Abstract
Background:
Incomplete efficiency of oral medications restricts their therapeutic success in pain control of the painful form of the diabetic peripheral neuropathy (DPN). Use of topical medications because of less systemic side effects is more acceptable. This study aimed to compare the effect of clonidine gel and capsaicin cream in relieving pain associated with DPN.
Materials and Methods:
This 12-week, randomized, double-blind and parallel-group trial was conducted to compare the efficacy and safety of topical clonidine and capsaicin. Totally, 139 patients with type 2 diabetes with a pain score of at least 4 as assessed by visual analog scale (VAS), were treated for up to 3 months. The endpoint of the study was the reduction in the median pain score from baseline, as assessed by the VAS at the 4 weekly follow-up visits.
Results:
The intention-to-treat population for the efficacy analysis consists of 69 patients receiving clonidine and 70 patients receiving capsaicin. Both drugs significantly relieved pain at 12 weeks (P < 0.001 for both) but no significant difference in the efficacy between the two treatments was observed (P = 0.931). Dermatologic complications were more common in capsaicin group (P = 0.001).
Conclusion:
The results of this study showed the comparable efficacy of clonidine gel in comparison with capsaicin cream in the treatment of pain due to DPN with less adverse events. More studies are required to better evaluate the efficacy and safety of this topical compound for relieving pain in DPN.
Keywords: Capsaicin, clonidine, efficacy, neuropathy, topical
INTRODUCTION
Painful form of the diabetic peripheral neuropathy (DPN) is one of the most commonly encountered neuropathic pain syndromes in clinical practice,[1] that is very uncomfortable for patients and despite recent improvements in its treatment, the pain is often inadequately controlled.[2] Several medications, mostly antidepressants, and antiepileptics, have been used with varying degrees of success in the treatment of painful diabetic neuropathy (PDN).[3] Incomplete efficiency of analgesics, systemic side effects, and cognitive impairment of these drugs due to central effects restrict their therapeutic success.[4] In this field, use of topical medications because of less systemic side effects is more acceptable. Topical capsaicin formulations those act by depletion of substance P and possibly other neurotransmitters from sensory nerve endings are widely used to manage neuropathic pain.[2,5] Also, topical form of clonidine that is an α2-adrenergic receptor agonist and was originally approved as an oral treatment for hypertension is used recently for pain relief.[6] Given the greater tendency to use of local medications in this area, this study aimed to compare the effect of clonidine gel and capsaicin cream in relieving pain associated with DPN.
MATERIALS AND METHODS
Study participants
Patients with type 2 diabetes, aged between 30 and 70 years, who had DPN were considered for the study. Patients with chronic daily pain for more than 3 months, who had a pain score of at least 4 as assessed by visual analog scale (VAS), were enrolled in the study. Patients with <1-year duration of diabetes, opium or alcohol use, use of other drugs for pain reduction, other causes of neuropathy, hepatic or renal failure (serum creatinine >1.5 mg/dl), clinically significant cardiovascular disease, HA1C ≥9%, pregnancy or lactation, ulcer or infection of foot, hypersensitivity to pepper were excluded from the study. Use of any medication for neuropathic pain was discontinued 2 weeks before enrolling the study and throughout it, but required therapies including insulin and oral hypoglycemic agents, were maintained throughout the study.
Study design and treatment
This 12-week, randomized, double-blind and parallel-group trial was conducted to compare the efficacy and safety of clonidine and capsaicin for pain associated with DPN. Laboratory tests, physical examinations, body weight, height, blood pressure and general characteristics including age, duration of diabetes, history of hypertension, pain duration and medications were recorded at the beginning of the study. PDN was confirmed by Neuropathy Symptom Score and Neuropathy Disability Score criteria.[7,8] Patients were randomized to clonidine or capsaicin group. Randomization was done by permuted-block design that involves randomizing patients to treatment groups in sequential blocks. Patients were then treated under double-blind conditions for up to 3 months. Written informed consent was obtained from each patient prior to enrollment.
The study drugs were clonidine 0.1% gel and capsaicin 0.75% cream in no label laminated tubes containing 100 g of material. Clonidine gel was prepared by dissolving the active ingredient in hydro alcoholic solution and then gelling agent (carbomer 941) was added under stirring (400 rpm). In the end, triethanolamine was added slowly until clonidine gel formed. Unichem Laboratories (India) via the Kimiara Company in Iran provided clonidine as free sample and capsaicin was provided by the Kish Medipharm pharmaceutical Company (Iran).
Clonidine or capsaicin was dispensed to patient according to randomized group assignment. Patients applied either of drugs topically below the ankles on the feet for 3 times a day.
The endpoint of the study was the reduction in the median pain score from baseline, as assessed by the VAS (0-10 points) [Figure 1]. Evaluations of the patients including assessment of the pain severity, vital signs and examination and questioning regarding the adverse effects were performed at each of the 4-weekly follow-up visits. Compliance was assessed by direct questioning. The investigator was accessible by telephone to all patients throughout the study.
Figure 1.
0-10 points visual analog scale
The study was approved by the Hamedan University of Medical Sciences Research Ethics Committee (D/P/16/35/9/1281). This clinical trial had been registered in Clinical Trials Registry - India (IRCT) (Registration ID: IRCT201207188308N1).
Statistical analysis
Previous study has shown the responder treatment in control group equal to 50%.[6] For sample size calculation, because of the type of this clinical trial (noninferiority trial), we assumed that the percentage “success” in both standard and experimental treatment groups is the same. According to G POWER software (G Power 3.0.10 for Windows), 138 patients were required to be 80% sure that the upper limit of a 95% two-sided confidence interval would exclude a difference in favor of the standard group of more than 25% (noninferiority limit was considered 25%). The efficacy analyses were performed on both per protocol analysis and the intention-to-treat (ITT) analysis. ITT population was defined as randomized patients who took at least one dose of medication and provided at least one baseline and one postbaseline efficacy assessment. The efficacy endpoint was the change in mean monthly pain score on the VAS. Values are expressed as means ± standard deviation and numbers and percentages. The patient VAS was compared using the Student's t-test. Cure rates between treatment groups and incidence of adverse events were compared by Chi-square test. In the study, participants were measured multiple times after baseline (week 4, week 8 and week 12) to see changes to the intervention. Therefore, repeated measure analysis was performed on VAS change scores (from baseline to week 12). For comparing variables at baseline, Mann-Whitney test was used for some continuous scale that didn’t have normal distribution such as pain duration and duration of diabetes, for other continuous variables with normal distribution, Student's t-test was performed, and, however, Chi-square test was used for comparing gender between the groups.
Missing data for subjects who terminated early were imputed using multiple imputations by the regression method. Multiple imputations involve drawing values of the parameters from a posterior distribution. Therefore, the multiple imputations simulate both the process generating the data and the uncertainty associated with the parameters of the probability distribution of the data.
A P < 0.05 was considered as significant. SPSS (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) was used to perform the analysis.
RESULTS
The study was conducted between April 2013 and February 2014. Patients’ demographic and baseline characteristics were comparable between two group of treatments those are presented in Table 1. The flow chart of patient enrollment and disposition is shown in Figure 2. The ITT population for the efficacy analysis consists of 69 patients receiving clonidine and 70 patients receiving capsaicin. A total of 46 (33%) patients discontinued therapy during the treatment period: 16 (23.1%) taking clonidine and 30 (42.85%) taking capsaicin. Discontinuation rate was higher in capsaicin group. Totally, 157 eligible patients of 258 screened patients enrolled the study. A total of 93 patients of 139 had completed the study with 66.9% compliance.
Table 1.
Demographic and baseline characteristics of patients in capsaicin and clonidine groups
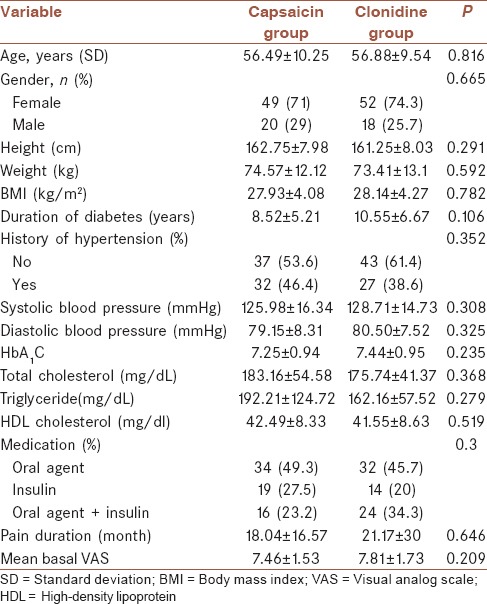
Figure 2.
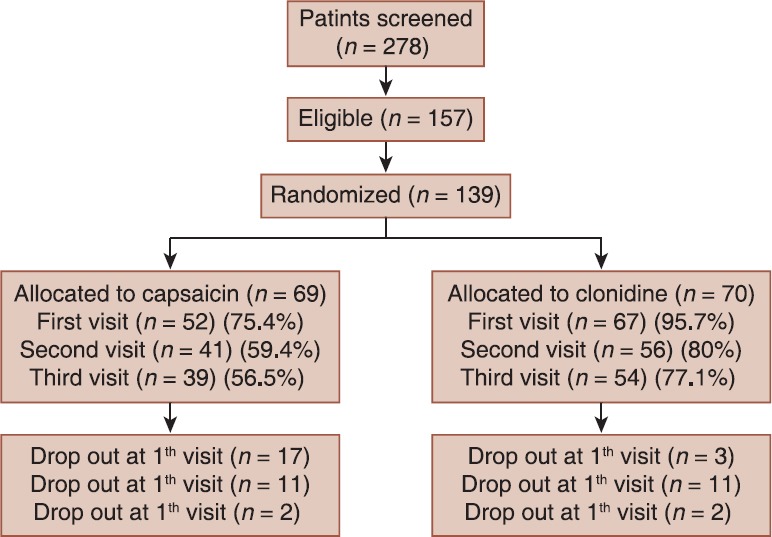
The flow chart of patient enrollment and disposition
Treatment responders (≥50% reduction from baseline on the VAS assessment) at week 12, were 40 (57.1%) and 28 (40.6%) of patients with clonidine and capsaicin, respectively (P = 0.051). As shown in Figure 3, in the intention to treat population both drugs significantly relieved pain at 12 weeks (P < 0.001 for both) but no significant difference in the efficacy between the two treatments was observed (P = 0.931). Also, the slope of the VAS decline had not a significant difference in both groups (P = 0.189). Therefore, there is not a significant time and treatment interaction. Per protocol analysis showed similar results.
Figure 3.
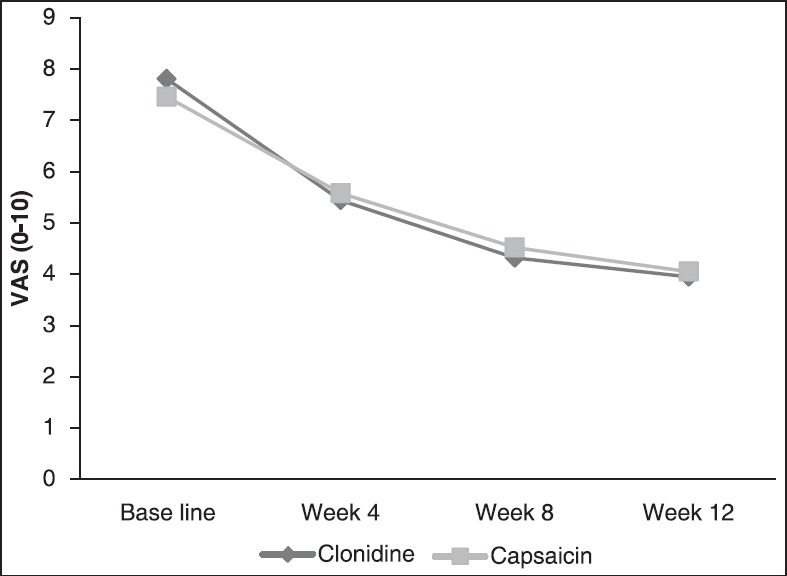
Efficacy of clonidine versus capsaicin on pain severity over 12 weeks of treatment visual analog scale
The number needed to treat, was 6, that calculated as 1/([percent not improved with capsaicin]−[percent not improved with clonidine]).
Systolic and diastolic blood pressures had not significant changes in the course of the treatment in the clonidine group (P = 0.547, 0.247 respectively).
Dermatologic complications were more common in capsaicin group: 40 (58%) patients in capsaicin group and 4 (5.7%) patients in clonidine group (P = 0.001). Skin dryness and then itching were the most common in the clonidine group (4.2% and 1.4% respectively). The most common side effects of capsaicin were itching, blister formation and erythema respectively (45.5%, 13.6% and 9.1%).
DISCUSSION
The overall results of this study indicated the comparable efficacy of clonidine gel in comparison with capsaicin cream in the treatment of pain due to peripheral neuropathy in type 2 diabetic patients. Less adverse events in treatment with the clonidine gel was an additional advantage.
With any drug treatment side effects is an important issue. Despite the established efficacy of some oral medications in neuropathic pain, side effects are experienced by most of the patients.
Clonidine, an α-2 receptor agonist, is an effective antinociceptive agent, that its effects are mediated via spinal and supraspinal sites.[9] However, its oral administration is limited by adverse effects including sedation, dry mouth, hypotension, and rebound hypertension.[10] Topical medications have some advantages in comparison with systemic drugs. Higher drug concentrations at the site of pain and avoiding adverse central side effects are some of the advantages.[11]
Capsaicin is the active compound present in chilli peppers that binds to nociceptors which controls the movement of sodium and calcium ions across the cell membrane. Initially, binding opens the ion channel, causing depolarization and the production of action potentials, which are usually perceived as itching, pricking or burning sensations. Repeated applications or high concentrations give rise to a long-lasting effect, which has been termed “defunctionalization,” probably owing to a number of different effects that together overwhelm the cell's normal functions and can lead to reversible degeneration of nerve terminals.[12] Capsaicin cream 0.075% is over-the-counter in US and is approved for DPN[13] but causes pain on the initial use that may be intolerable, though this generally subsides within 2 weeks.[12,14]
The current therapies for pain reduction in diabetic neuropathies have many adverse effects, while a recent systematic review and meta-analysis of the pharmacologic interventions for PDN reported adverse effects such as somnolence and dizziness with tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and anticonvulsants; xerostomia with tricyclic antidepressants; and peripheral edema and burning sensation with pregabalin and capsaicin.[15]
In an animal study by Ahmet Dogrul in Turkey, topical administration of clonidine to mice elicits antinociception without producing the undesirable central side effects of the systemic administration.[16]
In one study, transdermal patch of clonidine in patients with reflex sympathetic dystrophy, reduced hyperalgesia.[17] In a pilot study, application of clonidine (0.2 mg/g) cream in 17 patients with orofacial pain reduced the severity of burning.[18] The results of the two other studies demonstrated that a subset of patients with PDN benefits from transdermal clonidine administration.[19,20]
Campbell et al. compared the efficacy of topical clonidine with placebo, in a randomized, double-blind, placebo-controlled, parallel-group, multi-center trial.[6] In this study, 0.1% topical clonidine gel significantly reduced the level of foot pain in comparison with placebo (P = 0.01). Also, no severe adverse events were reported in treatment with clonidine. A limitation of this study was the use of one endpoint (VAS) for the efficacy measurement. Application of other endpoints such as tests for function, sleep, depression and anxiety could be helpful for better evaluation of the efficacy and safety. While the present study could not prove the better effect of clonidine gel in pain control of DPN patients, similar efficacy with capsaicin shows that this drug can be an alternative medication for pain relief in these patients.
CONCLUSION
In general, in our study clonidine was well tolerated and safe during this 12-week study. There were more discontinuations due to adverse events in the capsaicin treatment group than in the clonidine treatment group. This study compared the efficacy of clonidine gel with capsaicin cream, a FDA approved drug, but prolonged therapy and evaluation for a longer duration than the 12 weeks can better evaluate the benefits of this drug. More studies are required to better evaluate the efficacy and safety of this topical compound for relieving pain in DPN.
AUTHOR'S CONTRIBUTION
JK carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. FS and SAN provide assistance in the design of the study, coordinated and carried out all the experiments and participated in manuscript preparation. FEA provides assistance in the design of the study and performed the statistical analysis and participated in manuscript preparation. All authors have read and approved the content of the manuscript.
ACKNOWLEDGMENTS
This study was supported by the grant of Hamedan University of Medical Sciences (project number: D/P/16/35/2666). The authors are indebted to the patients that participated in this project. We gratefully acknowledge Dr. Jalal Kiani (Tehran University of Medical sciences) and Dr. Shiva Borzuie (Hamedan University of Medical sciences) for participation in study conduct. Also support of Mr. Rahbar, the trading development manager of the Kimiara Company in preparation of the clonidine raw material is appreciated. The authors would like to thank Mrs. Asgari for her contribution in clonidine gel preparation.
Footnotes
Source of Support: This study funded by the grant of Hamedan University of Medical Sciences (project number: D/P/16/35/2666).
Conflict of Interest: No conflict of interests.
REFERENCES
- 1.Marchettini P, Teloni L, Formaglio F, Lacerenza M. Pain in diabetic neuropathy case study: Whole patient management. Eur J Neurol. 2004;11(Suppl 1):12–21. doi: 10.1111/j.1471-0552.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- 2.Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: A randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497–503. doi: 10.1111/papr.12013. [DOI] [PubMed] [Google Scholar]
- 3.Huizinga MM, Peltier A. Painful diabetic neuropathy: A management centered review. Clin Diabetes. 2007;25:6–15. [Google Scholar]
- 4.Jorge LL, Feres CC, Teles VE. Topical preparations for pain relief: Efficacy and patient adherence. J Pain Res. 2010;4:11–24. doi: 10.2147/JPR.S9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand P, Bley K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107:490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell CM, Kipnes MS, Stouch BC, Brady KL, Kelly M, Schmidt WK, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815–23. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: The Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995;45:1115–21. doi: 10.1212/wnl.45.6.1115. [DOI] [PubMed] [Google Scholar]
- 8.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–4. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 9.Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000;90:400–7. doi: 10.1097/00000539-200002000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Puskas F, Camporesi EM, O’Leary CE, Hauser M, Nasrallah FV. Intrathecal clonidine and severe hypotension after cardiopulmonary bypass. Anesth Analg. 2003;97:1251–3. doi: 10.1213/01.ANE.0000083526.08033.20. [DOI] [PubMed] [Google Scholar]
- 11.Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Derry S, Sven-Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;2:CD007393. doi: 10.1002/14651858.CD007393.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler D, Fonseca V. From guideline to patient: A review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications. 2015;29:146–56. doi: 10.1016/j.jdiacomp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012;9:CD010111. doi: 10.1002/14651858.CD010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161:639–49. doi: 10.7326/M14-0511. [DOI] [PubMed] [Google Scholar]
- 16.Dogrul A, Uzbay IT. Topical clonidine antinociception. Pain. 2004;111:385–91. doi: 10.1016/j.pain.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–17. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JB, Grushka M, Le N. Topical clonidine for orofacial pain: A pilot study. J Orofac Pain. 1997;11:346–52. [PubMed] [Google Scholar]
- 19.Zeigler D, Lynch SA, Muir J, Benjamin J, Max MB. Transdermal clonidine versus placebo in painful diabetic neuropathy. Pain. 1992;48:403–8. doi: 10.1016/0304-3959(92)90092-P. [DOI] [PubMed] [Google Scholar]
- 20.Byas-Smith MG, Max MB, Muir J, Kingman A. Transdermal clonidine compared to placebo in painful diabetic neuropathy using a two-stage ‘enriched enrollment’ design. Pain. 1995;60:267–74. doi: 10.1016/0304-3959(94)00121-t. [DOI] [PubMed] [Google Scholar]


