Abstract
Cytochrome P450 2D6 (CYP2D6) is a major drug-metabolizing enzyme responsible for eliminating approximately 20% of marketed drugs. Studies have shown that differential transcriptional regulation of CYP2D6 may contribute to large interindividual variability in CYP2D6-mediated drug metabolism. However, the factors governing CYP2D6 transcription are largely unknown. We previously demonstrated small heterodimer partner (SHP) as a novel transcriptional repressor of CYP2D6 expression. SHP is a representative target gene of the farnesoid X receptor (FXR). The objective of this study is to investigate whether an agonist of FXR, 3-(2,6-dichlorophenyl)-4-(3′-carboxy-2-chlorostilben-4-yl)oxymethyl-5-isopropylisoxazole (GW4064), alters CYP2D6 expression and activity. In CYP2D6-humanized transgenic mice, GW4064 decreased hepatic CYP2D6 expression and activity (by 2-fold) while increasing SHP expression (by 2-fold) and SHP recruitment to the CYP2D6 promoter. CYP2D6 repression by GW4064 was abrogated in Shp(−/−);CYP2D6 mice, indicating a critical role of SHP in CYP2D6 regulation by GW4064. Also, GW4064 decreased CYP2D6 expression (by 2-fold) in primary human hepatocytes, suggesting that the results obtained in CYP2D6-humanized transgenic mice can be translated to humans. This proof of concept study provides evidence for CYP2D6 regulation by an inducer of SHP expression, namely, the FXR agonist GW4064.
Introduction
Cytochrome P450 2D6 (CYP2D6) is a major drug-metabolizing enzyme responsible for eliminating approximately 20% of clinically used medications. CYP2D6-mediated drug metabolism is known to exhibit large interindividual variability (Sachse et al., 1997; Zanger et al., 2001), in which the population is divided into four phenotype categories ranging from poor metabolizer (PM) to ultrarapid metabolizer (Hou et al., 1991; Dahl et al., 1992; Sachse et al., 1997; Zanger et al., 2001; Bertilsson et al., 2002). This interindividual variability is in part explained by genetic polymorphisms in the CYP2D6 gene. For example, polymorphisms associated with low or minimal expression of CYP2D6 protein (e.g., due to frame-shift mutation) or the expression of nonfunctional CYP2D6 proteins lead to the PM phenotype. On the other hand, individuals with multiple copies of the CYP2D6 gene present the ultrarapid metabolizer phenotype. Of note, these individuals comprise only a small portion (∼10%) of the population (Hou et al., 1991; Dahl et al., 1992; Sachse et al., 1997; Zanger et al., 2001; Bertilsson et al., 2002). Of note, in a non-PM population, urinary metabolic ratios of dextrorphan/dextromethorphan exhibit significant overlaps among individuals of different functional gene doses for CYP2D6 (Gaedigk et al., 2008), suggesting that CYP2D6 genotypes alone do not fully explain the large interindividual variability in CYP2D6 activity. The sources of CYP2D6 variability in the population remain unclear.
Previous studies have shown that the mRNA expression and activity levels of CYP2D6 are well correlated with each other (Carcillo et al., 2003; Temesvari et al., 2012), and the correlation coefficient ranges from 0.85 to 0.91. Such a high correlation between mRNA and enzyme activity levels was also observed for CYP3A4 (Temesvari et al., 2012), whose activity level is known to be governed by the transcriptional regulation of the gene. These results suggest that differential transcriptional regulation of CYP2D6 may contribute to the large interindividual variability in CYP2D6 activity. However, factors controlling transcriptional regulation of CYP2D6 expression remain poorly understood. We have recently demonstrated that small heterodimer partner (SHP) suppresses hepatocyte nuclear receptor 4α (HNF4α)-mediated transactivation of the CYP2D6 promoter and thus represses hepatic CYP2D6 expression (Koh et al., 2014a). Also, knockdown of SHP expression (by using small interfering RNA) in CYP2D6-humanized transgenic (Tg-CYP2D6) mice led to enhanced hepatic CYP2D6 expression (Koh et al., 2014a). What remains unknown is whether modulators of SHP expression alter hepatic CYP2D6 expression such that the large interindividual variability in CYP2D6 activity may be explained by differential SHP expression and/or activity.
SHP is a representative target gene of the farnesoid X receptor (FXR), a bile acid sensor (Parks et al., 1999). When hepatic concentrations of bile acids are high (e.g., in cholestasis), the ligand-activated FXR transactivates the SHP promoter (Goodwin et al., 2000). SHP in turn suppresses the expression of genes involved in bile acid synthesis and uptake in the liver (Wang et al., 2002; Ellis et al., 2003; Nishimaki-Mogami et al., 2004; Miao et al., 2009; Li and Chiang, 2014), protecting the liver from the toxicity of excess bile acids. The role of SHP (and FXR) in bile acid homeostasis has been extensively characterized by using selective agonists of FXR, such as GW4064 (Maloney et al., 2000). For example, in rats and human hepatocytes, GW4064 increased SHP expression and thus led to decreased expression of SHP target genes involved in bile acid homeostasis (e.g., CYP7A1 and CYP8B1) (Liu et al., 2003). In the present study, we aimed to verify the role of SHP in the regulation of CYP2D6 expression by examining how a known regulator of SHP expression, GW4064, alters CYP2D6 expression. We provide evidence that GW4064 represses CYP2D6 expression in an SHP-dependent manner.
Materials and Methods
Animals.
CYP2D6-humanized transgenic (Tg-CYP2D6) and Shp-null mice were previously described (Corchero et al., 2001; Park et al., 2011). Tg-CYP2D6 mice harbor the CYP2D6 gene along with its ∼2.5 kilobase promoter region in the mouse genome (Corchero et al., 2001). Both Tg-CYP2D6 and Shp-null mice were on the C57BL/6 background. Adult male mice (8 weeks of age; 20–25 g body weight) were used for the experiments. GW4064 (10 mg/kg) or vehicle (olive oil) was injected intraperitoneally in mice daily for 5 days (n = 4–5 per group). Mice were sacrificed on the sixth day, and liver tissues were collected. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
Chemicals and Reagents.
Debrisoquine, 4-hydroxydebrisoquin, and paraxanthine were purchased from Biomol (Plymouth Meeting, PA). GW4064, [3-(2-[2-chloro-4-([3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methoxy)phenyl]ethenyl)benzoic acid] was purchased from Sigma-Aldrich (St. Louis, MO).
Primary Human Hepatocytes.
Freshly isolated human hepatocytes, derived from three donors, were obtained from the Liver Tissue Cell Distribution System (Pittsburgh, PA; funded by National Institutes of Health Contract #HHSN276201200017C). Briefly, hepatocytes were shipped overnight in cold preservation media. Upon receipt, the media was replaced with serum-free Williams’ E media (without phenol red) containing 0.1 μM dexamethasone, 10 μg/ml gentamicin, 15 mM HEPES, 2 mM L-glutamine, and 1% insulin-transferrin-sodium selenite media supplement. Cells were allowed to recover from shipping for 10 hours at 37°C in an atmosphere containing 5% CO2. After recovery, the hepatocytes were treated with vehicle control (dimethylsulfoxide) or GW4064 (1 μM) for 48 hours. Cell lysates were collected to prepare RNAs and S9 fractions.
Western Blot.
Western blot was performed as described previously (Koh et al., 2014a). CYP2D6 and SHP protein expression levels were determined by using the respective antibodies (CYP2D6, catalog #458246, BD Gentest, Franklin Lakes, NJ; SHP, sc-30169, Santa Cruz Biotechnology, Dallas, TX).
Determination of CYP2D6 Activity.
Hepatic S9 fractions were prepared as described previously (Felmlee et al., 2008; Koh et al., 2014a). S9 fractions were incubated with debrisoquine (a CYP2D6 probe substrate) based on the report that mouse endogenous CYP2Ds play minor roles in debrisoquine hydroxylation (Koh et al., 2014a). The concentration of 4-hydroxydebrisoquine was measured by liquid chromatography–tandem mass spectrometry using paraxanthine as the internal standard (Koh et al., 2014a).
RNA Isolation and Quantitative Real Time-Polymerase Chain Reaction.
Total RNA was isolated from mouse liver tissues or primary human hepatocytes using Trizol (Life Technologies, Carlsbad, CA) and converted to cDNA using a high-capacity cDNA reverse transcription kit (Life Technologies). Using the cDNA as a template, quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the StepOnePlus real-time PCR system, with sequence-specific primers and PrimeTime probes. The PrimeTime quantitative PCR assay for mouse and human CYP8B1 genes was purchased from Integrated DNA Technologies (Mm.PT.58.12268653.g and Hs.PT.58.40608207.g, respectively; Coralville, IA). Primer sequences for all other target genes were described previously (Koh et al., 2014a). Results are expressed as fold changes by drug treatment by using the gene expression levels normalized to those of GAPDH (2–ΔΔCt method).
Chromatin Immunoprecipitation Assays.
Liver samples were subjected to chromatin immunoprecipitation (ChIP) assays as described previously (Koh et al., 2014a,b). Briefly, livers were finely minced and incubated in phosphate-buffered saline (PBS) containing 1% formaldehyde at room temperature for 15 minutes, and glycine was added to stop the cross-linking reaction. Cell pellets were resuspended in hypotonic buffer (15 mM HEPES, 60 mM KCl, 2 mM EDTA, 0.5% bovine serum albumin, 0.15 mM spermine, 0.5 mM spermidine, and 0.32 M sucrose, pH 8.0) and lysed by homogenization. Nuclei were pelleted and resuspended in nuclei lysis buffer (50 mM Tris-HCl, 2 mM EDTA, and 1% SDS, pH 8.0). The samples were sonicated to shear DNA to a length ranging from 100 to 500 base pairs. After centrifuge, the chromatin samples were immunoprecipitated using magnetic beads coated with 2-μg antibody (HNF4α, sc-6556x; RNA polymerase II, sc-899x; SHP, sc-30169, Santa Cruz) or immunoglobulin G (normal goat IgG, sc-2028; normal rabbit IgG, sc-2027, Santa Cruz) at 4°C overnight. The immune complex on the magnetic beads was collected and extensively washed, and the bound chromatin was eluted. Genomic DNA was purified by phenol chloroform extraction followed by Wizard SV gel and the PCR clean-up system (Promega, Madison, WI). qRT-PCR was performed using the following probes for Cyp8b1: 5′-AAGGCAGGCAAACATGGAGA-3′ (forward) and 5′- CAATGCAAAGGTTCCTGCCC-3′ (reverse). CYP2D6 probes were described previously (Koh et al., 2014a).
Statistical Analysis.
Values were reported as mean ± S.E.M. Statistical differences between the two groups were determined by using Student’s t test.
Results
FXR Agonist GW4064 Represses CYP2D6 Expression and Activity in Tg-CYP2D6 Mice.
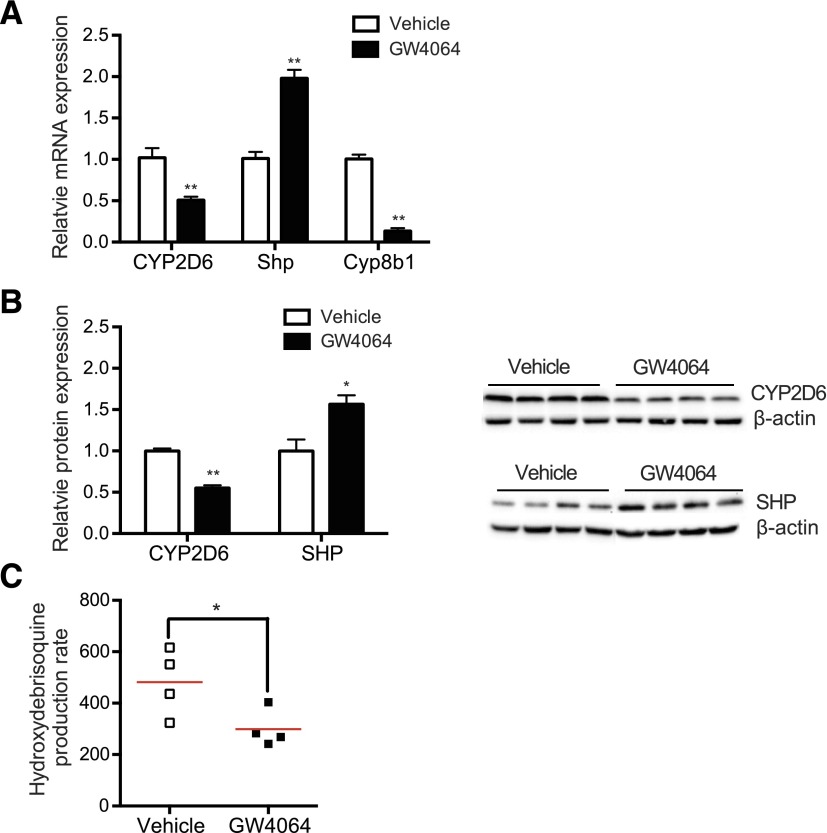
To determine whether GW4064 alters CYP2D6 expression and activity in vivo, GW4064 or vehicle control was intraperitoneally administered to Tg-CYP2D6 mice for 5 days, and hepatic CYP2D6 mRNA and protein levels were measured by qRT-PCR and Western blot, respectively. Cyp8b1, a gene known to be downregulated by SHP (Inoue et al., 2006), was used as a positive control. The results showed that GW4064 significantly decreased both mRNA and protein expression levels of CYP2D6 by ∼2-fold (Fig. 1A; Fig. 1B). CYP2D6 activity, determined by measuring the debrisoquine hydroxylation rate in the hepatic S9 fraction (Koh et al., 2014a), was also found to be decreased by ∼2-fold upon GW4064 treatment (Fig. 1C). GW4064 increased SHP expression ∼2-fold (Fig. 1A; Fig. 1B), which is consistent with previous results (Goodwin et al., 2000; Li et al., 2010).
Fig. 1.
GW4064 represses CYP2D6 expression in Tg-CYP2D6 mice. Tg-CYP2D6 mice were administered with GW4064 (10 mg/kg) or vehicle (olive oil) intraperitoneally daily for 5 days (n = 4 per group). (A) CYP2D6, Shp, and Cyp8b1 mRNA expression was determined by using qRT-PCR. (B) CYP2D6 and SHP protein expression levels were determined by Western blot. The image of the Western blot (right) and the quantified band intensities (after normalization by β-actin) (left) are shown. (C) S9 fractions were prepared from the liver tissues of Tg-CYP2D6 mice treated with GW4064 or vehicle control, and CYP2D6 phenotyping was performed using debrisoquine (200 μM). Data shown are 4-hydroxydebrisoquin production rates (in pmol/min per mg protein). Values are presented as mean ± S.E.M. (n = 4). *P < 0.05 and **P < 0.01 versus vehicle treatment.
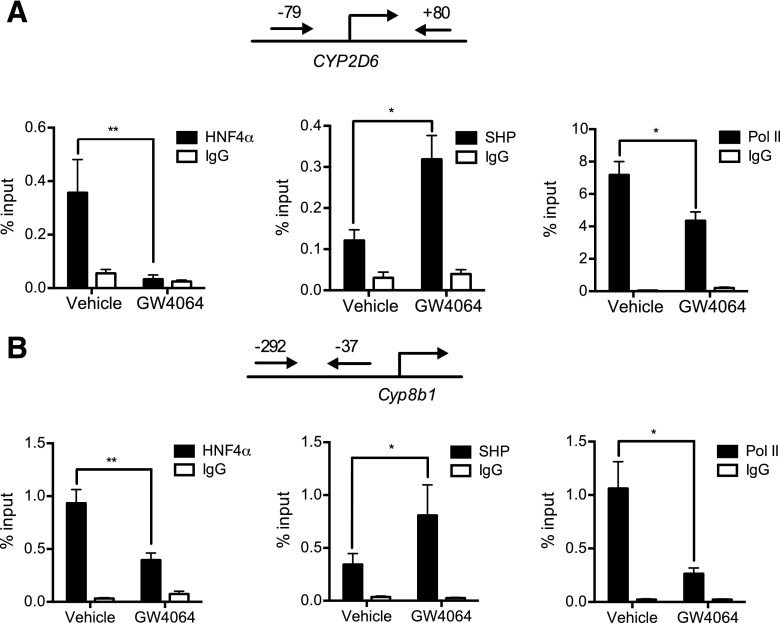
We previously showed that SHP represses HNF4α transactivation of the CYP2D6 promoter (Koh et al., 2014a). To determine whether GW4064 alters CYP2D6 promoter activity in mice, ChIP assays were performed using mouse liver tissues. Liver tissues were collected from GW4064 (or vehicle)-treated mice and subjected to ChIP using antibodies against SHP, HNF4α, or RNA polymerase II (Pol II) (a marker of transcription initiation). The protein-bound DNA was analyzed by using a primer set that can detect the HNF4α response element at −55/−43 of CYP2D6 (Cairns et al., 1996). As a positive and negative control, recruitment of the transcription factors to the Cyp8b1 promoter or a downstream region of CYP2D6 (+3913/+4368), respectively, was examined. The results demonstrated increased recruitment of SHP and decreased recruitment of HNF4α and Pol II to the CYP2D6 promoter (Fig. 2A). Similar trends were observed for SHP, HNF4α, and Pol II recruitment to the Cyp8b1 promoter region [which harbors the HNF4α response element (Inoue et al., 2006)] (Fig. 2B). Recruitment of the transcription factors to the downstream region of CYP2D6 was minimal and not affected by GW4064 (data not shown). Together, these results suggest that the repressive effect of GW4064 on CYP2D6 is potentially mediated by enhanced SHP expression.
Fig. 2.
GW4064 represses HNF4α transactivation of the CYP2D6 promoter. Recruitment of HNF4α, SHP, and RNA polymerase II onto (A) CYP2D6 promoter and (B) Cyp8b1 promoter were analyzed by ChIP assay in the livers of mice treated with GW4064 (10 mg/kg) or vehicle (olive oil). Values are presented as mean ± S.E.M. (n = 4). *P < 0.05 and **P < 0.01 versus vehicle treatment.
CYP2D6 Repression by GW4064 Is Abrogated in Shp(−/−);CYP2D6 Mice.
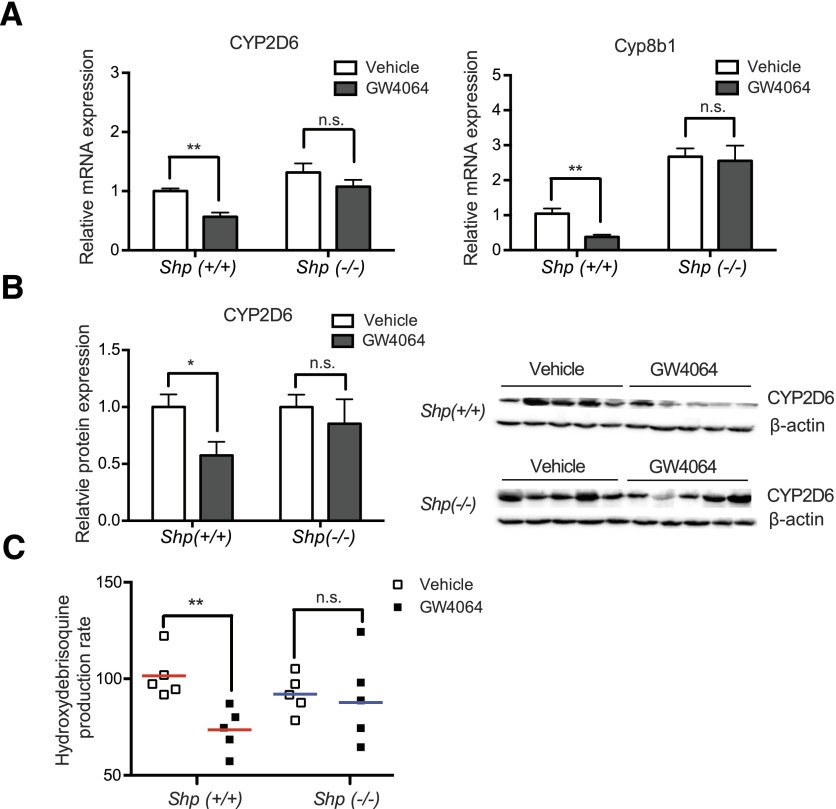
To examine the essentiality of SHP in CYP2D6 repression by GW4064, Tg-CYP2D6 mice were crossed with Shp-null mice, mice of Shp(+/+);CYP2D6 or Shp(−/−);CYP2D6 genotype were generated, and CYP2D6 repression by GW4064 or vehicle was compared between the mice of different genotypes. None of the Shp(+/+);CYP2D6 or Shp(−/−);CYP2D6 mice exhibited any prominent phenotypes, and all grew normally. Western blot results showed that SHP protein expression was abolished in Shp(−/−);CYP2D6 mice (data not shown). In the vehicle-treated mice, the basal mRNA expression levels of Cyp8b1 were higher in Shp(−/−);CYP2D6 as compared with Shp(+/+);CYP2D6 mice (Fig. 3A; P = 0.0004), whereas the basal CYP2D6 expression did not differ between the mice of different genotypes (Fig. 3A; P = 0.16). GW4064 treatment led to decreased expression of CYP2D6 and Cyp8b1 in Shp(+/+);CYP2D6 mice (Fig. 3A), which is similar to the results from Tg-CYP2D6 mice (Fig. 1A). These repressive effects of GW4064 on CYP2D6 and Cyp8b1 expression were abrogated in Shp(−/−);CYP2D6 mice (Fig. 3A), suggesting that GW4064 represses CYP2D6 transcription through SHP. The protein expression level of CYP2D6 was consistent with the decreased mRNA levels of CYP2D6 by GW4064 (Fig. 3B). Similarly, the decreased CYP2D6 activity levels (as determined by the debrisoquine hydroxylation rate) upon GW4064 treatment was abrogated in Shp(−/−);CYP2D6 mice (Fig. 3C).
Fig. 3.
CYP2D6 repression by GW4064 is abrogated in Shp(−/−);CYP2D6 mice. Shp(+/+);CYP2D6 or Shp(−/−);CYP2D6 mice were injected with GW4064 (10 mg/kg) or vehicle (olive oil) intraperitoneally daily for 5 days (n = 5 per group). (A) CYP2D6 and Cyp8b1 mRNA expression levels were measured by using qRT-PCR and normalized by those in vehicle-treated Shp(+/+);CYP2D6 mice. (B) CYP2D6 protein expression level was measured by Western blot. The image of Western blot (right) and the quantified band intensities (CYP2D6/β-actin) (left) are shown after normalization by CYP2D6 expression in the vehicle-treated mice of the respective genotype. (C) S9 from the mice was incubated with debrisoquine (200 μM), and 4-hydroxydebrisoquine concentrations were measured by using liquid chromatography–tandem mass spectrometry. Data shown are 4-hydroxydebrisoquin production rates (in pmol/min per mg protein). Values are presented as mean ± S.E.M. (n = 5). *P < 0.05; **P < 0.01; n.s., not statistically significant.
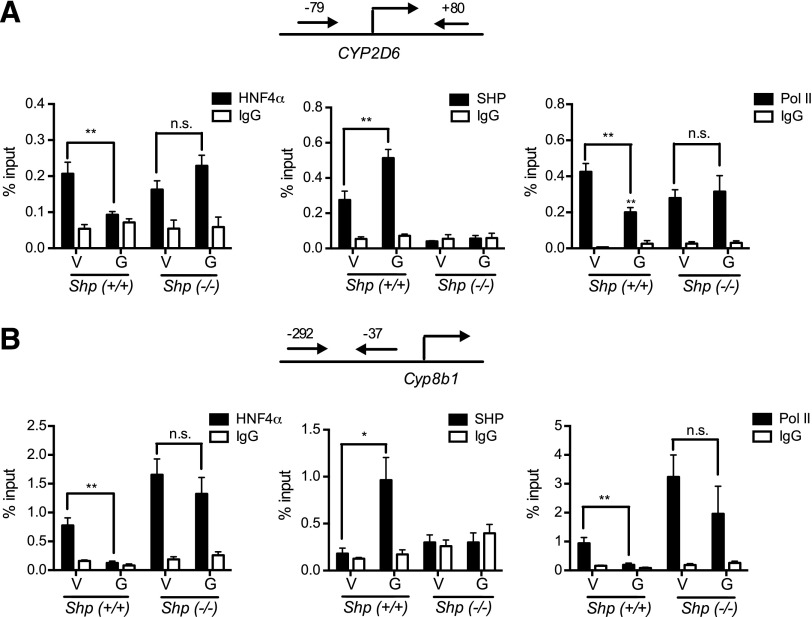
To determine whether Shp deletion leads to altered GW4064 effects on the HNF4α transactivation of the CYP2D6 promoter, ChIP assays were performed in the mouse liver tissues. In Shp(+/+);CYP2D6 mice, GW4064 decreased the recruitment of HNF4α and Pol II while increasing SHP recruitment to the CYP2D6 promoter (Fig. 4A), as in Tg-CYP2D6 mice (Fig. 2). These changes in transcription factor recruitment disappeared in Shp(−/−);CYP2D6 mice (Fig. 4A). Similar results were observed in the transcription factor recruitment to the Cyp8b1 promoter (Fig. 4B). Together, these results suggest an essential role of SHP in CYP2D6 repression by GW4064.
Fig. 4.
Repressed HNF4α transactivation of the CYP2D6 promoter is abrogated in Shp(−/−);CYP2D6 mice. Shp(+/+);CYP2D6 or Shp(−/−);CYP2D6 mice were injected with GW4064 (G, 10 mg/kg) or vehicle (V, olive oil) intraperitoneally daily for 5 days (n = 5 per group). Recruitment of HNF4α, SHP, and RNA polymerase II onto (A) CYP2D6 promoter and (B) Cyp8b1 promoter were analyzed by ChIP assay. Values are presented as mean ± S.E.M. (n = 5). *P < 0.05, **P < 0.01, and n.s., not statistically significant, versus vehicle-treated mice for each respective genotype.
SHP Represses CYP2D6 Expression in Primary Human Hepatocytes.
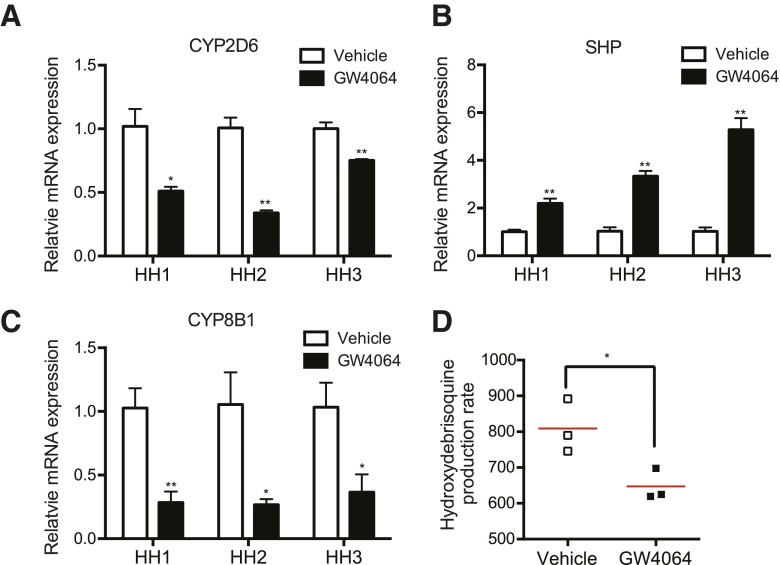
To determine whether GW4064 effects on CYP2D6 expression obtained in mice can be translated to humans, primary human hepatocytes were treated with GW4064 or vehicle for 48 hours, and CYP2D6 expression and activity were examined. The results showed that GW4064 treatment decreased CYP2D6 mRNA expression by 1.5- to 2-fold (Fig. 5A) while enhancing SHP expression (Fig. 5B) in human hepatocytes. The mRNA expression levels of positive control gene CYP8B1 were also increased in GW4064-treated human hepatocytes (Fig. 5C). In one batch of human hepatocytes (i.e., human hepatocyte 2), CYP2D6 activity levels were measured by using debrisoquine as a probe drug for CYP2D6. CYP2D6 activity in GW4064-treated cells was significantly lower (Fig. 5D) but to a small extent (∼20%), as expected from the long degradation half-life of the CYP2D6 protein (i.e., 51 hours) (Venkatakrishnan and Obach, 2005). Together, these results indicate that as in Tg-CYP2D6 mice, GW4064 represses CYP2D6 expression in human hepatocytes.
Fig. 5.
GW4064 represses CYP2D6 expression and activity in primary human hepatocytes. Primary human hepatocytes (HHs) from three different donors were treated with GW4064 (1 μM) or vehicle control (DMSO) for 48 hours. (A–C) CYP2D6, SHP, and CYP8B1 mRNA expression was determined by using qRT-PCR. (D) HH2 was incubated with debrisoquine (200 μM), and 4-hydroxydebrisoquine concentrations in the culture media were measured by using liquid chromatography–tandem mass spectrometry. Data shown are 4-hydroxydebrisoquin production rates (in pmol/min per mg protein). Values are presented as mean ± S.E.M. and calculated from three independent experiments. *P < 0.05 and **P < 0.01 versus vehicle treatment.
Discussion
Previously, we have identified SHP as a novel regulator of CYP2D6 transcription. SHP represses HNF4α transactivation of the CYP2D6 promoter (Koh et al., 2014a). Because SHP is a representative target gene of FXR, in this study, we examined whether upregulation of SHP by a selective FXR agonist (GW4064) alters CYP2D6 expression. Our data showed that GW4064 represses CYP2D6 expression in Tg-CYP2D6 mice as well as human hepatocytes.
Results from this study demonstrated a key role of SHP in CYP2D6 repression by GW4064. The decrease in CYP2D6 expression in GW4064-treated mice was accompanied by increases in SHP expression as well as SHP recruitment to the CYP2D6 promoter. The essential role of SHP in GW4064 action on CYP2D6 expression was further verified in Shp(−/−);CYP2D6 mice in that CYP2D6 repression by GW4064 was abrogated in the mice. Considering that SHP induction is a class action of FXR agonists, these results suggest that FXR activation by other drugs or diseases (e.g., cholestasis) is also expected to repress CYP2D6 expression. Indeed, we found that treatment of human hepatocytes with cholic acid (a major bile acid elevated in cholestasis) led to a significant decrease in CYP2D6 expression (data not shown). Cholestasis is often triggered by drugs, such as rifampicin, erythromycin, ethinylestradiol, and oxypenicilins (Zhang et al., 2014). Although it remains to be determined whether cholestasis represses CYP2D6-mediated drug metabolism in humans, the results from this study provide a mechanistic basis for the possibility. Additionally, this study provides evidence that supports important roles of SHP in the regulation of CYP2D6 expression and that differential SHP expression and/or activity may potentially contribute to interindividual variability in CYP2D6-mediated drug metabolism in humans. Whether or to what extent different SHP modulators affect hepatic CYP2D6 expression and activity remains to be examined.
Results from our previous study in Tg-CYP2D6 mice showed that CYP2D6 expression was increased in mice with SHP knocked down (by using small interfering RNA) (Koh et al., 2014a), indicating that decreased SHP expression leads to CYP2D6 induction. Interestingly, however, the basal expression levels of CYP2D6 in Shp(−/−);CYP2D6 mice did not differ from those in Shp(+/+);CYP2D6 mice (Fig. 3A). Previous studies have shown that gene knockout in mice can lead to multiple compensational changes in gene expression (Picciotto and Wickman, 1998), and altered expression of transcription factors in Shp(−/−) mice may compensate for the loss of SHP in regulating CYP2D6 expression. For example, NR0B1 is a nuclear receptor that lacks the DNA-binding domain similarly to SHP (Benoit et al., 2006), and it is known to repress HNF4α transactivation of a hepatic gene (Nedumaran et al., 2009). Our qRT-PCR experiment, however, revealed that NR0B1 mRNA expression is undetectable in mouse liver tissues (data not shown). Also, SHP-interacting leucine zipper protein (SMILE) [initially identified as an SHP-interacting protein (Xie et al., 2008)] has been shown to repress HNF4α transactivation of target genes in the absence of SHP (Xie et al., 2009). Our results showed, however, that the mRNA expression levels of SMILE did not differ between Shp(−/−);CYP2D6 and Shp(+/+);CYP2D6 male mice (data not shown; in female mice, SMILE mRNA expression was even lower in Shp(−/−);CYP2D6 mice as compared with Shp(+/+);CYP2D6 mice). Together, these results suggest that basal CYP2D6 expression in Shp(−/−);CYP2D6 mice may be governed by as-yet-unknown factors.
Many FXR agonists are currently under development for different hepatic or metabolic diseases, including primary biliary cirrhosis, nonalcoholic steatohepatitis, and diabetes (Thomas et al., 2008). For example, obeticholic acid (i.e., INT-747), a potent selective FXR agonist, is in phase III trials for primary biliary cirrhosis (Pellicciari et al., 2005). Also, the hepatoprotective effects of GW4064 and its analogs have been shown in cholestatic rats and mice with gallstones (Liu et al., 2003; Moschetta et al., 2004; Akwabi-Ameyaw et al., 2008; Bass et al., 2011; Porez et al., 2012). Our results suggest that drug-drug interactions between CYP2D6 substrates and FXR agonists may occur if these FXR agonists are approved and clinically used. Considering the long degradation half-lives of the CYP2D6 protein (Venkatakrishnan and Obach, 2005), it remains difficult to quantitatively predict the clinical outcome of these interactions based on the results from human hepatocytes. On the other hand, the results from Tg-CYP2D6 mice suggest that FXR activation could lead to ∼2-fold decreases in CYP2D6 activity. The time course and magnitude of this potential drug-drug interaction remain to be examined.
In conclusion, we showed that the FXR agonist GW4064 represses CYP2D6 expression through inducing SHP expression. This suggests that potential drug-drug interactions may occur between CYP2D6 substrates and FXR agonists that are currently under development for hepatic and metabolic disorders. Our results also provide a mechanistic basis to identify potential factors (e.g., bile acids) that may contribute to the interindividual variability in CYP2D6 activity.
Abbreviations
- ChIP
chromatin immunoprecipitation
- FXR
farnesoid X receptor
- GW4064
3-(2,6-dichlorophenyl)-4-(3′-carboxy-2-chlorostilben-4-yl)oxymethyl-5-isopropylisoxazole
- HNF4α
hepatocyte nuclear receptor 4α
- PM
poor metabolizer
- Pol II
RNA polymerase II
- qRT-PCR
quantitative real-time polymerase chain reaction
- SHP
small heterodimer partner
- SMILE
SHP-interacting leucine zipper protein
- Tg-CYP2D6
CYP2D6-humanized transgenic
Authorship Contributions
Participated in research design: Pan, Jeong.
Conducted experiments: Pan.
Contributed new reagents or analytic tools: Lee.
Performed data analysis: Pan, Jeong.
Wrote or contributed to the writing of the manuscript: Pan, Lee, Jeong.
Footnotes
This work was supported by the National Institutes of Health [Grants HD065532 and DK093774].
References
- Akwabi-Ameyaw A, Bass JY, Caldwell RD, Caravella JA, Chen L, Creech KL, Deaton DN, Jones SA, Kaldor I, Liu Y, et al. (2008) Conformationally constrained farnesoid X receptor (FXR) agonists: Naphthoic acid-based analogs of GW 4064. Bioorg Med Chem Lett 18:4339–4343. [DOI] [PubMed] [Google Scholar]
- Bass JY, Caravella JA, Chen L, Creech KL, Deaton DN, Madauss KP, Marr HB, McFadyen RB, Miller AB, Mills WY, et al. (2011) Conformationally constrained farnesoid X receptor (FXR) agonists: heteroaryl replacements of the naphthalene. Bioorg Med Chem Lett 21:1206–1213. [DOI] [PubMed] [Google Scholar]
- Benoit G, Cooney A, Giguere V, Ingraham H, Lazar M, Muscat G, Perlmann T, Renaud JP, Schwabe J, Sladek F, et al. (2006) International Union of Pharmacology. LXVI. Orphan nuclear receptors. Pharmacol Rev 58:798–836. [DOI] [PubMed] [Google Scholar]
- Bertilsson L, Dahl ML, Dalén P, Al-Shurbaji A. (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns W, Smith CA, McLaren AW, Wolf CR. (1996) Characterization of the human cytochrome P4502D6 promoter. A potential role for antagonistic interactions between members of the nuclear receptor family. J Biol Chem 271:25269–25276. [DOI] [PubMed] [Google Scholar]
- Carcillo JA, Adedoyin A, Burckart GJ, Frye RF, Venkataramanan R, Knoll C, Thummel K, Roskos L, Wilson JW, Sereika S, et al. (2003) Coordinated intrahepatic and extrahepatic regulation of cytochrome p4502D6 in healthy subjects and in patients after liver transplantation. Clin Pharmacol Ther 73:456–467. [DOI] [PubMed] [Google Scholar]
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. (2001) The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol 60:1260–1267. [DOI] [PubMed] [Google Scholar]
- Dahl ML, Johansson I, Palmertz MP, Ingelman-Sundberg M, Sjöqvist F. (1992) Analysis of the CYP2D6 gene in relation to debrisoquin and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther 51:12–17. [DOI] [PubMed] [Google Scholar]
- Ellis E, Axelson M, Abrahamsson A, Eggertsen G, Thörne A, Nowak G, Ericzon BG, Björkhem I, Einarsson C. (2003) Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology 38:930–938. [DOI] [PubMed] [Google Scholar]
- Felmlee MA, Lon HK, Gonzalez FJ, Yu AM. (2008) Cytochrome P450 expression and regulation in CYP3A4/CYP2D6 double transgenic humanized mice. Drug Metab Dispos 36:435–441. [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. (2008) The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83:234–242. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526. [DOI] [PubMed] [Google Scholar]
- Hou ZY, Pickle LW, Meyer PS, Woosley RL. (1991) Salivary analysis for determination of dextromethorphan metabolic phenotype. Clin Pharmacol Ther 49:410–419. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Yu AM, Yim SH, Ma X, Krausz KW, Inoue J, Xiang CC, Brownstein MJ, Eggertsen G, Björkhem I, et al. (2006) Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J Lipid Res 47:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KH, Pan X, Shen HW, Arnold SL, Yu AM, Gonzalez FJ, Isoherranen N, Jeong H. (2014a) Altered expression of small heterodimer partner governs cytochrome P450 (CYP) 2D6 induction during pregnancy in CYP2D6-humanized mice. J Biol Chem 289:3105–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KH, Pan X, Zhang W, McLachlan A, Urrutia R, Jeong H. (2014b) Krüppel-like factor 9 promotes hepatic cytochrome P450 2D6 expression during pregnancy in CYP2D6-humanized mice. Mol Pharmacol 86:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Thomas AM, Hart SN, Zhong X, Wu D, Guo GL. (2010) Farnesoid X receptor activation mediates head-to-tail chromatin looping in the Nr0b2 gene encoding small heterodimer partner. Mol Endocrinol 24:1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY. (2014) Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66:948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, et al. (2003) Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 112:1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, et al. (2000) Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem 43:2971–2974. [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Lee J, Comstock C, Knudsen KE, Kemper JK. (2009) Functional specificities of Brm and Brg-1 Swi/Snf ATPases in the feedback regulation of hepatic bile acid biosynthesis. Mol Cell Biol 29:6170–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschetta A, Bookout AL, Mangelsdorf DJ. (2004) Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 10:1352–1358. [DOI] [PubMed] [Google Scholar]
- Nedumaran B, Hong S, Xie YB, Kim YH, Seo WY, Lee MW, Lee CH, Koo SH, Choi HS. (2009) DAX-1 acts as a novel corepressor of orphan nuclear receptor HNF4alpha and negatively regulates gluconeogenic enzyme gene expression. J Biol Chem 284:27511–27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaki-Mogami T, Une M, Fujino T, Sato Y, Tamehiro N, Kawahara Y, Shudo K, Inoue K. (2004) Identification of intermediates in the bile acid synthetic pathway as ligands for the farnesoid X receptor. J Lipid Res 45:1538–1545. [DOI] [PubMed] [Google Scholar]
- Park YJ, Kim SC, Kim J, Anakk S, Lee JM, Tseng HT, Yechoor V, Park J, Choi JS, Jang HC, et al. (2011) Dissociation of diabetes and obesity in mice lacking orphan nuclear receptor small heterodimer partner. J Lipid Res 52:2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Costantino G, Fiorucci S. (2005) Farnesoid X receptor: from structure to potential clinical applications. J Med Chem 48:5383–5403. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Wickman K. (1998) Using knockout and transgenic mice to study neurophysiology and behavior. Physiol Rev 78:1131–1163. [DOI] [PubMed] [Google Scholar]
- Porez G, Prawitt J, Gross B, Staels B. (2012) Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res 53:1723–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse C, Brockmöller J, Bauer S, Roots I. (1997) Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 60:284–295. [PMC free article] [PubMed] [Google Scholar]
- Temesvári M, Kóbori L, Paulik J, Sárváry E, Belic A, Monostory K. (2012) Estimation of drug-metabolizing capacity by cytochrome P450 genotyping and expression. J Pharmacol Exp Ther 341:294–305. [DOI] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. (2008) Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K, Obach RS. (2005) In vitro-in vivo extrapolation of CYP2D6 inactivation by paroxetine: prediction of nonstationary pharmacokinetics and drug interaction magnitude. Drug Metab Dispos 33:845–852. [DOI] [PubMed] [Google Scholar]
- Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, et al. (2002) Redundant pathways for negative feedback regulation of bile acid production. Dev Cell 2:721–731. [DOI] [PubMed] [Google Scholar]
- Xie YB, Lee OH, Nedumaran B, Seong HA, Lee KM, Ha H, Lee IK, Yun Y, Choi HS. (2008) SMILE, a new orphan nuclear receptor SHP-interacting protein, regulates SHP-repressed estrogen receptor transactivation. Biochem J 416:463–473. [DOI] [PubMed] [Google Scholar]
- Xie YB, Nedumaran B, Choi HS. (2009) Molecular characterization of SMILE as a novel corepressor of nuclear receptors. Nucleic Acids Res 37:4100–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Fischer J, Raimundo S, Stüven T, Evert BO, Schwab M, Eichelbaum M. (2001) Comprehensive analysis of the genetic factors determining expression and function of hepatic CYP2D6. Pharmacogenetics 11:573–585. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Limaye PB, Renaud HJ, Klaassen CD. (2014) Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice. Toxicol Appl Pharmacol 277:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]