Abstract
Background:
One of the major causes of death in schizophrenia is a metabolic syndrome. The clozapine has the highest rate of weight gain among antipsychotics. It has been shown that metformin can promote weight loss. We aimed to investigate the effect of metformin as an adjunctive therapy with clozapine to prevent metabolic syndrome in patients with schizophrenia.
Materials and Methods:
A total of 37 patients consisting metformin group (19 cases) and a group of placebo consisting of 18 cases were evaluated. A brief psychiatric rating scale score (BPRS) and metabolic profiles was determined for all patients. All of the variables were also determined at 2, 8, 16, and 20 weeks after the onset of the study.
Results:
The mean age of the group of metformin was 47.2 ± 10.4 compared with 45.8 ± 10.2 for the group of placebo. The difference in mean waist circumference and serum level of triglyceride at baseline compared with the end of study showed a statistically significant difference between two groups (P = 0. 000). A statistically significant difference was also observed in a comparison of mean difference of weight and body mass index at baseline compared with end of study (P = 0. 000). There was a statistically significant difference of fasting blood sugar (P = 0.011) and serum high-density lipoprotein (P = 0.000) between two groups but this difference was not significant for mean BPRS scores, mean systolic and diastolic blood pressure, serum level of triiodothyronine, thyroxin and thyroid stimulating hormone, serum low-density lipoprotein and serum cholesterol.
Conclusion:
Metformin could be considered an adjunctive therapy with clozapine to prevent metabolic syndrome in schizophrenic patients.
Keywords: Clozapine, metformin, schizophrenia
INTRODUCTION
Schizophrenia is a mental disorder with a prevalence of 1% in general population.[1] The etiology is not fully understood, but some investigations have reported differences in brain mass in patients with schizophrenia compared with control group.[2] The mortality rate is significantly higher in patients with schizophrenia and they experience a lower life expectancy compared with general population.[3,4] One of the major causes of death in schizophrenia is cardiovascular diseases.[5] The modifying risk factors for cardiovascular disease include: High blood cholesterol and triglyceride, high fasting blood glucose, and central obesity, among others that collectively known as metabolic syndrome.[6] On the other hand, obesity is more common in patients with schizophrenia than in general population.[7] One explanation is that schizophrenic patients more commonly consume high fat-low fiber diet.[8] Another explanation is that second generation antipsychotics that are widely used for the treatment of schizophrenia are accompanied by a higher incidence of weight gain and metabolic syndrome that may result in being noncompliant with medications.[9,10] The prevalence of metabolic syndrome in schizophrenic patients ranging from 11% to 69% based on the medication that was used by the patient.[11] The prevalence of metabolic syndrome among Iranian patients with schizophrenia is 27.4%, 37.6%, and 38.7% that were reported in different studies.[12] Atypical antipsychotics also showed an increase in blood glucose and cholesterol levels and the rise is specifically significant for clozapine.[13] The prevalence of metabolic syndrome among patients who are taking clozapine is ranging from 46% to 62%.[11] The clozapine has also the highest rate of weight gain among antipsychotics.[14] Clozapine is metabolized by liver enzymes and has a half-life of 9.1-17.4 h.[15] The weight gain is most likely due to the effect of clozapine on H-1 receptor in hypothalamus.[16] If more serious side effects, including: Agranulocytosis, myocarditis, cardiomyopathy, and a prolonged QTc interval occurs, discontinuation of medication may be warranted,[17] but since clozapine is an efficacious treatment for schizophrenia, especially in treatment-resistant cases, it is not always comfortable to ignore the therapeutic effects of this medicine.[18,19] Therefore, defining new treatment protocols to decrease drug-induced side effects is mandatory.
Metformin is primarily used for the treatment of diabetes mellitus. It decrease the amount of serum glucose through a few mechanism including: Enhancing glycemic control effects of insulin, gluconeogenesis and glycogenolysis suppression and antagonizing the effects of glucagon.[20] It has been shown that metformin can promote weight loss.[21] Metformin is also successfully used as an adjunctive therapy with atypical antipsychotics to decrease weight gain.[22] Best results were obtained when metformin was used in combination with lifestyle changes.[23] In animal models, metformin could neutralize some metabolic effects of clozapine when used in combination with clozapine.[24] In human subjects, some studies have reported a decrease in mean waist circumference, serum level of triglyceride, and BMI in the group of metformin compared with placebo in patients who were treated by antipsychotics but the number of these studies are still very limited and as of our knowledge, no study of this kind has not previously been done in Iranian population.[23,25,26,27]
On the other hand, some characteristics of clozapine can be influenced by ethnicity. Therefore we decided to investigate the effects of metformin as add-on treatment with clozapine to prevent metabolic syndrome in patients with schizophrenia in Iranian population.
MATERIALS AND METHODS
We conducted a double-blind placebo-controlled clinical trial. The duration of study was 20 weeks and it was done on 37 patients who were hospitalized in either Ibn-e-Sina or Hejazi Psychiatric Hospital (Mashhad, Iran) with a definitive diagnosis of schizophrenia between December 2008 and June 2010. Our Institutional Ethics Committee approved the research project, and informed consent was obtained from patients or their surrogates (research project number: 2214). Potential candidates for the study were interviewed by two psychiatrists and if the diagnosis of schizophrenia was confirmed using diagnostic and statistical manual of mental disorders, 4th edition, text revision (DSM-IV-TR) criteria for schizophrenia and they met our inclusion criteria, the patients would receive clozapine combined by either placebo or metformin by a nurse who was unaware of the study groups.[28]
The sample size was calculated with considering a confidence interval level of 95%, α = 0.05 and a sample power of 95%. 60 patients were initially included in this study and they were randomly assigned into a group of metformin and a group of placebo, but later in the study, 11 patients in the group of metformin and 12 patients in the group of placebo were dropped out of the study because of serious drug side effects and ultimately 19 cases in the group of metformin and 18 cases in the group of placebo continued the study to the end [Figure 1].
Figure 1.
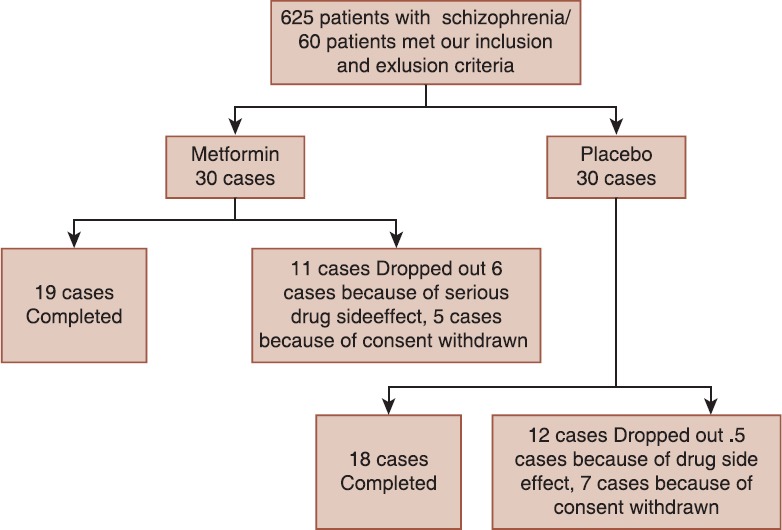
Consort diagram of participant flow
The inclusion criteria for the study were as follows:
Patients with a diagnosis of schizophrenia, according to the DSM-IV-TR criteria.
Age between 18 and 75.
Patients receiving clozapine and with a BMI of >25.
Patients with following criteria were not included in the study:
Patients receiving hormonal medications.
Patients with other serious medical or mental illness, including seizure based on patients’ medical records or examination by a neurologist.
Chronic diseases, including high blood pressure or diabetes mellitus.
Patients with an abnormal liver functions test, kidney function test, thyroid function test or fasting blood sugar (FBS) at the onset of the study.
Female patients who were breastfeeding or had a positive beta-human chorionic gonadotropin test.
Patients who were substance abuser or patients who were taking any medications during the 1-month before the study.
The exclusion criteria were as follows:
Severe drug-caused side effects, including: High blood pressure, seizure, severe agitation or severe lightheadedness.
Medical problems that warranted therapy, including: Diarrhea, urinary tract infection, trauma or cardiovascular disease.
Patients who discharged from the hospital by their own consent.
Patients who refused to complete the study and follow-up.
A clinical interview was done by a psychiatrist to confirm the diagnosis, to determine if the patient meets our inclusion criteria and to ascertain the dose and the duration of clozapine and the quantity of cigarette smoke used by the patient. A baseline brief psychiatric rating scale (BPRS) score was determined for all patients by a psychiatrist. Metabolic profiles, including: FBS, triglyceride, cholesterol, thyroid function tests, BUN, and Cr were measured by central lab of the mentioned hospitals that are using automated laboratory instruments Body weight, BMI and waist circumference were measured by a trained health staff. Demographic parameters were obtained from patients’ medical records. Patients who were qualified for the study were randomly assigned into a group of metformin 500 mg (Aria, Iran) and a group of placebo. Since previous studies have shown that metformin with a low dose of 500 mg/day is well effective for metabolic syndrome.[21] Randomization was done using Balanced block randomization method. Within 1-week after the onset of the study, the dose of metformin was increased to 1000 mg, one tablet in the morning and one tablet in the afternoon. The patients in the group of placebo were also treated the same way. Potential drug side effects were evaluated at baseline and at weeks 2, 8, 16, and 20 after the onset of the study by a psychiatrist using checklist.
Since there is little evidence to suggest that the weight gain and metabolic syndrome related to clozapine is dose dependent,[29] metformin was added to the patient's previous clozapine regimen. Metformin was discontinued at week 16 and reevaluation was done at week 20. BPRS scores were determined at baseline and at the end of the study (week 20).
Data analysis
Statistical analysis was done using SPSS for windows version 20.0 (SPSS, Chicago, IL, USA). Since all patients were hospitalized and we did not have any missing data, we followed the per protocol strategy for data analysis and we used Chi-square, independent t-test, Fisher exact test and Kolmogorov–Smirnov and ANOVA with repeated measures test. P < 0.05 was considered significant.
RESULTS
A total of 60 patients were initially included in this study and they were randomly assigned into a group of metformin and a group of placebo, but later in the study, 11 patients in the group of metformin and 12 patients in the group of placebo were dropped out of the study and ultimately 19 cases in the group of metformin and 18 cases in the group of placebo continued the study to the end [Figure 1].
Ten patients in the group of metformin and seven patients in the group of placebo were male. Statistical analysis using a Chi-square test showed no statistically significant differences between two groups. 12 patients in the group of metformin and 10 patients in the group of placebo were single. According to Fisher exact test, there was no statistical difference in marital status between two groups. Five patients in the group of metformin and eight patients in the group of placebo were smokers. The Chi-square test demonstrated no statistical differences between two groups. Evaluation of educational level between two groups was done using a Fisher exact test that showed no statistical difference between two groups. The above findings suggest that gender, marital status, smoking, and educational level are equal in both groups and does not influence the results. These are illustrated in Table 1.
Table 1.
Comparison of demographic characteristics and quantitative parameters at baseline between two groups of patients
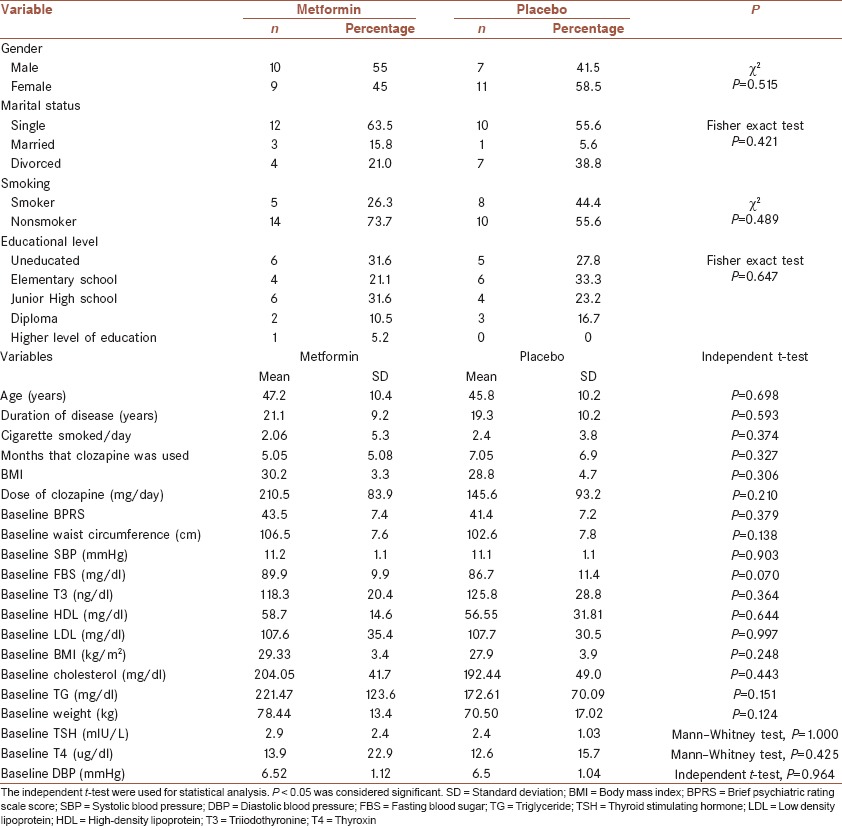
For quantitative data analysis, normal distribution was evaluated using Kolmogorov-Smirnov test. All quantitative variables had a normal distribution except for: The amount of cigarette smoked, the level of systolic blood pressure (SBP) at 2, 8, and 20 weeks after the onset of the study, the level of diastolic blood pressure (DBP) at the baseline and at weeks 8 and 20 after the onset of the study, the amount of FBS at week 2 and the serum levels of thyroxin (T4) and thyroid-stimulating hormone (TSH) at baseline and 2 weeks after the onset of the study.
Comparison of quantitative parameters at baseline was done using t-tests. The mean age of the group of metformin was 47.2 ± 10.4 compared with 45.8 ± 10.2 for the group of placebo. Comparison of mean age between two groups using t-test demonstrated no statistical significant difference between two groups. Similar results were also observed in comparison of mean duration of disease, mean number of cigarette smoked per day, mean months of clozapine use prior to the onset of the study, the mean daily dose of clozapine, the mean BPRS scores at baseline, the mean waist circumference at baseline, the mean SBP and DBP, the mean FBS, the mean serum level of triiodothyronine (T3), T4, and TSH, low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol and triglyceride, and the mean body weight and body mass index. This statistical analysis supports the idea that the randomization was successful [Table 1].
The BPRS scores were calculated at the end of the study (week 20). Mean BPRS score for the group of metformin was 43.5 ± 7.4 and in the group of placebo it was 41.4 ± 7.2. Comparison of mean BPRS scores using the t-test showed no statistical significant differences between two groups. Waist circumference was measured at baseline and at 2, 8, 16, and 20 weeks after the onset of the study. Comparison of mean waist circumferences between two groups using ANOVA with repeated measures test demonstrated a statistically significant differences between two groups at 2, 8, 16, 20 weeks, but the difference in mean waist circumference between the onset of the study and waist circumference at the end of the study was significantly higher for the group of metformin compared with placebo (P = 0.000) demonstrating that the metformin is effective in decreasing the waist circumference in patients treated with clozapine [Table 2]. Comparison of mean SBP and DBP between two groups was done at the baseline and at 2, 8, 16, and 20 weeks after the onset of the study using the ANOVA with repeated measures test that showed no statistical differences between two groups. Comparison of mean difference in SBP and DBP at baseline and at the end of the study between the two groups also demonstrated no significant difference between two groups. Comparison of serum level of FBS between two groups using ANOVA with repeated measures demonstrated no statistical difference over time. The same test was used for evaluation of serum level of high-density lipoprotein that demonstrated a statistically significant difference between two groups (P = 0.048). T3, T4, and TSH, serum low density lipoprotein and serum cholesterol at baseline and at 2, 8, 16 weeks after the onset of the study between two groups demonstrated no statistical significant difference between two groups. The mean difference between baseline and endpoint also was not statistically significant for these values. The results were observed in the serum level of triglyceride in comparison of two groups at baseline and at weeks 2, 8, and 16 and 20 shows statistically significant differences at weeks 8, 16, 20.
Table 2.
Comparison of different parameters between and within two groups
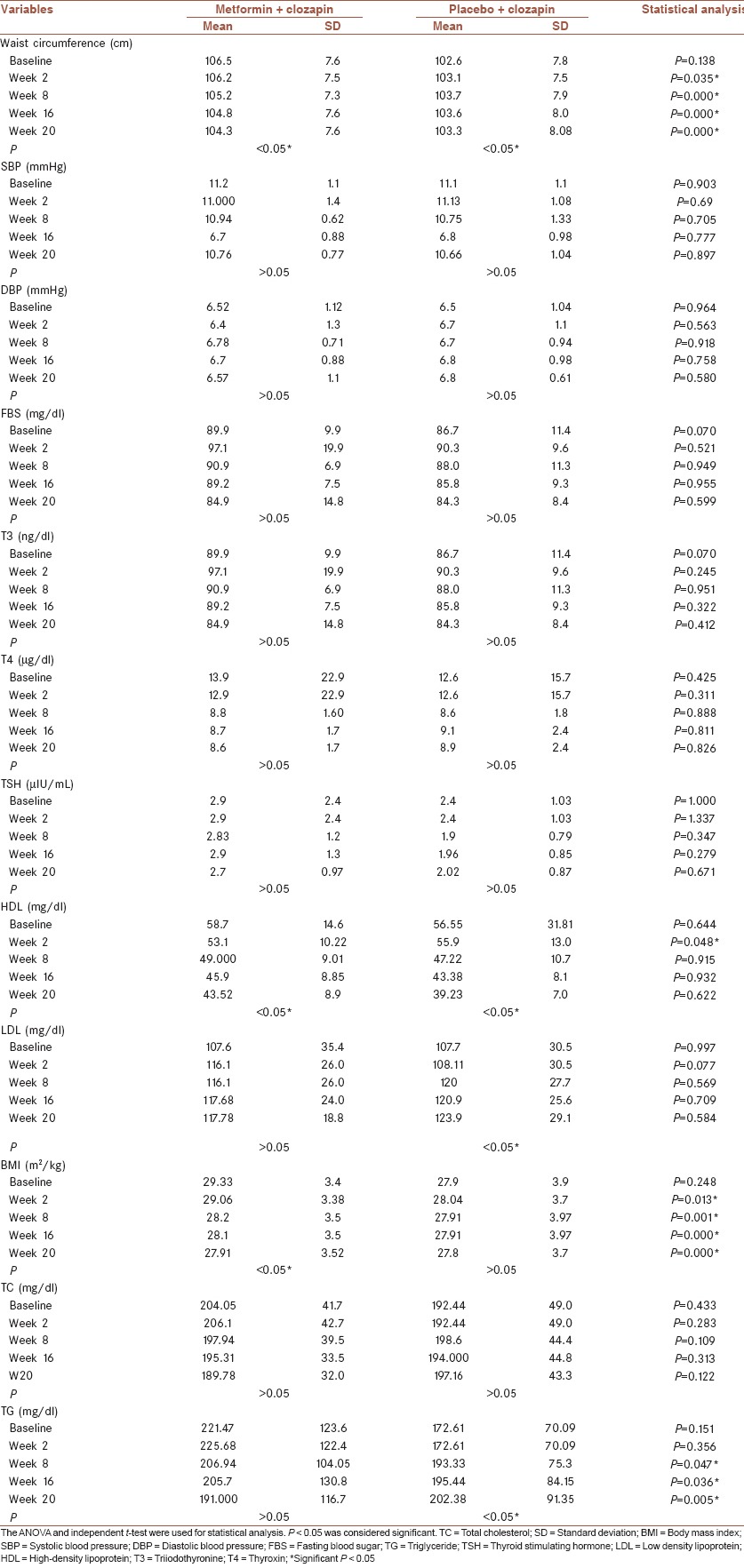
Body mass index of participants in the group of metformin at baseline was 78.44 ± 13.4 and 29.33 ± 3.4, respectively, while for the group of placebo, it was 70.50 ± 17.02 and 27.9 ± 3.9. These are illustrated in Table 2.
The most common drug-induced side effect was GI upset and nausea.
Collectively patients in the group of metformin compared with placebo showed significantly more decrease in baseline weight, BMI, TG, and waist circumferences compared with study endpoint.
DISCUSSION
In this study for the first time in Iranian population, the effect of metformin as add-on treatment with clozapine in schizophrenic patients was evaluated. In a review of literature, we found very limited studies, including a case that was reported by Ozenoglu et al. in 2007[25] and two studies that were conducted by Wu et al. and Shin et al. in 2008,[23,26] that specifically evaluated the effect of metformin on side effects induced by clozapine in patients with schizophrenia. For this reason, we had some limitations in comparison of our results with findings in previous studies.
In Wu et al.'s study, 128 patients were participated. 40 cases were treated with a combination of clozapine and metformin and all other patients were treated by either olanzapine, risperidone or sulpiride. In the group of metformin, a decrease of 2.10 cm in mean waist circumference was observed while in the group of placebo, an increase of 2.2 cm in mean waist circumference was reported. Shin et al., have reported a decrease of 2 cm in mean waist circumference. These findings are compatible with our findings. In our study, we showed a decrease of 2.21 cm in mean waist circumference in patients in the group of metformin.[23,26] In our study, metformin had no effect on SBP or DBP, which is compatible with some previous studies.[23,24,25,26] In our study, there was a statistically significant decrease in serum levels of triglyceride in the group of metformin compared with placebo. Similar results have been reported by Carrizo et al.[27] while in the study that was conducted by Ozenoglu et al., the serum level of TG was not significantly different between the two study groups.[26] In the context of thyroid function tests, we did not find any statistically significant difference between two groups. Our findings are compatible with those reported by Ozenoglu et al.[25] In our study, we reported a statistically significant difference in serum HDL between two groups but no statistically significant difference in serum cholesterol, triglyceride or LDL. Carrizo et al., reported an increase in serum HDL and a decrease in serum LDL but Wu et al. and Ozenoglu et al., did not report any changes in serum levels of HDL.[25,26,27] Shin et al. conducted a study on patients aged between 10 and 18 and they reported a decrease in serum levels of triglyceride with no changes in HDL or LDL which is similar to our findings.[26] In this study, we observed an increase in serum levels of LDL in both groups, but this increase was not statistically significant. This finding is compatible with Ozenoglu et al.[25] Similar results were also reported by Baptista et al. in metformin as an adjunctive treatment with Olanzapine.[30] In our study, a statistically significant decrease in BMI was observed in the group of metformin compared with placebo. This decrease was not significant in studies conducted by Shin et al. and Carrrizo et al. but it was significant in studies conducted by Wu et al. and Ozeroglu et al. We also showed a weight loss between 1 and 8 kg in the group of metformin compared with placebo which is compatible with those reported by Ozeroglu et al. in which they reported a weight loss of 27 kg in a schizophrenic patient who was treated with metformin and clozapine. Shin et al. and Wu et al. also reported similar results in their studies. In a double-blind clinical trial that was conducted by Carrizo et al. on 61 patients, similar to our study, metformin was perfectly tolerated and a weight loss has been reported in both metformin and placebo.[25,26,27] Incompatible findings in previous studies could be attributable to the fact that the number of studies published in the literature in this context was very limited and some, different psychiatric disorders other than schizophrenia that were considered in these studies, differences in duration of studies, the dose of metformin or the type of antipsychotic that was used in these studies. Furthermore, the limitations of our study, including short duration of study, small sample size, and a large number of dropouts from the study and use of a fixed dose of 1000 mg metformin might also have influenced our results.
Collectively, our study confirmed the effect of metformin on waist circumference, body weight and BMI, and the serum level of triglyceride. Since this study was done on Iranian population, in order to clarify the role of metformin as an adjunctive therapy with clozapine, more studies in other populations with different doses of metformin is mandatory.
AUTHOR'S CONTRIBUTIONS
PH contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
AAM contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
FB contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
MNM contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
KAR contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
EH contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
AAR contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENT
The authors would like to thank Research Council of the Mashhad University of Medical Sciences for providing the fund of this study. This work was supported by a grant from Mashhad University of Medical Sciences (research project number: 2214).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sawa A, Snyder SH. Schizophrenia: Diverse approaches to a complex disease. Science. 2002;296:692–5. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 2.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–8. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 4.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–48. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 5.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–21. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Dickerson FB, Brown CH, Kreyenbuhl JA, Fang L, Goldberg RW, Wohlheiter K, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113:306–13. doi: 10.1111/j.1600-0447.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 8.Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: A systematic review. J Psychiatr Res. 2013;47:197–207. doi: 10.1016/j.jpsychires.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 10.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2010;123:225–33. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra N, Grover S, Chakrabarti S, Kulhara P. Metabolic syndrome in schizophrenia. Indian J Psychol Med. 2013;35:227–40. doi: 10.4103/0253-7176.119471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezaei O, Khodaie-Ardakani MR, Mandegar MH, Dogmehchi E, Goodarzynejad H. Prevalence of metabolic syndrome among an Iranian cohort of inpatients with schizophrenia. Int J Psychiatry Med. 2009;39:451–62. doi: 10.2190/PM.39.4.i. [DOI] [PubMed] [Google Scholar]
- 13.Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160:290–6. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- 14.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 15.Jann MW, Grimsley SR, Gray EC, Chang WH. Pharmacokinetics and pharmacodynamics of clozapine. Clin Pharmacokinet. 1993;24:161–76. doi: 10.2165/00003088-199324020-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456–9. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen J, Correll CU, Manu P, Kane JM. Termination of clozapine treatment due to medical reasons: When is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74:603–13. doi: 10.4088/JCP.12r08064. [DOI] [PubMed] [Google Scholar]
- 18.Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull. 1988;24:62–7. [PubMed] [Google Scholar]
- 19.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 20.Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: Therapeutic and cellular mechanisms. Drugs. 1999;58(Suppl 1):31–9. doi: 10.2165/00003495-199958001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Golay A. Metformin and body weight. Int J Obes (Lond) 2008;32:61–72. doi: 10.1038/sj.ijo.0803695. [DOI] [PubMed] [Google Scholar]
- 22.Das C, Mendez G, Jagasia S, Labbate LA. Second-generation antipsychotic use in schizophrenia and associated weight gain: A critical review and meta-analysis of behavioral and pharmacologic treatments. Ann Clin Psychiatry. 2012;24:225–39. [PubMed] [Google Scholar]
- 23.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: A randomized controlled trial. JAMA. 2008;299:185–93. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Wang G, Liu H, Yan C. Effect of combined treatment with clozapine and metformin on fasting blood glucose, insulin level, and expression of the glucose transporter-2 (GLUT2) in Sprague-Dawley rats. Shanghai Arch Psychiatry. 2013;25:149–56. doi: 10.3969/j.issn.1002-0829.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozenoglu A, Ugurlu S, Balci H, Eker E. Nutritional approach to metabolic changes arising out of schizophrenia therapy: Case report. Intern Med. 2007;46:1213–8. doi: 10.2169/internalmedicine.46.6323. [DOI] [PubMed] [Google Scholar]
- 26.Shin L, Bregman H, Breeze JL, Noyes N, Frazier JA. Metformin for weight control in pediatric patients on atypical antipsychotic medication. J Child Adolesc Psychopharmacol. 2009;19:275–9. doi: 10.1089/cap.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrizo E, Fernández V, Connell L, Sandia I, Prieto D, Mogollón J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: A 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113:19–26. doi: 10.1016/j.schres.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Jablensky A. The diagnostic concept of schizophrenia: Its history, evolution, and future prospects. Dialogues Clin Neurosci. 2010;12:271–87. doi: 10.31887/DCNS.2010.12.3/ajablensky. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70:1041–50. doi: 10.4088/jcp.08r04392. [DOI] [PubMed] [Google Scholar]
- 30.Baptista T, Rangel N, Fernández V, Carrizo E, El Fakih Y, Uzcátegui E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: A multicentric, double-blind, placebo-controlled trial. Schizophr Res. 2007;93:99–108. doi: 10.1016/j.schres.2007.03.029. [DOI] [PubMed] [Google Scholar]


