Case Report
Patient 1.
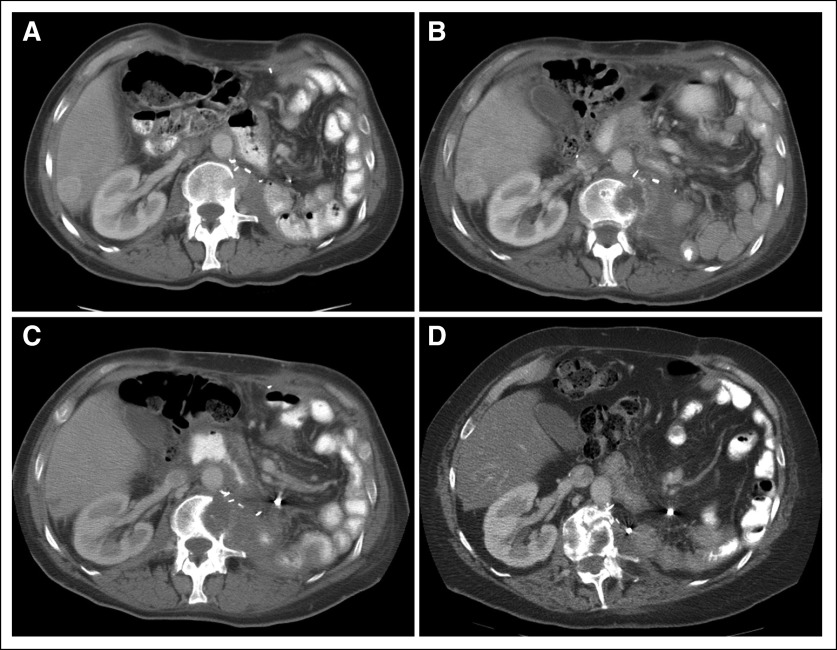
A 56-year-old man presented with weight loss and hematuria. Evaluation revealed a large left renal mass with extensive retroperitoneal lymphadenopathy and caval tumor thrombus. After two cycles of high-dose bolus interleukin-2 (IL-2), he underwent total resection of his abdominal disease. Pathology revealed a 15-cm conventional (clear cell) renal cell carcinoma (RCC)—Fuhrman nuclear grade 4—with involvement of the renal vein and left adrenal gland and intrahepatic caval tumor thrombus. Follow-up computed tomography scans showed tumor recurrence in the cava, liver, and second lumbar vertebral body (Fig 1A). He was enrolled onto a phase II study of sorafenib (BAY 43-9006; Bayer Pharmaceuticals, Wayne, NJ) 400 mg orally twice daily. After 9 weeks of therapy, imaging studies showed progression of liver metastases (Fig 1B); he also required palliative radiation to the lumbar spine. He was then enrolled onto a phase II study of gemcitabine plus capecitabine and received gemcitabine 1,000 mg/m2 over 30 minutes on days 1, 8, and 15 and capecitabine 830 mg/m2 twice per day for 21 days of a 28-day cycle. After six cycles of therapy, he achieved a partial response in the liver with resolution of the caval thrombus and return to full physical function (Fig 1C). Therapy with gemcitabine and capecitabine was continued for 26 cycles then stopped because of fatigue and pancytopenia. The patient has remained progression free for more than 6 years, without additional therapy (most recent staging, June 2010; Fig 1D).
Fig 1.
Patient 2.
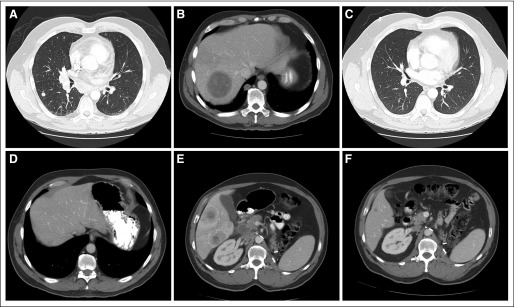
A 49-year-old man presented with gross hematuria and was found to have a left renal mass and lung nodules. The patient underwent cytoreductive left radical nephrectomy, with retroperitoneal lymph node dissection. Pathology demonstrated a conventional (clear cell) RCC—Fuhrman nuclear grade 4—with extensive necrosis, extending to the perinephric adipose tissue and renal vein, and metastasis to one retroperitoneal lymph node. He received two cycles of high-dose bolus IL-2, with disease progression in the lungs and mediastinum. He also developed a 5-cm metastatic lesion in segment VII of the liver. He then received bevacizumab 10 mg/kg intravenously every 2 weeks plus erlotinib 150 mg orally daily. Two months later, imaging studies revealed marked disease progression, especially in the liver (Figs 2A, 2B), and hypercalcemia. Chemotherapy was then administered with gemcitabine 1,200 mg/m2 intravenously over 2 hours biweekly plus capecitabine 1,750 mg/m2 divided twice daily for 21 days of a 28-day cycle. He achieved partial response after 2 months of treatment and complete response (CR) after 16 months of treatment. Three months after documentation of CR, chemotherapy was discontinued. Figures 2C and 2D show continued CR in the lung, mediastinum, and liver, off therapy. Twenty-one months after stopping all therapy, the patient developed recurrent disease in the mediastinum, bilateral hilar, and retroperitoneal lymph nodes. Sunitinib was administered 50 mg daily on a 4-week on, 2-week off, schedule. After two cycles, computed tomography scans of the chest, abdomen, and pelvis showed progressive intrathoracic lymph node metastases and new liver metastases (Fig 2E). Chemotherapy with gemcitabine and capecitabine was restarted. After two cycles, imaging studies showed improvement of metastatic disease in all sites. At last follow-up, after 10 months of this chemotherapy regimen, he achieved a near CR (Fig 2F). The patient is currently physically active and continues to receive gemcitabine and capecitabine at reduced doses because of grade 2 hand-foot syndrome.
Fig 2.
Discussion
Targeted therapy is the current standard of care for the systemic treatment of metastatic RCC. The multitargeted tyrosine kinase inhibitors sunitinib and pazopanib as well as the combination of bevacizumab plus interferon alfa are the accepted first-line therapies for patients with metastatic clear cell RCC at good to intermediate risk.1–4 The mammalian target of rapamycin (mTOR) inhibitor temsirolimus is the accepted first-line therapy for patients with advanced poor-risk RCC.5 Sorafenib, sunitinib, and pazopanib are accepted second-line therapies after cytokine failure; the mTOR inhibitor everolimus is the approved treatment after sorafenib and/or sunitinib failure.6,7 Approximately 20% of patients with metastatic RCC have primary resistance to anti–vascular endothelial growth factor (VEGF) therapies. Furthermore, there is no established third-line therapy for patients who develop progressive disease after VEGF- and mTOR-targeted therapies or for patients who are intolerant of these targeted agents. Metastatic RCC has traditionally been considered unresponsive to cytotoxic chemotherapy.8 The combination of gemcitabine and capecitabine has been studied in several case series and four single-arm phase II trials in patients with metastatic RCC refractory to immunotherapy and, to a lesser extent, refractory to targeted therapy. Response rates have ranged from 8.4% to 16%, stable disease rates from 48% to 67%, and median progression-free survival from 4.6 to 7.6 months.9–12
We report two patients with metastatic RCC who achieved durable remissions after treatment with the chemotherapy regimen of gemcitabine and capecitabine after failure of targeted therapies. One patient has been in remission more than 6 years since rapid disease progression while receiving high-dose IL-2 and sorafenib. The second patient demonstrated a second tumor response on rechallenge with this chemotherapy regimen after rapid disease progression while receiving two prior anti-VEGF therapies (bevacizumab and sunitinib). The mechanisms of resistance of RCC to anti-VEGF agents and mTOR inhibitors are poorly understood. Clinicopathologic studies have demonstrated that higher tissue levels of phosphorylated Akt in nephrectomy specimens from patients with metastatic RCC subsequently treated with sorafenib are associated with shorter progression-free survival.13 A study evaluating nephrectomy specimens from patients with metastatic RCC who received bevacizumab-based therapy before nephrectomy demonstrated that critical components of the PI3-kinase pathway are upregulated in individuals with shorter progression-free survival.14 Although these data do not prove a direct mechanistic association between PI3-kinase pathway upregulation and poor clinical outcome in patients treated with antiangiogenic agents, they provide hypothesis-generating studies for testing.
Gemcitabine (2′,2′-difluorodeoxycytidine, LY188011, dFdCyd) is a pyrimidine antimetabolite. Cell-cycle kinetic studies with gemcitabine have shown specificity for proliferation in the S phase of the cell cycle, with no effect on progress through the early G1, G2, or M phase.15 We postulate that c-Myc activation may provide a specific vulnerability to this class of agents by driving cells to accumulate higher levels of abnormal DNA in the presence of gemcitabine, generating in effect a synthetic lethal phenotype. Evan et al16 reviewed mechanisms by which increased DNA damage in conjunction with c-Myc activation could lead to susceptibilities to DNA-damaging processes. In patients with rapidly progressing disease with high cell-cycle activity, RCC tumors may be more susceptible to cytotoxic chemotherapy. A DNA analog like gemcitabine, in the background of the pre-existing gross chromosomal abnormalities seen in RCC, may be sufficient to drive a c-Myc–dominant tumor—the DNA repair mechanisms of which are down-regulated—into apoptosis.17,18 The combination of gemcitabine and capecitabine is an effective and well-tolerated regimen, with the potential for achieving durable remissions in metastatic RCC. We believe this regimen should be evaluated in the treatment paradigm of metastatic RCC refractory to targeted therapies.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 9.Waters JS, Moss C, Pyle L, et al. Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with metastatic renal carcinoma. Br J Cancer. 2004;91:1763–1768. doi: 10.1038/sj.bjc.6602209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadler WM, Halabi S, Rini B, et al. A phase II study of gemcitabine and capecitabine in metastatic renal cancer: A report of Cancer and Leukemia Group B protocol 90008. Cancer. 2006;107:1273–1279. doi: 10.1002/cncr.22117. [DOI] [PubMed] [Google Scholar]
- 11.Tannir NM, Thall PF, Ng CS, et al. A phase II trial of gemcitabine plus capecitabine for metastatic renal cell cancer previously treated with immunotherapy and targeted agents. J Urol. 2008;180:867–872. doi: 10.1016/j.juro.2008.05.017. discussion 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Veldhuizen PJ, Hussey M, Lara PN, Jr, et al. A phase II study of gemcitabine and capecitabine in patients with advanced renal cell cancer: Southwest Oncology Group Study S0312. Am J Clin Oncol. 2009;32:453–459. doi: 10.1097/COC.0b013e3181925176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonasch E, Corn P, Pagliaro LC, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma: Clinical and biomarker analysis. Cancer. 2010;116:57–65. doi: 10.1002/cncr.24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsavachidou-Fenner D, Tannir N, Tamboli P, et al. Gene and protein expression markers of response to combined antiangiogenic and epidermal growth factor targeted therapy in renal cell carcinoma. Ann Oncol. 2010;21:1599–1606. doi: 10.1093/annonc/mdp600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertel LW, Boder GB, Kroin JS, et al. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) Cancer Res. 1990;50:4417–4422. [PubMed] [Google Scholar]
- 16.Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 17.Monzon FA, Alvarez K, Gatalica Z, et al. Detection of chromosomal aberrations in renal tumors: A comparative study of conventional cytogenetics and virtual karyotyping with single-nucleotide polymorphism microarrays. Arch Pathol Lab Med. 2009;133:1917–1922. doi: 10.5858/133.12.1917. [DOI] [PubMed] [Google Scholar]
- 18.Monzon FA, Hagenkord JM, Lyons-Weiler MA, et al. Whole genome SNP arrays as a potential diagnostic tool for the detection of characteristic chromosomal aberrations in renal epithelial tumors. Mod Pathol. 2008;21:599–608. doi: 10.1038/modpathol.2008.20. [DOI] [PubMed] [Google Scholar]