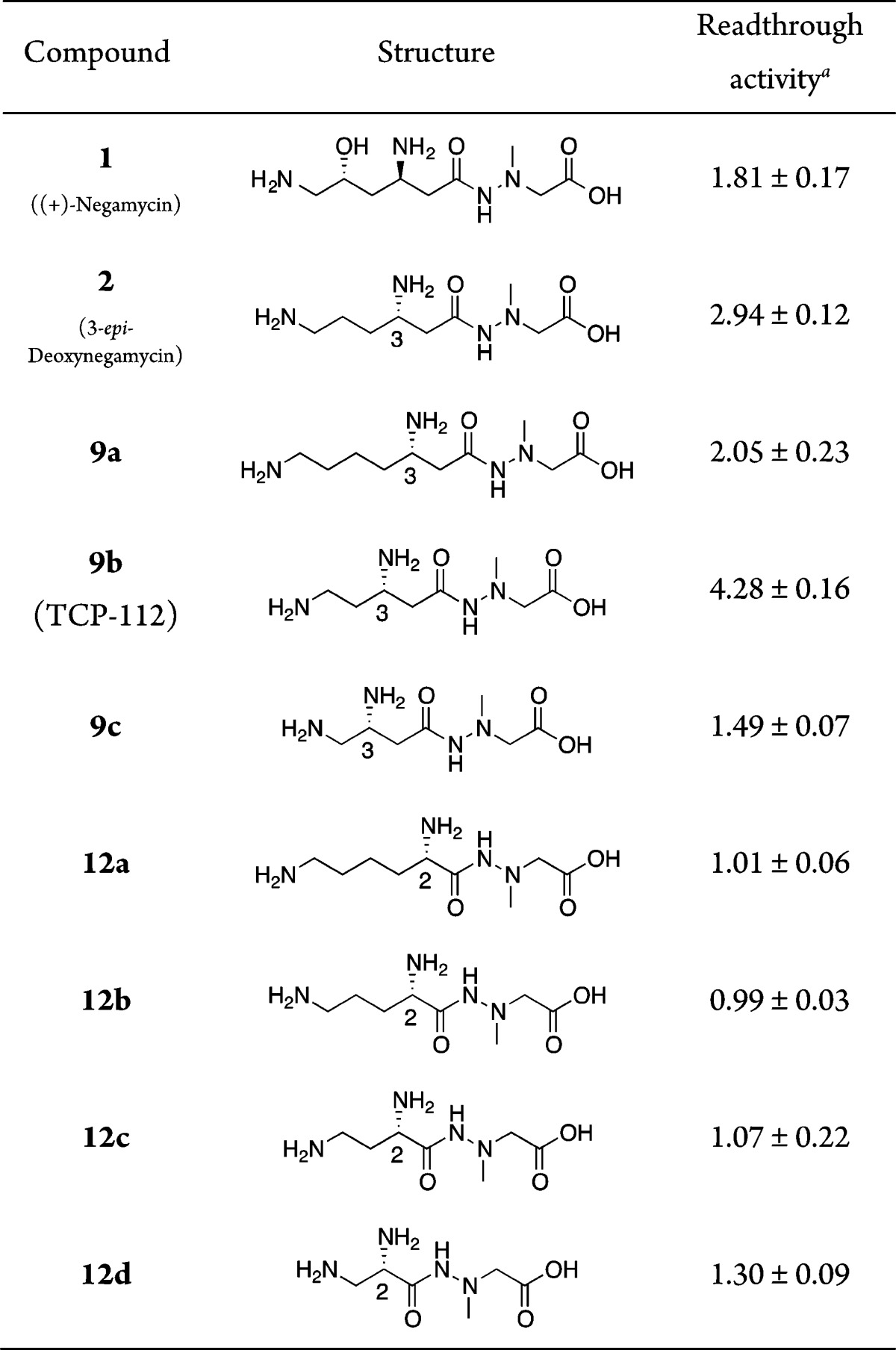
Cell-based readthrough activity (ratio) relative to control (D-MEM) in COS-7 cells; compounds are evaluated at a concentration of 200 μM. Data are determined in triplicate. Values are the mean ± SD.
Table 2. Readthrough Activity of Derivatives 15a–d and 17a–l.
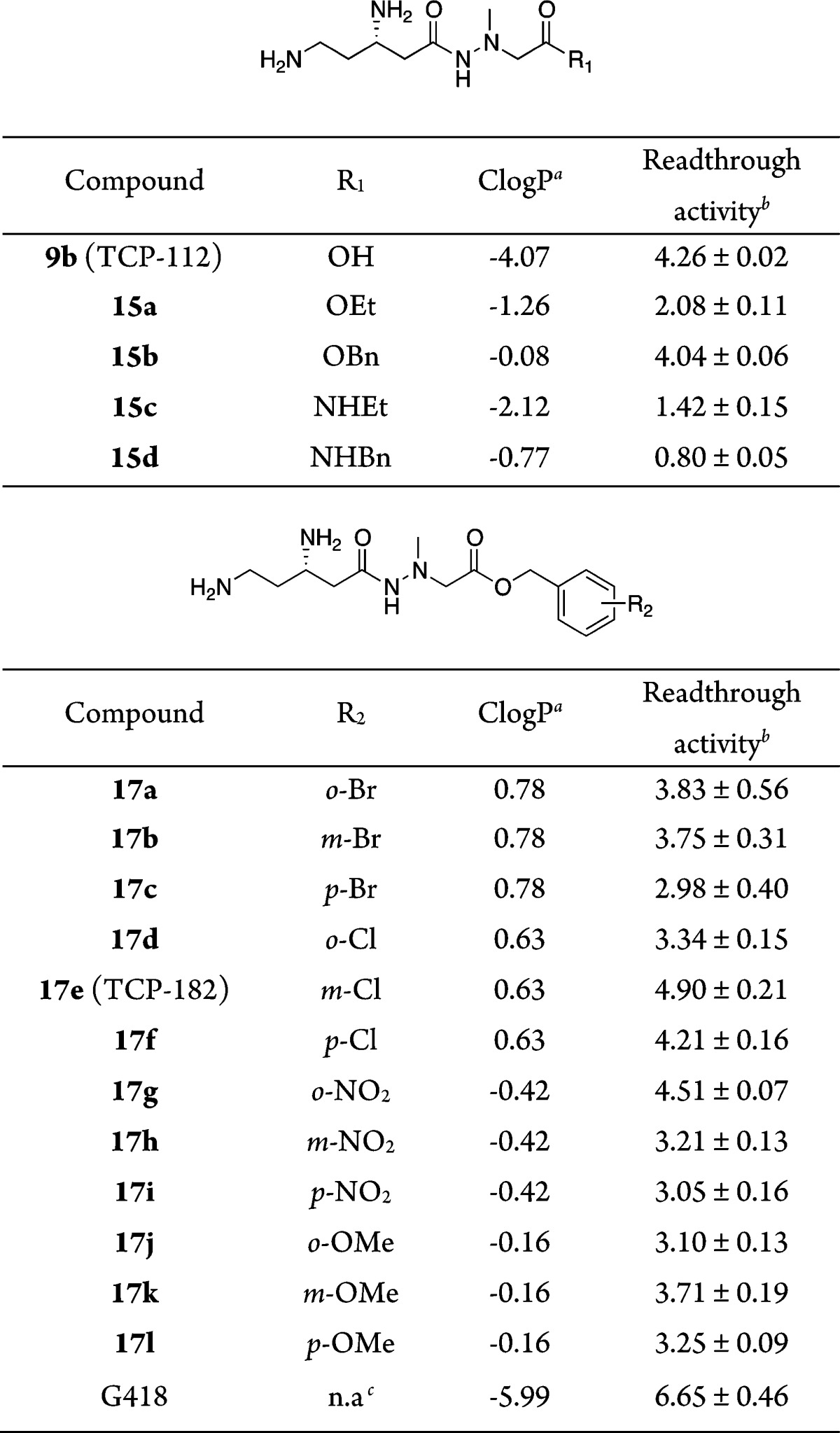
Value of the calculated log P (ClogP) determined by CS ChemBioDraw Ultra 12.0.
Cell-based readthrough activity (ratio) relative to 9b (= 4.26) in COS-7 cells;22 compounds are evaluated at a concentration of 200 μM. Data are determined in triplicate. Values are the mean ± SD.
n.a.: not applicable.
Scheme 1. Synthesis of Derivatives 9a–c and 12a–d.
Reagents and conditions: (A) Synthesis of 9a–c: (a) isobutylchloroformate, N-methylmorpholine, THF, −15 °C, 10 min, then, NaBH4, THF/H2O, −15 °C, 10 min; (b) MsCl, Et3N, CH2Cl2, rt, overnight, 58–66%; (c) KCN, 18-crown-6, DMF, 100 °C, 2 h, 51% (for 6a) or acetonitrile, 40 °C, overnight (for 6b) or acetonitrile, reflux, 3 h (for 6c); (d) KOH, EtOH/H2O, 80 °C, 45 min–overnight; (e) PTSA·H2N–N(Me)–CH2CO2t-Bu 7, HOBt·H2O, Et3N, EDC·HCl, DMF, rt, overnight, 38–39% (2 steps); (f) 4 M HCl/dioxane, rt, 1 h, then, RP-HPLC, 52–69%. (B) Synthesis of 12a–d: (a) 7, HOBt·H2O, Et3N, EDC·HCl, DMF, rt, overnight, 62%–quant.; (b) 4 M HCl/dioxane, rt, 1 h, then, RP-HPLC, 22–69%.
To prepare 12a–d, which are α-amino acid derivatives on the left side of the molecule, a series of hydrazides 11a–d were synthesized by coupling Boc-protected amino acid derivative 4a–c or 10 and hydrazine unit 7 using the same coupling procedure as shown above. Then the hydrazides 11a–d were deprotected with 4 M HCl/dioxane and purified by RP-HPLC to afford desired derivatives 12a–d.
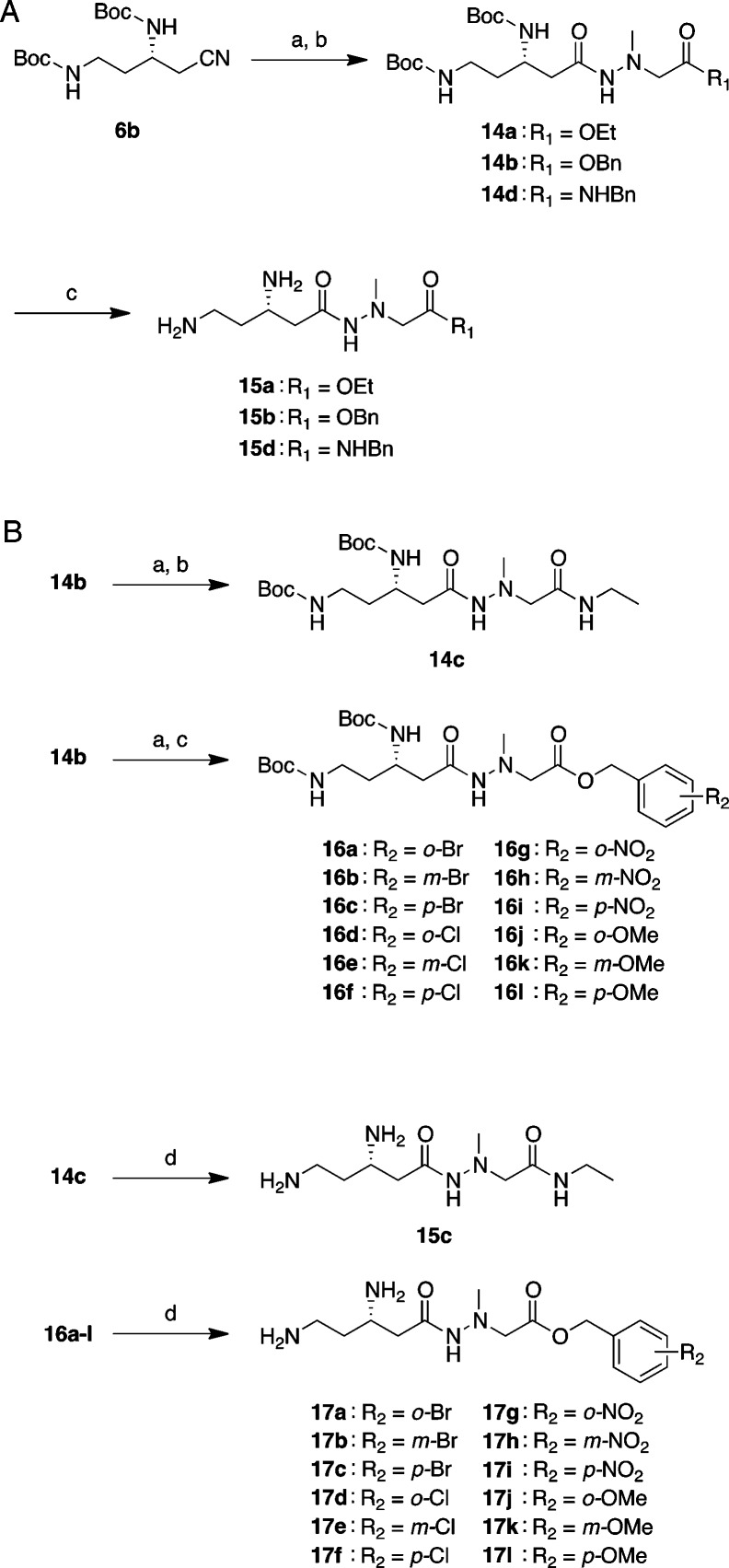
Ester or amide derivatives 15a,b and 15d at the carboxyl-terminal (15a, ethyl ester; 15b, benzyl ester; 15d, benzyl amide) were synthesized from intermediate 6b (Scheme 2A). Nitrile 6b was hydrolyzed by treatment with KOH in ethanol/water (2:1) followed by coupling with hydrazine unit 13a–c using the EDC-HOBt method to obtain corresponding hydrazides 14a,b and 14d. Finally, the deprotection of the Boc groups with 4 M HCl/dioxane and purification by RP-HPLC gave desired ester or amide derivatives 15a,b and 15d in 36–50% yields. Moreover, the ethyl amide derivative 15c and a series of substituted-benzyl ester derivatives 17a–l (Tables 1 and 2 and Scheme 2B) were synthesized by replacing the benzyl ester group of 14b with ethyl amide and a substituted-benzyl group, respectively. Namely, after deprotection of the benzyl group under an atmosphere of hydrogen over Pd/C (10%) in methanol, the resulting carboxylic acid was coupled with ethylamine·HCl and a series of substituted-benzyl alcohols by the EDC-HOBt method or N,N′-dicyclohexylcarbodiimide (DCC) in the presence of 4-dimethylaminopyridine (DMAP) to obtain hydrazides 14c and 16a–l. Deprotection of the Boc groups with 4 M HCl/dioxane and purification by RP-HPLC produced the desired ethyl amide derivative 15c and substituted-benzyl ester derivatives 17a–l in 23–90% yields.
Scheme 2. Synthesis of 15a–d and 17a–l.
Reagents and conditions: (A) Synthesis of 15a–b and 15d: (a) KOH, EtOH/H2O, 80 °C, 45 min–4 h; (b) ethyl or benzyl 2-(1-methylhydrazinyl)acetate 13a,b or N-benzyl-2-(1-methylhydrazinyl)acetamide 13c, HOBt·H2O, Et3N, EDC·HCl, DMF, rt, overnight, 26–57% (2 steps); (c) 4 M HCl/dioxane, rt, 1 h, then, RP-HPLC, 36–50%. (B) Synthesis of 15c and 17a–l: (a) Pd/C, H2, MeOH, rt, 15 min; (b) ethylamine·HCl, HOBt·H2O, Et3N, EDC·HCl, DMF, rt, overnight, 70% (2 steps); (c) substituted benzyl alcohol, DMAP, DCC, rt, overnight, 23–68% (2 steps); (d) 4 M HCl/dioxane, rt, 1 h, then, RP-HPLC, 23–90%.
The readthrough activities of synthesized 3-epi-deoxynegamycin (2) derivatives were evaluated using a cell-based reporter assay involving COS-7 cells transfected with a dual-reporter plasmid encoding β-galactosidase and luciferase genes connected with a TGA-containing nucleotide sequence as a PTC, according to a previously described procedure.19 In this reporter assay system, β-galactosidase, which is positioned upstream of the PTC, was expressed constitutively. Luciferase, which is positioned downstream of the PTC, was expressed only when PTC readthrough occurred. By measuring both enzymatic activities of cell lysates, the readthrough activities of the compounds were obtained as ratios of the luciferase activity over β-galactosidase activity relative to the basal level (ratio = 1) without any compound (see the Supporting Information for a detailed description of the assay method). (+)-Negamycin (1) and G418 were used as positive controls. All compounds were evaluated at a concentration of 200 μM.
Synthesized native 3-epi-deoxynegamycin (2) shows a stronger readthrough activity toward TGA nonsense codon than (+)-negamycin (1) as previously reported (Table 1).19 Compared to native analogue 2, one carbon longer derivative 9a at the left side shows a weaker readthrough activity, whereas one carbon shorter derivative 9b interestingly exhibits a stronger readthrough activity with a ratio of 4.28. However, two carbon shorter derivative 9c displays a lower activity than (+)-negamycin (1). These results suggest that the appropriate configuration of the amino group in the side chain of the β-amino acid residue on the left side of 2 is important for the potent activity. These modifications led to the discovery of a new negamycin derivative with a more potent readthrough activity than 1 and 2.
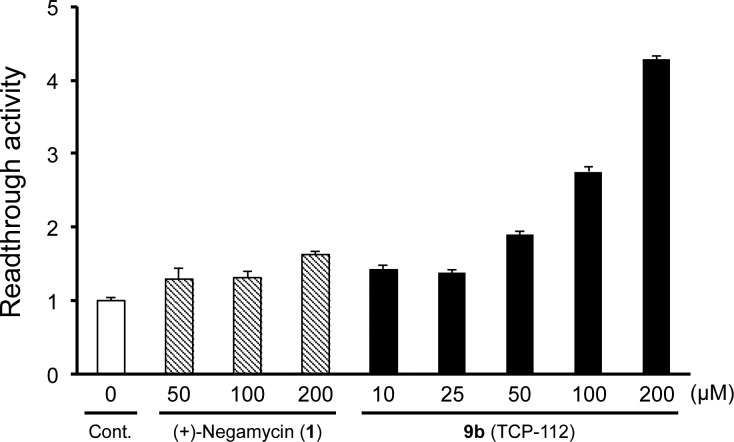
To determine the effect of the amino group at the 3-position of the left side, we synthesized a series of 2-amino derivatives with different chain lengths. The readthrough activity of these α-amino acid derivatives (12a–d) is lost (Table 1), suggesting that the configuration at the 3-amino group is an important factor for activity. Furthermore, the readthrough activity of potent derivative 9b is dose dependent between 25–200 μM (Figure 2). However, 9b does not show antimicrobial activity (MIC) against Gram-positive and Gram-negative microorganisms (Staphylococcus aureus NBRC13276, MIC = 1024 μg/mL; and Escherichia coli NBRC3972, MIC ≥ 1024 μg/mL), indicating that 9b maintains a similar pharmacological selectivity as native analogue 2.19
Figure 2.
Cell-based readthrough activity (TGA) of 9b in COS-7 cells. Error bars indicate SD (n = 3).
In general, the carboxylic acid of negamycins seems to be important for the biological activity because all known natural analogues of (+)-negamycin have an unmodified carboxylic acid. However, the hydrophilic nature of 1 (calculated log P (ClogP) = −4.29) or its derivatives does not seem to increase the cell penetration ability. Therefore, we then focused on the carboxylic acid in the right side. Four types of ester and amide derivatives 15a–d were synthesized based on the structure of derivative 9b (ClogP = −4.07). Ethyl ester 15a (ClogP = −1.26) interestingly shows a moderate but significant readthrough activity22 (Table 2). More hydrophobic benzyl ester 15b (ClogP = −0.08) maintains a similar potency to 9b. However, amide derivative 15c having lower ClogP value (ClogP = −2.12) and 15d having a similar ClogP value (ClogP = −0.77) to 15a do not show any readthrough activity. The observed difference in activity between ester and amide derivatives with the same kind of alkyl group suggests that the ester-type derivatives may act as prodrugs, which are converted into corresponding carboxylic acid derivative 9b in the cellular assay.
Because the benzyl ester shows a potent activity, we then synthesized a series of substituted-benzyl ester derivatives with either electron withdrawing halogens and a nitro group or an electron donating methoxy group. In addition, we determined the ClogP value as a parameter for hydrophobicity, which is an important factor when estimating the cell membrane permeability. Compared to benzyl ester 15b, substituted benzyl derivatives with halogen atoms increase the ClogP value, while substitution of nitro and methoxy groups do not. However, the observed readthrough activity is not related to the ClogP values. Most derivatives in this modified series have lower activities than benzyl ester 15b (4.04). Only electron withdrawing m- or p-Cl and o-NO2 derivatives (17e–g) show a more potent activity than 15b with values of 4.90, 4.21, and 4.51, respectively. Although this value is still lower than that of G418 (6.65, TGA), which is an aminoglycoside with the most potent readthrough activity among known native products,23,24 it is considerably improved.
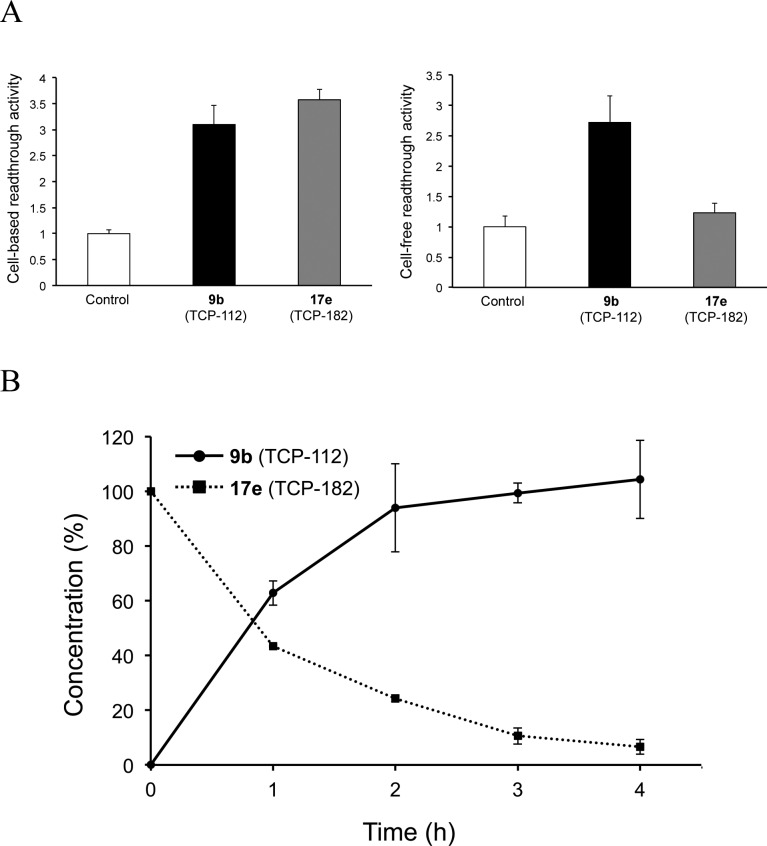
Because derivative 17e exhibits both potency and hydrophobicity, we examined whether it could serve as a prodrug. A cell-free readthrough assay (TGA) system consisting of a cell-free protein expression system with a human cell line-derived lysates (Human Cell-Free Protein Expression System, Takara Bio Inc. Japan) was established.19 This in vitro system can directly evaluate the readthrough activity of the compounds without the influence of cell penetration and metabolism of the compounds. Derivative 9b displays a potent cell-free readthrough activity (2.72) against nonsense codon TGA (20 μM) along with the cell-based assay (3.10) (Figure 3A). However, cell-free readthrough activity of the m-Cl benzyl ester 17e is drastically decreased (1.23) compared to the cell-based assay (3.58), clearly indicating that 17e acts as a prodrug, which is hydrolyzed to 9b outside or inside of the cells in the cell-based assay. The slightly higher activity of 17e compared to 9b in the cell-based assay may be due to the slight increase in the cell penetration ability. These results imply that the prodrug strategy at the carboxylic acid part should effectively contribute to the readthrough activity.
Figure 3.
(A) Readthrough activity in cell-based and cell-free assays. Readthrough activity (ratio) of the compound against that of control (= 1) is shown. Cell, COS-7; compound concentration, 200 μM (cell-based assay, n = 3) or 20 μM (cell-free assay, n = 3). Error bars indicate ± SD. (B) Hydrolysis of 17e by porcine liver esterase. Compound concentration (%) is determined by RP-HPLC. Error bars indicate ± SD (n = 3).
To confirm whether 17e acts as a prodrug, it was treated with porcine liver esterase, a carboxylic-ester hydrolase (110 μunits of enzyme/mL), in a 100 mM phosphate buffer (pH 7.4) at 37 °C. The enzymatic stability was analyzed by RP-HPLC, and a new metabolite peak was collected and identified by high-resolution mass spectrometry. We detected the time-dependent production of 9b along with the degradation of 17e (Figure 3B). Additionally, we confirm that derivative 17e is stable in an environment of 100 mM phosphate buffer (pH 7.4) without esterase at 37 °C (94% remaining after a 6 h incubation). These results suggest that substituted benzyl ester derivatives function as a prodrug to produce parent drug 9b in the living cell system.
In conclusion, to develop a promising readthrough drug candidate, we performed a SAR study of 3-epi-deoxynegamycin (2) and discovered more potent derivatives 9b (designated as TCP-112) and its benzyl ester-type prodrugs. Particularly, the in vitro studies, including the cell-free readthrough assay and degradation with esterase, demonstrate that m-Cl benzyl ester 17e (designated as TCP-182), which has an increased hydrophobicity, functions as a prodrug of 9b in the living cell system. These new derivatives should be promising therapeutic candidates for nonsense mutation-mediated genetic diseases like DMD. Currently additional SAR studies are underway to develop more efficient derivatives.
Acknowledgments
The authors thank Mr. Shuntaro Ikezawa, Tokyo University of Pharmacy and Life Sciences for the technical assistance.
Supporting Information Available
Synthesis procedures, characterization of derivatives, biological assay protocols, and NMR data. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00121.
This work was supported by the Japan Society for the Promotion of Science (JSPS), KAKENHI including a Grant-in-Aid for Scientific Research (B) 23390029, a Grant-in-Aid for Young Scientists (B) 25860092, a Grant-in-Aid for Research Activity Start-up 25893258, and a Grant-in-Aid for Scientific Research on Innovative Areas “Chemical Biology of Natural Products” from The Ministry of Education, Culture, Sports, Science and Technology, and Platform for Drug Discovery, Informatics and Structural Life Science, and Intramural Research Grant (26-8) for Neurological and Psychiatric Disorders of NCNP.
The authors declare no competing financial interest.
Supplementary Material
References
- Keeling K. M.; Bedwell D. M. Pharmacological suppression of premature stop mutations that cause genetic diseases. Curr. Pharmacogenomics 2005, 3, 259–269. [Google Scholar]
- Bakker E.; Veenema H.; Den Dunnen J. T.; van Broeckhoven C.; Grootscholten P. M.; Bonten E. J.; van Ommen G. J.; Pearson P. L. Germinal mosaicism increases the recurrence risk for new duchenne muscular dystrophy mutations. J. Med. Genet. 1989, 26, 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer P. A.; Dietz H. C. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 1999, 8, 1893–1900. [DOI] [PubMed] [Google Scholar]
- Campbell K. P.; Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature 1989, 338, 259–262. [DOI] [PubMed] [Google Scholar]
- Mendell J. R.; Moxley R. T.; Griggs R. C.; Brooke M. H.; Fenichel G. M.; Miller J. P.; King W.; Signore L.; Pandya S.; Florence J.; Schierbecker J.; Robison J.; Kaiser K.; Mandel S.; Arfken C.; Gilder B. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N. Engl. J. Med. 1989, 320, 1592–1597. [DOI] [PubMed] [Google Scholar]
- Biggar W. D.; Harris V. A.; Eliasoph L.; Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul. Disord. 2006, 16, 249–255. [DOI] [PubMed] [Google Scholar]
- Howard M.; Frizzell R. A.; Bedwell D. M. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat. Med. 1996, 2, 467–469. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Correction of genetic disease by making sense from nonsense. J. Clin. Invest. 1999, 104, 367–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellais S.; Le Goff C.; Dagoneau N.; Munnich A.; Cormier-Daire V. In vitro readthrough of termination codons by gentamycin in the Stüve–Wiedemann Syndrome. Eur. J. Hum. Genet. 2010, 18, 130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis E. R.; Cordier L.; Shoturma D. I.; Leland S. E.; Sweeney H. L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest. 1999, 104, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F.; Mogg A. E. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res. 1985, 13, 6265–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman I.; Glikin D.; Smolkin B.; Hainrichson M.; Belakhov V.; Baasov T. Repairing faulty genes by aminoglycosides: development of new derivatives of Geneticin (G418) with enhanced suppression of diseases-causing nonsense mutations. Bioorg. Med. Chem. 2010, 18, 3735–3746. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq M. P.; Tulkens P. M. Aminoglycosides: nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43, 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin T.; Cortopassi G. Proposed molecular and cellular mechanism for aminoglycoside ototoxicity. Antimicrob. Agents Chemother. 1994, 38, 2517–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch E. M.; Barton E. R.; Zhuo J.; Tomizawa Y.; Friesen W. J.; Trifillis P.; Paushkin S.; Patel M.; Trotta C. R.; Hwang S.; Wilde R. G.; Karp G.; Takasugi J.; Chen G.; Jones S.; Ren H.; Moon Y. C.; Corson D.; Turpoff A. A.; Campbell J. A.; Conn M. M.; Khan A.; Almstead N. G.; Hedrick J.; Mollin A.; Risher N.; Weetall M.; Yeh S.; Branstrom A. A.; Colacino J. M.; Babiak J.; Ju W. D.; Hirawat S.; Northcutt V. J.; Miller L. L.; Spatrick P.; He F.; Kawana M.; Feng H.; Jacobson A.; Peltz S. W.; Sweeney H. L. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007, 447, 87–91. [DOI] [PubMed] [Google Scholar]
- Hamada H.; Takeuchi T.; Kondo S.; Ikeda Y.; Naganawa H.; Maeda K.; Okami Y.; Umezawa H. A new antibiotic, negamycin. J. Antibiot. 1970, 23, 170–171. [DOI] [PubMed] [Google Scholar]
- Arakawa M.; Shiozuka M.; Nakayama Y.; Hara T.; Hamada M.; Kondo S.; Ikeda D.; Takahashi Y.; Nonomura Y.; Sheykholeslami K.; Kondo K.; Kaga K.; Kitamura T.; Suzuki-Miyagoe Y.; Takeda S.; Matsuda R. Negamycin restores dystrophin expression in skeletal and cardiac muscles of mdx mice. J. Biochem. 2003, 134, 751–758. [DOI] [PubMed] [Google Scholar]
- Kondo S.; Yoshida K.; Ikeda T.; Iinuma K.; Honma Y.; Hamada M.; Umezawa H. 3-epi-Deoxynegamycin and leucyl-3-epi-deoxynegamycin produced by Streptomyces. J. Antibiot. 1977, 30, 1137–1139. [DOI] [PubMed] [Google Scholar]
- Taguchi A.; Hamada K.; Kotake M.; Shiozuka M.; Nakaminami H.; Pillaiyar T.; Takayama K.; Yakushiji F.; Noguchi N.; Usui T.; Matsuda R.; Hayashi Y. Discovery of natural products possessing selective eukaryotic readthrough activity: 3-epi-deoxynegamycin and its leucine adduct. ChemMedChem. 2014, 9, 2233–2237. [DOI] [PubMed] [Google Scholar]
- Hayashi Y.; Regnier T.; Nishiguchi S.; Sydnes M. O.; Hashimoto D.; Hasegawa J.; Katoh T.; Kajimoto T.; Shiozuka M.; Matsuda R.; Node M.; Kiso Y. Efficient total synthesis of (+)-negamycin, a potential chemotherapeutic agent for genetic diseases. Chem. Commun. 2008, 2379–2381. [DOI] [PubMed] [Google Scholar]
- König W.; Geiger R. New method for the synthesis of peptides: activation of the carboxyl group with dicyclohexylcarbodiimide by using 1-hydroxybenzotriazoles as additives. Chem. Ber. 1970, 103, 788–798. [DOI] [PubMed] [Google Scholar]
- The readthrough activity of derivatives 15a–c, 17a–l, and G418 in Table 2 is indicated according to the standard value of 9b (4.26), which is derived from 16 independent cell-based evaluations in order to correct measurement errors among 96-well plates.
- Manuvakhova M.; Keeling K.; Bedwell D. M. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA 2000, 6, 1044–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floquet C.; Rousset J. P.; Bidou L. Readthrough of premature termination codons in the adenomatous polyposis Coli gene restores its biological activity in human cancer cells. PLoS One 2011, 6, No.e24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.