Abstract
Background:
Depending on the site of irradiation, about 40-80% of patients undergoing radiotherapy (RT) will experience nausea and/or vomiting. The current study aimed to investigate the efficacy of ondansetronas as a single agent and with a combination to aprepitant on preventing RT-induced nausea and vomiting (RINV).
Materials and Methods:
In a clinical randomized controlled trial (from September 2010 to September 2011), conducted in Radiation Oncology Department of Seyed-al-Shohada Hospital, Isfahan University of Medical Sciences, 40 abdominopelvic malignancies cancer patients were allocated into two aliquots using block randomization of size. Patients in the first group (group I) received ondansetron alone while those patients in the remaining group (group II) received ondansetron and aprepitant. Then, developing of RINV and its severity and benefit of adding aprepitant to ondansetron, in comparison with ondansetron as a single drug therapy were evaluated.
Results:
The average age of the patients in group I was 61.15 ± 12.27 years while in group II it was 50.1 ± 13.27 years. No statistically significant gender differences were found between the two groups. In patients treated with ondansetron single drug therapy (group I), frequency and grade of RINV were significantly more than the group treated simultaneously by aprepitant and ondansetron (group II) (odds ratio [OR] = 21.2; P < 0.01). Compared with RT alone, the patients whom underwent RT along with chemotherapy showed lower probability of experiencing RINV (OR = 0.13; P < 0.05).
Conclusion:
The present study indicated a significant superiority of combination of ondansetron and aprepitant in management of RINV, in patients undergoing RT, compared to ondansetron as a single agent therapy. More accurate follow-up studies are needed for the evaluation of the efficacy of ondansetron with combination to aprepitant on preventing the RINV.
Keywords: Aprepitant, nausea and vomiting, ondanseton, radiotherapy, radiotherapy-induced nausea and vomiting
INTRODUCTION
Generally, about one-third of patients undergoing radiotherapy (RT) have experienced RT-induced nausea and vomiting (RINV).[1,2,3] In this regards, the radiation oncologists have attitude of prescribing antiemetic drugs as a rescue, with a wide range of doses and schedules.[4,5,6] They also prescribe 5-hydroxytryptamine (5-HT3) antagonists rather than other antiemetic drugs that were generally being used.[4,5,6,7,8,9,10]
Patients submitted to total body irradiation (TBI), half body irradiation (HBI) or abdominal RT are at major risk of RINV. However, few randomized controlled clinical trials have evaluated the efficacy of various antiemetic drugs in preventing RINV.[2,3]
It is generally accepted that the rate of prophylaxis is low, and consequently radiation oncologists often wait for some degree of nausea or vomiting before initiating antiemetic therapy.[2,3] This notion is supported by the most recent study by the Italian group for Antiemetic Research in Radiotherapy, which showed that only 12.4% of RT patients, including 41% and 28% of the patients underwent upper abdomen and brain treatment, respectively, received any kind of prophylaxis.[3] Enblom et al. have performed a cross-sectional study and found that only 17% of patients undergoing RT had received any antiemetic therapy within 1-week of being questioned, a figure that would have included both prophylactic and rescue therapy. Even more concerning, one-third of these patients still viewed their therapy as insufficient for their needs.[4]
Ondansetron, a 5-TH3 receptor antagonist, is widely used to prevent postoperative and pregnancy nausea and vomiting.[5] An European survey on 200 radiation oncologists from France, Italy, Germany, Spain and the UK suggested that, 5-HT3 antagonists are under-used in patients receiving RT.[5] Only 52% of patients who received highly emetogenic RT (RF site of gastrointestinal or abdominal) actually received a 5-HT3 antagonist. There are also differences in the prescribing procedure between the evaluated countries. A 5-HT3 antagonist was more frequently prescribed if the patient received radiation with chemotherapy (46%) than in RT alone (33%).[5] Similar results are demonstrated by Goldsmith in the United States.[3,5]
The efficacy of 5-HT3 antagonists either as a single agent therapy or combination with dexamethasone (DEX) in the management of RINV in single fractionation and fractionated RT to upper abdomen were established by several studies.[7,8,9,10,11]
Aprepitant is a neurokin in receptor inhibitor and recently it has been found to have an important role in control and management of RINV, especially late onset and long-lasting cases where 5-HT3 antagonists are not as efficient as first few days of treatment.[12,13]
Beyond the above-mentioned studies and a few small surveys, there is a limited literature that characterizes typical RINV management in daily practice.[1] On the other hand, current practice guidelines for the use of antiemetics in RT are quite different when classifying radiation emetogenic risk categories and giving indications for the use of antiemetic drugs.[1] This diversity of recommendations reflects the limited amount of high-level evidence available to date.[2,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]
The current study aimed to investigate the efficacy of ondansetronas a single agent and with combination to aprepitant on preventing the RINV.
MATERIALS AND METHODS
Study design and participants
This study was a clinical randomized controlled trial (from September 2010 to September 2011), conducted in Radiation Oncology Department of Seyed-al-Shohada Hospital, affiliated to Isfahan University of Medical Sciences, on some abdominopelvic cancer patients with no sex limitation that received RT to abdomen and/or abdominopelvic area of their body.
Patients with pathologic malignancy of abdomen that need RT or concomitant chemoradiation therapy were eligible for the enrollment. Inclusion criteria for enrolling the patients in our study were as follows: Age ≥18 years, life expectancy more than 3 months, Karnofski performance scale (KPS) ≥70, liver function according to Child-Pugh A and B. In addition, nonpregnant women were considered eligible for arrival, and concomitant use of Cisplatin, Capecitabine, 5FU, hormonal therapy and also race and nationality were not regarded as criteria for nonarrival to study.
Sample size was determined according to comparing prevalence of RINV in two parallel groups of a randomized trial.[7,8,10] Considering the type one error rate and statistical power of 5% and 80%, respectively, for detecting 40% difference in prevalence of RINV between two studied groups the required sample size was determined as 15 patients in each group. However for compensating the possible attrition, 20 patients were considered. All patients completed the study. Written informed consent was recorded from subjects who agreed to participate in the study. Ethical approval was obtained from The Local Research Ethics Committee in School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran before recruitment. The study was performed in accordance with published guidelines and is reported in accordance with the consort statement.
Intervention
The included patients were randomly divided into two groups using block randomization of size two mechanisms [Figure 1]. 20 patients were treated using ondansetron (group I [O]) and 20 patients treated with ondansetron along with aprepitant (group II [OA]). Patients were treated by a dose of 180-200 cGy per fraction, 5 days/week with at least total dose of 3000 cGy by Cobalt or Linear Accelerator.
Figure 1.
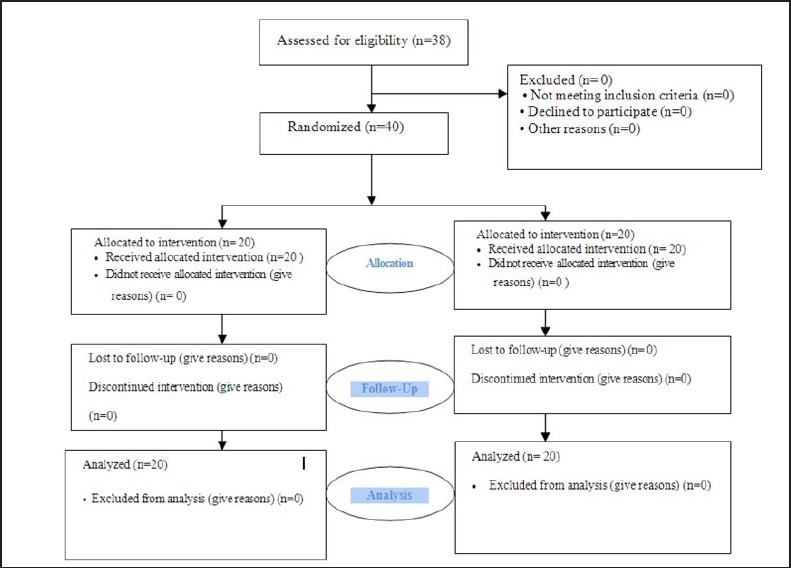
Consort diagram of the study deign
Firstly, before any medical intervention, patients were visited by physician. Then, patients in group I received 8 mg ondansetron orally 2 h before each RT course. Patients in group II were received ondansetron the same as group I and 125 mg aprepitant orally on Saturday and 80 mg on Monday and Wednesday during the course of treatment. Neither patient nor physician did not know about treatment type and group of patients. The primary endpoint was symptoms of RINV and its severity was determined according to Radiation Therapy Oncology Group criteria for RINV [Figure 2].[1,2,3] The patients were examined and asked for symptoms and signs of RINV and its degree at the end of the week. The other studied variables were age, gender, KPS, chemoradiotherapy (CRT), field size as well as radiation dose. Detailed information about these variables in two studied groups is presented in Table 1.
Figure 2.
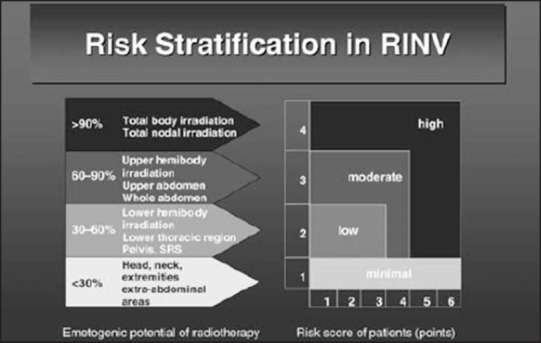
Risk evaluation and the primary endpoint of radiotherapy-induced nausea and vomiting, determined according to Radiation Therapy Oncology Group criteria
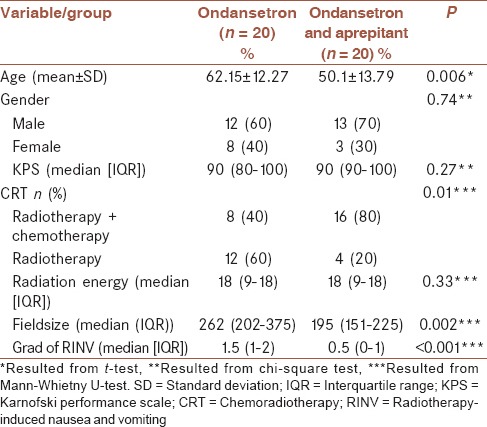
Table 1.
Basic and clinical characteristics of study samples in both studied groups
Statistical analysis
Quantitative data were expressed as mean (±standard deviation) or median (interquartile range), while qualitative were denoted data as frequency (percent). Independent t-test or Mann-Whietny U-test were used for comparing the groups in terms of quantitative data. Chi-square or Fisher exact test were used for comparing the studied groups in terms of qualitative variables. Ordinal logistic regression was used for investigation of treatment between groups I and II, on the grades of RINV. Proportional odds assumption in ordinal logistic regression was tested using appropriate Chi-square test. All statistical analyses were conducted using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). P ≤ 0.05 was considered as statistically significant.
RESULTS
Table 1 shows the characteristics of patients in both studied groups. As can be seen from this table, there was no statistically significant differences between the two groups in terms of gender distribution and KPS, while the mean age was significantly higher in group I compared to group II (P < 0.01). There were significant differences between the two groups in terms of experiencing RINV (P < 0.001). Group II, in which the patients received ondansetron and aprepitant simultaneously showed lower RINV [Table 1].
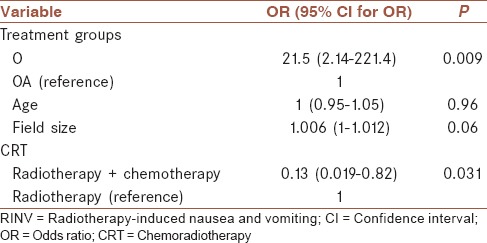
Ordinal logistic regression was applied for investigating the effect of treatment modalities between groups I and II on grade of RINV, considering the impact of some important confounding factors. The effect of treatment modalities and some important confounding variables on grade of RINV is shown in Table 2. Compared to group II, patients in group I had greater odds (21.5 [(95% confidence interval: 2.14-221.4]; P < 0.01) of experiencing higher grade of RINV. As shown in Table 2, patients who underwent RT along with chemotherapy compared with RT alone had lower odds (odds ratio [OR] = 0.13; P < 0.05) for experiencing the higher grades of RINV. The association between age and field size, in the presence of other variables were not statistically significant (P ≥ 0.05).
Table 2.
The effect of treatment modalities and some important confounding variables on grade of RINV
DISCUSSION
The present study indicates a significant superiority of combination of ondansetron and aprepitant in management of RINV in moderate emetogenic risk RT compared with ondansetron as single agent therapy.
Current oncology practice guidelines for the use of antiemetic in RT are quite different when classifying radiation emetogenic risk categories and giving indications for the use of antiemetic drugs. This diversity of recommendations reflects the limited amount of high-level evidence available to date (i.e., few randomized controlled trials and small number of patients entered in each trial).[2,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]
In order to achieve an optimal treatment strategy to prevent nausea and/or vomiting, it could be useful to develop a risk-adjusted treatment for RINV. Therefore, the individual risk of the patient to develop nausea and/or vomiting should be taken into consideration as well as the emetogenicity of the radiotherapeutic regimen and any simultaneous administration of chemotherapy.[1,3] Patient factors are known to influence the risk of emesis in cancer patients.[3,4,5] As an instance, previous chemotherapy-induced emesis is a significant prognostic factor for developing RINV.[6,7,8,9] Individual risk profiles according to patient-related emetogenic risk factors are age, gender, alcohol consumption, previous experience of nausea and vomiting and anxiety.[1,2,3]
There are three randomized clinical trials in patients with fractionated RT and one with single-fraction RT investigating the efficacy of non-5-HT3 antagonists in RT of the upper abdomen. There was no difference among the various compounds used, and the antiemetic efficacy was limited.[9,10,11] The only double-blind study on corticosteroids suggested that, the use of DEX resulted in a significantly better control of RINV than placebo.[12] There are a number of trials with 5-HT-3 antagonists for patients treated with total-body or upper abdominal irradiation.[11,12,13,14,15] The 5-HT3 antagonists gave a significantly greater protection from RT-induced emesis than placebo or non-5-HT3 antagonists.[11,12,13,14,15]
The emetogenic potential of RT is divided into high, moderate, low and minimal, as is the emetogenicity of cytotoxic drugs.[3,4,5] Using this tool, it is possible to develop a risk-adjusted treatment for RINV.[3,4,5]
In this study, 40 patients were enrolled and the result was statistically significant. It should be noted that, considering the irradiated site and emetogenic risk, upper-abdomen irradiation showed as the “most emetogenic” regimen. Unfortunately, RINV was not evaluable in patients submitted to TBI or HBI due to the small number of patients who received these therapies during the survey.
The included patients were randomly divided into two groups. In the first group, in this study called group I, 20 patients were treated using ondansetron and in the second one the patients treated with ondansetron along with aprepitant. Results of this study showed that, there was significant differences between the two groups in terms of experiencing RINV (P < 0.001). Group II, in which the patients received ondansetron and aprepitant simultaneously showed lower RINV [Table 1].
Considering the patient characteristics, there were no statistically significant differences between the two groups in terms of gender distribution and KPS, while the mean age was significantly higher in group I compared to group II (P < 0.01).
This study not only evaluated the role of addition of aprepitant to ondansetron in management of RINV, but also assessed other emetogenic risk factors such as age, sex, CRT, field size [Table 1].
Considering the well-recognized difficulties in recruiting patients into research, this study achieved a reasonable inclusion rate. However, more accurate follow-up studies are needed for the evaluation of the efficacy of ondansetron as a single agent and with combination to aprepitant on preventing the RINV.
CONCLUSIONS
Data presented here suggest a benefit of ondansetron medication with combination to aprepitant on decreasing the probability of RT-induced nausea and vomiting. The present study indicated a significant superiority of combination of ondansetron and aprepitant in management of RINV, in patients undergoing RT, compared to ondansetron as single agent therapy. By improving RINV, better treatment results and consequently better quality of life is expected.
AUTHOR'S CONTRIBUTIONS
HE, SH, and SMS were the principal investigators of the study. HE, SH, and SMS participated in preparing the design of the study and collecting the data. SMS, ST, and PA participated in preparing the design of the study, revisited the manuscript and critically evaluated the intellectual contents. AF conducted the analysis of data. SMS, AF, and GM participated in preparing the final draft of the manuscript, revisited the manuscript and critically evaluated the intellectual contents. SMS and AF coordinated in study design and data collection. SMS and GM participated in the preparation of the final draft of the manuscript, revisited the manuscript and critically evaluated the intellectual contents. All authors have read and approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
ACKNOWLEDGMENTS
This study was supported by a grant (grant number; 289275) from Isfahan University of Medical Science, Isfahan, Iran. We would like to thank the University authorities who offered critical administrative support and managerial services in carrying out the study, and also all of the researchers for their help and support.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Feyer PC, Stewart AL, Titlbach OJ. Aetiology and prevention of emesis induced by radiotherapy. Support Care Cancer. 1998;6:253–60. doi: 10.1007/s005200050163. [DOI] [PubMed] [Google Scholar]
- 2.Feyer P, Zimmermann JS, Titlbach OJ, Buchali A, Hinkelbein M, Budach V. Radiotherapy-induced emesis. An overview. Strahlenther Onkol. 1998;174(Suppl 3):56–61. [PubMed] [Google Scholar]
- 3.Maranzano E, DeAngelis V, Pergolizzi S, Constantini S, Lupattelli M, Petal F. Radiation-induced emesis (RIE): Results of the second observational multicenter Italian trial. Radiother Oncol. 2010;1:36–41. [Google Scholar]
- 4.Enblom A, Bergius Axelsson B, Steineck G, Hammar M, Borjeson S. One third of patients with radiotherapy-induced nausea consider their antiemetic treatment insufficient. Support Care Cancer. 2009;17:23–32. doi: 10.1007/s00520-008-0445-x. [DOI] [PubMed] [Google Scholar]
- 5.Feyer PC, Seegenschmiedt MH. Antiemetic patterns of care for radiotherapy induced nausea and vomiting. ECCO-12. 2003:929. [Google Scholar]
- 6.Goodin S, Cunningham R. 5-HT(3)-receptor antagonists for the treatment of nausea and vomiting: A reappraisal of their side-effect profile. Oncologist. 2002;7:424–36. doi: 10.1634/theoncologist.7-5-424. [DOI] [PubMed] [Google Scholar]
- 7.Franzén L, Nyman J, Hagberg H, Jakobsson M, Sorbe B, Nyth AL, et al. A randomised placebo controlled study with ondansetron in patients undergoing fractionated radiotherapy. Ann Oncol. 1996;7:587–92. doi: 10.1093/oxfordjournals.annonc.a010675. [DOI] [PubMed] [Google Scholar]
- 8.Priestman TJ, Roberts JT, Upadhyaya BK. A prospective randomized double-blind trial comparing ondansetron versus prochlorperazine for the prevention of nausea and vomiting in patients undergoing fractionated radiotherapy. Clin Oncol (R Coll Radiol) 1993;5:358–63. doi: 10.1016/s0936-6555(05)80086-x. [DOI] [PubMed] [Google Scholar]
- 9.Lanciano R, Sherman DM, Michalski J, Preston AJ, Yocom K, Friedman C. The efficacy and safety of once-daily Kytril (granisetron hydrochloride) tablets in the prophylaxis of nausea and emesis following fractionated upper abdominal radiotherapy. doi: 10.1081/cnv-100107736. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute of Canada Clinical Trials Group (SC) Wong RK, Paul N, Ding K, Whitehead M, Brundage M, et al. 5-hydroxytryptamine-3 receptor antagonist with or without short-course dexamethasone in the prophylaxis of radiation induced emesis: A placebo-controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19) J Clin Oncol. 2006;24:3458–64. doi: 10.1200/JCO.2005.04.4685. [DOI] [PubMed] [Google Scholar]
- 11.Priestman TJ, Roberts JT, Lucraft H, Collis CH, Adams M, Upadhyaya BK, et al. Results of a randomized, double-blind comparative study of ondansetron and metoclopramide in the prevention of nausea and vomiting following high-dose upper abdominal irradiation. Clin Oncol (R Coll Radiol) 1990;2:71–5. doi: 10.1016/s0936-6555(05)80790-3. [DOI] [PubMed] [Google Scholar]
- 12.Aapro M, Molassiotis A, Dicato M, Peláez I, Rodríguez-Lescure Á, Pastorelli D, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER) Ann Oncol. 2012;23:1986–92. doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 13.Haller D, Wagman L, Camphausen K, Hoskins W. 15th ed. New Zealand: The Oncology Group, UBM Medica; 2012. Cancer Management: A Multidisciplinary Approach: Medical, Surgical and Radiation Oncology. [Google Scholar]
- 14.Prentice HG, Cunningham S, Gandhi L, Cunningham J, Collis C, Hamon MD. Granisetron in the prevention of irradiation-induced emesis. Bone Marrow Transplant. 1995;15:445–8. [PubMed] [Google Scholar]
- 15.Priestman TJ, Roberts JT, Upadhyaya BK. A prospective randomized double-blind trial comparing ondansetron versus prochlorperazine for the prevention of nausea and vomiting in patients undergoing fractionated radiotherapy. Clin Oncol (R Coll Radiol) 1993;5:358–63. doi: 10.1016/s0936-6555(05)80086-x. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer TR, Friedman CJ, Bushnell W, Frankel SR, Raschko J. Double-blind, randomized, parallel-group study on the efficacy and safety of oral granisetron and oral ondansetron in the prophylaxis of nausea and vomiting in patients receiving hyperfractionated total body irradiation. Bone Marrow Transplant. 2000;26:203–10. doi: 10.1038/sj.bmt.1702479. [DOI] [PubMed] [Google Scholar]
- 17.Kirkbride P, Bezjak A, Pater J, Zee B, Palmer MJ, Wong R, et al. Dexamethasone for the prophylaxis of radiation-induced emesis: A National Cancer Institute of Canada Clinical Trials Group phase III study. J Clin Oncol. 2000;18:1960–6. doi: 10.1200/JCO.2000.18.9.1960. [DOI] [PubMed] [Google Scholar]
- 18.Bey P, Wilkinson PM, Resbeut M, Bourdin S, Le Floch O, Hahne W, et al. A double-blind, placebo-controlled trial of i.v. dolasetron mesilate in the prevention of radiotherapy-induced nausea and vomiting in cancer patients. Support Care Cancer. 1996;4:378–83. doi: 10.1007/BF01788845. [DOI] [PubMed] [Google Scholar]
- 19.Jordan K, Schmoll HJ, Aapro MS. Comparative activity of antiemetic drugs. Crit Rev Oncol Hematol. 2007;61:162–75. doi: 10.1016/j.critrevonc.2006.08.003. [DOI] [PubMed] [Google Scholar]


