Abstract
Background:
There is accumulating evidence for a possible protective role of vitamin D in the development and disease course of multiple sclerosis. Whether vitamin D is also effective in treating patients with optic neuritis (ON) is not known. The aim of this study was to evaluate the effect of oral vitamin D on the thickness of retinal nerve fiber layer (RNFL) in vitamin D deficient patients with ON by optical coherence tomography.
Materials and Methods:
A Phase II placebo-controlled randomized clinical trial conducted between July 2011 and November 2012 included 52 patients with confirmed unilateral ON aged 15-38 years and low serum 25-hydroxyvitamin D levels. The main outcome measures were changes in thickness of RNFL and macula 6 months after treatment. Patients were randomly allocated to receive 6 months of treatment with adding either 50,000 IU/week vitamin D or placebo.
Results:
In the 27 patients treated with vitamin D, the mean (standard deviation [SD]) thickness of RNFL decreased from 111.3 (18.9) μm at baseline to 91.4 (13.3) at the end of study period (P < 0.001). Correspondingly, in the 25 patients treated with placebo, the mean (SD) thickness of RNFL decreased from 113.7 (21.5) μm at baseline to 96.1 (12.3) at the end of study period (P < 0.01). In both groups, the mean thickness of the macula did not changed (P > 0.05). Average thickness of RNFL at the end of trial did not differ between groups.
Conclusion:
Adding vitamin D to routine disease therapy had no significant effect on the thickness of RNFL or macula in patients with ON. This trial is registered on www.clinicaltrials.gov (ID NCT01465893).
Keywords: 25-hydroxyvitamin D, efficacy, Iran, optic neuritis, optical coherence tomography, vitamin D
INTRODUCTION
Optic neuritis (ON), an immune-mediated inflammatory disorder of the optic nerve, causes loss of vision usually due to swelling and destruction of the myelin sheath around the axons of optic nerve. Direct axonal damage may also play a role in nerve destruction in many cases. It is characterized by sudden partial or complete loss of vision, dyschromatopsia, pain with or without optic disc swelling and afferent papillary defect in asymmetric or unilateral cases and may present as an isolated episode or as the first presentation of multiple sclerosis (MS).[1,2]
Studies in unilateral ON patients show a decrease in thickness of retinal nerve fiber layer (RNFL) due to axonal loss and macular volume,[3,4,5,6,7] occurring within 1-6 months after ON[4,5] and a single event of ON typically results in a mean loss of about 18-22% of RNFL thickness compared to the fellow eye.[5,6]
Vitamin D plays an important role in bone formation and mineral homeostatis. Studies have also suggested that vitamin D affect immune and central nervous system development and function and has immune-regulatory capacity.[8,9,10,11] Some in-vitro and animal studies suggest that vitamin D supplementation reduced inflammatory infiltration in the CNS by suppressing function of antigen-presenting cells.[12] There is accumulating evidence for a possible protective role of vitamin D in the development and disease course of MS.[13,14,15,16,17] Whether vitamin D is also effective in treating patients with ON is not known. We hypothesize that vitamin D treatment reduces axonal loss in ON patients primarily by its properties to modulate inflammation, and perhaps also by its neuroprotective properties.
Thickness of RNFL and macula can accurately be measured using optical coherence tomography (OCT). It is a fast, noninvasive, noncontact, transpupillary imaging method with highly reproducible procedures.[6,7,18,19,20,21] Several studies have suggested that peripapillary RNFL and macular thickness analysis may be used to detect axonal loss in ON and MS.[19,22] Estimation of the retinal thickness from the OCT is a technique that offers great potential in this field.[6,7,18,19,22,23,24] The rapid changes in RNFL after acute ON make it useful for testing neuroprotective strategies over a short time frame.
In the present exploratory Phase II trial, we examined the therapeutic effect of vitamin D on the change of RNFL measurements in ON. Because vitamin D is cheap, easy to administer, and safe; even modest therapeutic effectiveness of this drug would be useful in the treatment of ON. The objective of this trial therefore was to test the hypothesis that vitamin D treatment will reduce thinning of RNFL in ON patients, as measured by OCT.
MATERIALS AND METHODS
This is an exploratory Phase II randomized parallel-group clinical trial to evaluate the effect of oral vitamin D on the retinal changes in patients with ON.
Patients
The original study sample comprised of 74 consecutive patients with unilateral acute ON with symptoms present less <72 h who sought treatment at our neurology and ophthalmology outpatient clinics of Isfahan University of Medical Sciences, Iran, between July 2011 and November 2012: of these, 52 returned for follow-up. ON was diagnosed by ophthalmologists on the basis of clinical presentation, presence Of decreased visual acuity and assessment of visual evoked potentials. Entry criteria were either gender, age between 15 and 50 years, unilateral ON, serum 25-hydroxyvitamin D (25(OH)D) level <20 ng/ml, nonfulfillment of the McDonald criteria for MS[25] at the time of inclusion in the study and a willingness to continue current medications for the duration of the study. Assessments of serum 25(OH)D level was carried out, and used to detect vitamin D insufficiency. Serum 25(OH)D was measured using a commercially available radioimmunoassay kit (DiaSorin, Stillwater, MN, USA). The normal range for serum 25(OH)D level was 30-100 ng/ml. Exclusion criteria were evidence of substantial abnormalities in neurological, psychiatric, cardiac, endocrinological, hematologic, hepatic, renal, or metabolic functions, use of medication that could influence the prognosis of ON (e.g., β-interferon) during the 6 months follow-up period, vitamin D supplement, any condition predisposing to hypercalcemia, nephrolithiasis, renal insufficiency and pregnancy as determined by history, physical examination, and screening blood tests. Patients who demonstrated poor compliance with instructions to take vitamin D or the placebo, or who failed to attend for follow-up visits, OCT scanning, and 25(OH)D measurements during the study were excluded. Women of child-bearing potential were required to use a clinically-accepted method of contraception. Tenets of the current version of the declaration of Helsinki and standards of good clinical practice according to the International Conference on Harmonization of Technical Requirements for registration of pharmaceuticals for human use were followed, the study protocol was approved by the Ethics Committee of Isfahan University of Medical Sciences (approval number 290090 dated April 28, 2011) and the nature of the trial was explained to all participants. After a detailed discussion with the neurologist, patients made a final decision and each participant provided written informed consent. This trial is registered on www.clinicaltrials.gov (ID NCT01465893).
Randomization scheme
A total of 83 patients were screened for the study, but nine patients were excluded because they declined to participate or did not meet inclusion criteria. Seventy-four patients were assigned randomly to one of the two self-administer treatment groups. Twenty-two patients were failed to attend for follow-up visits. Fifty-two patients (4 [7.7%] men, 48 [92.3%] women) completed the study without interruption. Patients were randomized according to a preexisting list produced by a computer program differed from a random number generator only in that it assigned equal numbers of participants into each treatment group. All patients received an intravenous single daily dose of methylprednisolone (1000 mg/day) for 3 days, followed by oral prednisone (1 mg/kg/day) for 11 days. The first treatment group received a single dose of 50,000 IU adjunct vitamin D3 (trade name Vitamin D3, Zahravi Pharm. Co., Tabriz, Iran) per week in the form of oral pearls and continued for 6 months. The second group received a similar pearl-shaped placebo for 6 months. Compliance with the study treatment was verified by asking the patients about missed doses and by counting unused pearls. All patients underwent pretreatment evaluation to record demographic data, complete neurologic and medical history, the finding of physical and neurologic examination, and previous treatment. Figure 1 illustrates the patient allocation algorithm. In the final sample of participants, mean (SD) age was 27.0 (5.2) years (range 15-38 years).
Figure 1.
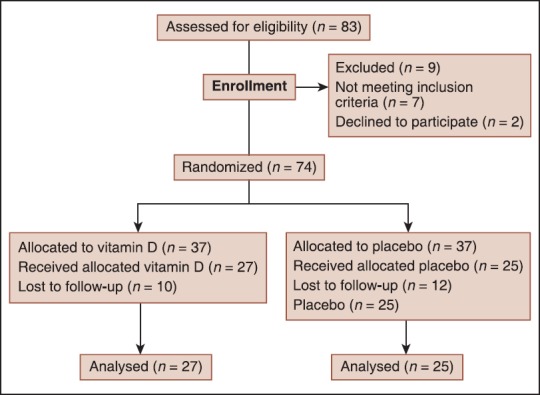
Design of the trial to compare oral vitamin D (50,000 IU/week) versus placebo in patients with optic neuritis
Ophthalmologic examination
Participants underwent a complete ophthalmologic examination, including best-corrected monocular visual acuity assessment, standard automated perimetry, and dilated fundoscopy using the biomicroscopic indirect ophthalmoscope. Participants underwent spectral domain OCT scanning of the macular area without dilating the pupil, using commercially available equipment (3D OCT-1000, Topcon Corp., Tokyo, Japan) on the same day of the ophthalmic evaluation. RNFL images were acquired by taking three circular 3.4 mm scans, centered on the optic disc, the mean of which was used to express RNFL thickness. Macular thickness maps were acquired by making six radial scans centered on the fovea, and by construction of a map from these scans. OCT was performed twice at the baseline and follow-up visit, and the mean value at each visit was used for the purpose of statistical analysis. OCT was done within 72 h of ON symptoms present. Criteria for acceptable 3D OCT-1000 fundus images included an absence of large eye movements, defined as an abrupt shift completely disconnecting a large retinal vessel, a consistent signal intensity level across the scan, and an absence of black bands (caused by blinking) throughout the examination. Six months later the participants were again examined, with OCT measurements performed in a similar fashion to the first visit. No patient had any other known ophthalmological disease.
Patient evaluation
The trial was single-blinded in that patients were unaware of the type of treatment each patient received. Masking of the active and placebo treatments was preserved by creating treatments that looked identical. The hospital pharmacist was informed of all randomization assignments and was responsible for labeling the study drug and maintaining a master list linking participants and their treatment assignments. Participants were evaluated by a qualified neurologist (MS) at baseline and 6 months after the start of the therapy to evaluate the development of side-effects of the medications, compliance, and disease activity.
The primary outcome measure was mean changes in RNFL measurements from baseline to 6 months after receiving vitamin D or placebo as measured by the OCT. Mean changes in the macular thickness were also measured for both groups.
Statistical analysis
The sample size was calculated when the study was designed and was based on the comparison of two means. We estimated that 5 μm mean difference in RNFL may be clinically significant. We calculated that 33 patients per treatment group would be required to provide the study with approximately 80% power to detect (with a two-sided alpha of 0.05) a mean difference in RNFL between vitamin D and placebo groups assuming a standard deviation (SD) of 9, given an anticipated dropout rate of 20%.
Between-group comparison of changes was made using Mann–Whitney U-tests. Within group comparisons were undertaken using Wilcoxon sign-rank test, to determine differences between baseline and 6 months assessment of RNFL. Comparisons between proportions were undertaken using Chi-square or Fisher's exact test. Results are expressed as mean (SD) and P < 0.05 was considered statistically significant. All statistical tests were two-sided. The analyses were undertaken using SPSS for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
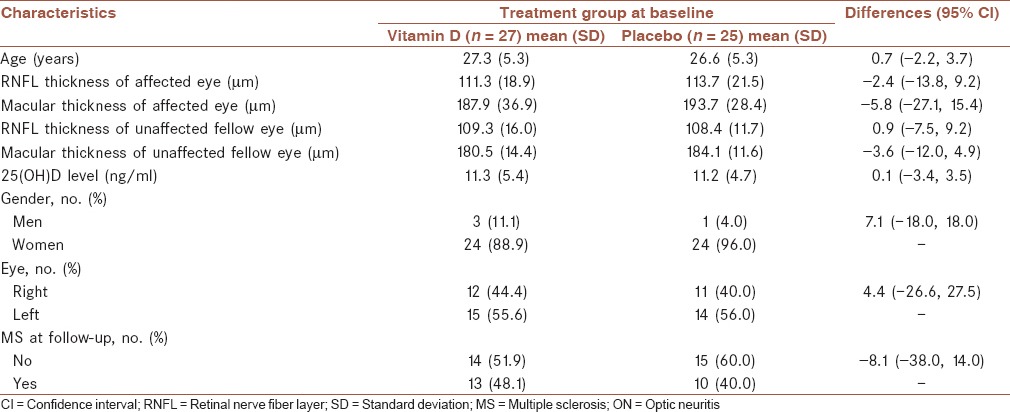
Seventy-four patients entered the study and 22 dropped out. Patient compliance with treatment was good. All 52 patients who completed treatment were available for follow-up at 6 months. The two treatment groups were generally well matched at baseline with regard to age, gender, thickness of RNFL, and macular thickness of affected and unaffected contralateral eyes, 25(OH)D level and other ON characteristics. ON involved the right eye in 23 patients (44.2%) and the left eye in the remaining 29 (55.8%) patients. Mean (SD) age in the vitamin D and placebo groups were 27.3 (5.3) and 26.6 (5.3) years, respectively. Mean (SD) RNFL thickness at the start of treatment was 111.3 (19.9) μm in the vitamin D group and 113.7 (21.1) μm in the placebo group [Table 1].
Table 1.
Characteristics of patients with ON who received vitamin D or placebo at baseline
Vitamin D treatment was well-tolerated and most of the adverse events reported were mild in severity. The most common side-effects of vitamin D were constipation (n = 5), dyspepsia (n = 4), fatigue (n = 3), and headache (n = 2). The most common side-effects of placebo were also constipation (n = 4), dyspepsia (n = 2), fatigue (n = 5), and headache (n = 1). There were no instances of urinary dysfunction or symptomatic nephrolithiasis. No, dose adjustment was necessary. There were no substantial differences between vitamin D and placebo groups in frequency or pattern of adverse events.
Mean thickness of RNFL and the macula before and after receiving vitamin D or placebo are shown in Table 2. In both groups, the average RNFL thickness decreased significantly. Of the 27 patients treated with vitamin D, the mean (SD) RNFL thickness decreased from 111.3 (18.9) μm at baseline to 91.4 (13.3) μm at the end of study period (P < 0.001). In the 25 patients treated with placebo, the mean (SD) RNFL thickness decreased from 113.7 (21.5) μm at baseline to 96.1 (12.3) at the end of study period (P < 0.001). After 6 months, the mean difference in change in RNFL thickness between vitamin D and placebo group was −4.7 (95% CI: −11.5, 2.8) μm (25%), indicating there is no evidence of an effect on the ON during the first 6 months of therapy in patients who received vitamin D compared to those who received the placebo.
Table 2.
Comparison of thickness of the macula and RNFL in 52 patients with ON before and 6 months after treatment with vitamin D and placebo
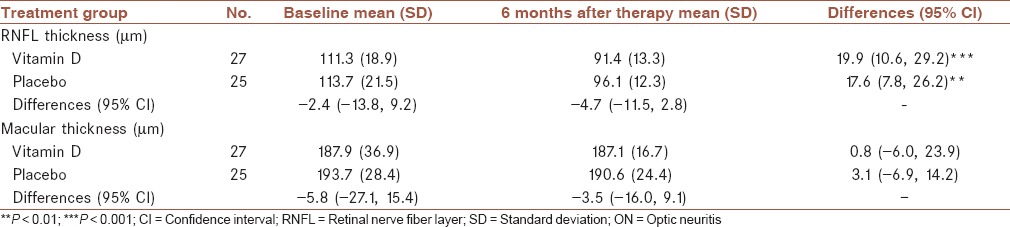
Although not statistically significant, the mean thickness of the macula appeared to increase in unaffected eyes of both groups. In both groups, the mean macular thickness did not changed in affected eyes (P > 0.05). There was no significant difference in the macula at the end of the study period between the vitamin D and placebo groups.
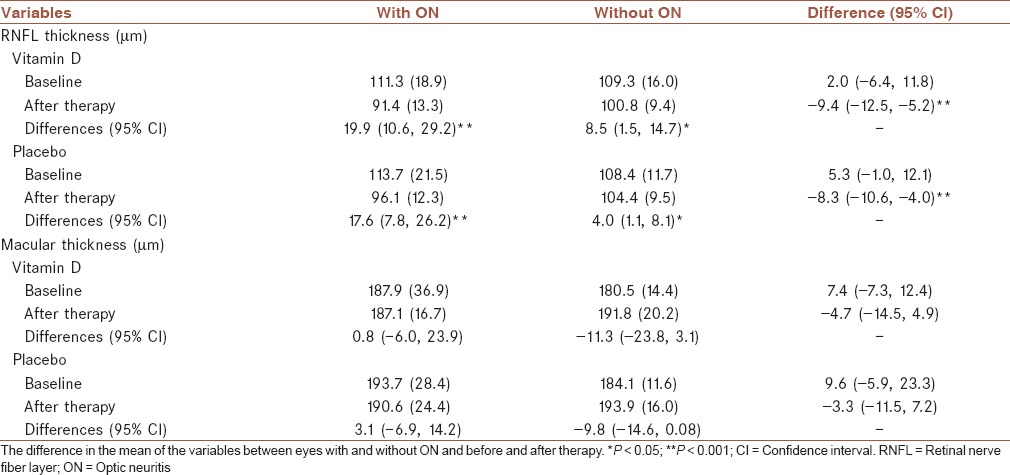
In both groups, the mean thickness of RNFL of the affected eyes compared with the unaffected contralateral eyes significantly decreased 6 months after vitamin D or placebo treatment. In vitamin D group, the difference in thickness of RNFL of the affected eyes compared with the unaffected contralateral eyes averaged (95% CI) −8.9 (−12.5, −5.2; P < 0.001) μm. Correspondingly, in the placebo group, the difference in thickness of RNFL of the affected eyes compared with the unaffected contralateral eyes averaged (95% CI) −7.3 (−10.6, −4.0; P < 0.001) μm [Table 3]. The mean change between pre- and post-study RNFL values in the affected eyes is almost similar at 19.9 and 17.6 microns in the two groups. In both groups, slight, but statistically significant, decrease in RNFL thickness was also observed between baseline and follow-up in unaffected contralateral eyes.
Table 3.
Comparison of thickness of the macula and RNFL in 52 pairs of eyes with and without ON before and 6 months after treatment with vitamin D and placebo
DISCUSSION
In this exploratory Phase II study, we found no significant difference in mean thickness of RNFL or macula between participants who took the placebo versus those who received adjunct oral vitamin D during 6 months. No unusual or unexpected safety risks were found with vitamin D therapy in our study population with ON. Previous studies have shown that vitamin D is fairly safe,[14] especially in terms of its effects on the gastrointestinal tract.[14] We did not find any significant differences between the active drug and the placebo in safety, and there were no distinct patterns in adverse events.
While the efficacy of vitamin D for treatment of MS has been examined in a few small trials with variable results,[15,16,17,23,26,27] to the best of our knowledge, no other studies are available comparing vitamin D with placebo in the preventing the progression of ON and this is the first randomized clinical trial to compare the effect of adjunct vitamin D in the preventing the progression of ON.
Several studies have performed OCT to assess RNFL thickness and macular volume in acute ON.[3,4,5,6,7,18,23] Our findings are consistent with other studies that found substantial reductions in RNFL thickness following a clinical episode of acute ON.[3,4,5,6,7,18,23] Serial and single time point studies at variable intervals following the episode indicate that most of this RNFL loss is evident 3-6 months following the onset of visual dysfunction.
We found that RNFL was statistically thinner in ON when compared with contralateral normal eyes 6 months after treatment with vitamin D or placebo. This finding is consistent with the hypothesis that optic nerve axonal loss results in retrograde axonal degeneration.[28]
Our findings are consistent with other studies that reported thinning of the RNFL in clinically unaffected eyes (i.e., eyes in which there has not been a previous clinical episode of ON) of people with MS when compared with healthy controls.[3,18,23,29] The reductions are less marked than those seen following ON, but nonetheless are statistically significant.
Albeit, this study is only placebo-controlled trials to date of effect of oral vitamin D on the thickness of RNFL and macula in ON patients the sample sizes may be small to allow detection of small changes in thickness of RNFL and macula between groups, and was limited by the loss to follow-up of 22/74 of the original baseline cohort. However, because the percentages and reasons for patients being lost to follow-up were not different between the vitamin D supplemented and placebo group, it is unlikely that the dropout rate affected the outcome of our study. The efficacy should therefore be tested in a larger sample. The present results clearly need to be replicated and extended across multiple centers and investigators. The mean macular thickness increased from baseline to 6 months in the unaffected eyes (presumably a chance effect) while macular thickness decreased slightly in the affected eyes and it could be argued that the mean macular thickness in unaffected eyes “regressed to the mean.” Although substantial evidence supports the safety of even large dose of vitamin D, such evidence is based on studies of limited size and duration. The best level of 25(OH)D for health is uncertain. Many experts believe that blood levels of 25(OH)D above 30 ng/mL are adequate.[30] A few studies also suggested that levels higher than 30 ng/mL may further help protect patients with MS.[29] Therefore, our study suggests that the dose of 50,000 IU/week vitamin D in patients with insufficient serum 25(OH)D level may be considered relatively safe.
CONCLUSION
This exploratory Phase II comparative trial of vitamin D supplementation and placebo showed that adding vitamin D to routine therapy had no significant effect on the thickness of RNFL or macula.
AUTHORS CONTRIBUTIONS
MS conceived and designed the study, recruited samples and contributed to discussion and revision of the manuscript, MJ analyzed the data and wrote the manuscript, ME contributed to the trial design and discussion and revision of the manuscript, ARD, HR and GAN conducted patients ophthalmologic visits and performed OCT. All authors discussed the results and reviewed and edited the manuscript.
ACKNOWLEDGMENTS
This study was partially supported by grants from the Isfahan University of Medical Sciences, Iran (Grant number: 290090). No pharmaceutical company supported it financially.
Footnotes
Source of Support: This study was partially supported by grants from the Isfahan University of Medical Sciences, Iran (Grant number: 290090)
Conflict of Interest: None declared.
REFERENCES
- 1.Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953–62. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson P, Larsson EM, Maly-Sundgren P, Perfekt R, Sandberg-Wollheim M. Predicting the outcome of optic neuritis: Evaluation of risk factors after 30 years of follow-up. J Neurol. 2005;252:396–402. doi: 10.1007/s00415-005-0655-9. [DOI] [PubMed] [Google Scholar]
- 3.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7. [PubMed] [Google Scholar]
- 4.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 5.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: A prospective study using optical coherence tomography. Mult Scler. 2008;14:893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- 6.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, et al. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol. 2008;64:325–31. doi: 10.1002/ana.21474. [DOI] [PubMed] [Google Scholar]
- 7.Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–91. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 8.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 9.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 10.Deluca HF, Cantorna MT. Vitamin D: Its role and uses in immunology. FASEB J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 11.Niino M. Vitamin D and its immunoregulatory role in multiple sclerosis. Drugs Today (Barc) 2010;46:279–90. doi: 10.1358/dot.2010.46.4.1476498. [DOI] [PubMed] [Google Scholar]
- 12.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 14.Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–9. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: Decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses. 1986;21:193–200. doi: 10.1016/0306-9877(86)90010-1. [DOI] [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Lesaux J, Rice GP, Kremenchutzky M, Ebers GC. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1294–6. doi: 10.1136/jnnp.2004.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achiron A, Barak Y, Miron S, Izhak Y, Faibel M, Edelstein S. Alfacalcidol treatment in multiple sclerosis. Clin Neuropharmacol. 2003;26:53. doi: 10.1097/00002826-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302–8. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119:1135–42. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- 21.Paunescu LA, Schuman JS, Price LL, Stark PC, Beaton S, Ishikawa H, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–24. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naismith RT, Tutlam NT, Xu J, Klawiter EC, Shepherd J, Trinkaus K, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72:1077–82. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277–87. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- 24.Moura FC, Medeiros FA, Monteiro ML. Evaluation of macular thickness measurements for detection of band atrophy of the optic nerve using optical coherence tomography. Ophthalmology. 2007;114:175–81. doi: 10.1016/j.ophtha.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaygannejad V, Janghorbani M, Ashtari F, Dehghan H. Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: Preliminary findings of a randomized placebo-controlled trial. Mult Scler Int 2012. 2012:452541. doi: 10.1155/2012/452541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soilu-Hänninen M, Aivo J, Lindström BM, Elovaara I, Sumelahti ML, Färkkilä M, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–71. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 28.Shindler KS, Ventura E, Dutt M, Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res. 2008;87:208–13. doi: 10.1016/j.exer.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–92. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- 30.Solomon AJ, Whitham RH. Multiple sclerosis and vitamin D: A review and recommendations. Curr Neurol Neurosci Rep. 2010;10:389–96. doi: 10.1007/s11910-010-0131-5. [DOI] [PubMed] [Google Scholar]


